Precision Phenomapping of Acute Coronary Syndromes to Improve Patient Outcomes
Abstract
:1. Introduction
2. Precision and Personalised Medicine
3. Why Intensify Precision Medicine for Acute Coronary Syndromes
4. Deep Precision Mapping of Acute Coronary Syndromes
5. Current Precision Treatments of Acute Coronary Syndromes
6. Precision Research Applied to Acute Coronary Syndromes
7. Precision Mapping of Healthy Phenotypes
8. Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 29, ehaa575. [Google Scholar]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST- elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar]
- Jernberg, T.; Johanson, P.; Held, C.; Svennblad, B.; Lindbäck, J.; Wallentin, L. SWEDEHEART/RIKS-HIA. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011, 305, 1677–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tea, V.; Bonaca, M.; Chamandi, C.; Iliou, M.C.; Lhermusier, T.; Aissaoui, N.; Cayla, G.; Angoulvant, D.; Ferrières, J.; Schiele, F.; et al. FAST-MI investigators. Appropriate secondary prevention and clinical outcomes after acute myocardial infarction according to atherothrombotic risk stratification: The FAST-MI 2010 registry. Eur. J. Prev. Cardiol. 2019, 26, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Maseri, A.; Andreotti, F. Targeting new thrombolytic regimens at specific patient groups: Implications for research and cost-containment. Eur. Heart J. 1997, 18, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Navarese, E.P.; Zheng, B.; Meng, Q.; Liu, N.; Ge, H.; Pan, Q.; Yu, Y.; Ma, X. Analytics with artificial intelligence to advance the treatment of acute respiratory distress syndrome. J. Evid. Based Med. 2020, 13, 301–302. [Google Scholar] [CrossRef]
- National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Leopold, J.A.; Loscalzo, J. Emerging role of precision medicine in cardiovascular disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Longo, D.L. Precision medicine—personalized, problematic, and promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crea, F.; Libby, P. Acute coronary syndromes: The way forward from mechanisms to precision treatment. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Becker, R.C. Atherothrombotic disorders: New insights from hematology. Circulation 2005, 111, 1855–1863. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. The complex molecular genetics of familial hypercholesterolaemia. Nat. Rev. Cardiol. 2019, 16, 9–20. [Google Scholar] [CrossRef]
- Cesario, A.; D’Oria, M.; Scambia, G. La Medicina Personalizzata tra Ricerca e Cura; FrancoAngeli s.r.l.: Milano, Italy, 2021; p. 62. [Google Scholar]
- Becker, R.; Andreotti, F. Proteomics, metabolomics and progenitor cells in acute coronary syndromes. J. Thromb. Thrombolysis 2006, 22, 85–88. [Google Scholar] [CrossRef]
- Ferrannini, G.; Manca, M.L.; Magnoni, M.; Andreotti, F.; Andreini, D.; Latini, R.; Maseri, A.; Maggioni, A.P.; Ostroff, R.M.; Williams, S.A.; et al. Coronary artery disease and type 2 diabetes: A proteomic study. Diabetes Care 2020, 43, 843–851. [Google Scholar] [CrossRef]
- Available online: https:www.escardio.org/Research/Big-Data-Heart (accessed on 20 January 2021).
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uemura, S.; Souteyrand, G.; Virmani, R.; Motreff, P.; Di Vito, L.; Biondi-Zoccai, G.; Halperin, J.; Fuster, V.; Ozaki, Y.; et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc. Imaging 2013, 6, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective anti-thrombotic therapy without stenting: Intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 2017, 38, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A.; et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes: The PHARMCLO Trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Vrey, E.; Heestermans, T.; Tjon Joe Gin, M.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Yang, J.; Yu, X.; Liu, L. Genotype-guided antiplatelet therapy compared with standard therapy for patients with acute coronary syndromes or undergoing percutaneous coronary intervention: A systematic review and meta-analysis. Thromb. Res. 2020, 193, 130–138. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. Effect of Dalcetrapib vs Placebo on Cardiovascular Risk in a Genetically Defined Population with a Recent Acute Coronary Syndrome (dal-GenE). Available online: https://clinicaltrials.gov/ct2/show/NCT02525939 (accessed on 20 January 2021).
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Iervolino, A.; Andreotti, F. Is it time to introduce anti-inflammatory drugs into secondary cardiovascular prevention: Evidence from clinical trials. Vessel Plus 2021, 5. [Google Scholar] [CrossRef]
- US National Library of Medicine. The Microbiome as a Target for Precision Medicine in Atherosclerosis (MIGATER). Available online: https://clinicaltrials.gov/ct2/show/NCT03434483 (accessed on 20 January 2021).
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: Inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. Biomarker-Based Prognostic Assessment. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04044066 (accessed on 20 January 2021).
- Tardif, J.C.; Rhéaume, E.; Lemieux Perreault, L.P.; Grégoire, J.C.; Feroz Zada, Y.; Asselin, G.; Provost, S.; Barhdadi, A.; Rhainds, D.; L’Allier, P.L.; et al. Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib. Circ. Cardiovasc. Genet. 2015, 8, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, M.; Yonetsu, T.; Kurihara, O.; Nakajima, A.; Lee, H.; Soeda, T.; Minami, Y.; Higuma, T.; Kimura, S.; Takano, M.; et al. Circadian variations in pathogenesis of ST-segment elevation myocardial infarction: An optical coherence tomography study. J. Thromb. Thrombolysis 2021, 51, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Kluft, C. Circadian variation of fibrinolytic activity in blood. Chronobiol. Int. 1991, 8, 336–351. [Google Scholar] [CrossRef]
- Myocardial Infarction Genetics CARDIoGRAMExome Consortia Investigators; Stitziel, N.O.; Stirrups, K.E.; Masca, N.G.; Erdmann, J.; Ferrario, P.G.; König, I.R.; Weeke, P.E.; Webb, T.R.; Auer, P.L.; et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N. Engl. J. Med. 2016, 374, 1134–1144. [Google Scholar]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Gunnarsdottir, K.; Halldorsson, G.H.; Sigurdsson, A.; Arnadottir, G.A.; Jonsson, H.; Olafsdottir, E.F.; Niehus, S.; Kehr, B.; Sveinbjörnsson, G.; et al. Lifelong reduction in LDL (Low-Density Lipoprotein) cholesterol due to a gain-of-function mutation in LDLR. Circ. Genom. Precis. Med. 2021, 14, e003029. [Google Scholar] [CrossRef] [PubMed]
- Peter, I.S. The function of architecture and logic in developmental gene regulatory networks. Curr. Top. Dev. Biol. 2020, 139, 267–295. [Google Scholar] [PubMed]
- Hartman, R.J.G.; Owsiany, K.; Ma, L.; Koplev, S.; Hao, K.; Slenders, L.; Civelek, M.; Mokry, M.; Kovacic, J.C.; Pasterkamp, G.; et al. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation 2021, 143, 713–726. [Google Scholar] [CrossRef] [PubMed]
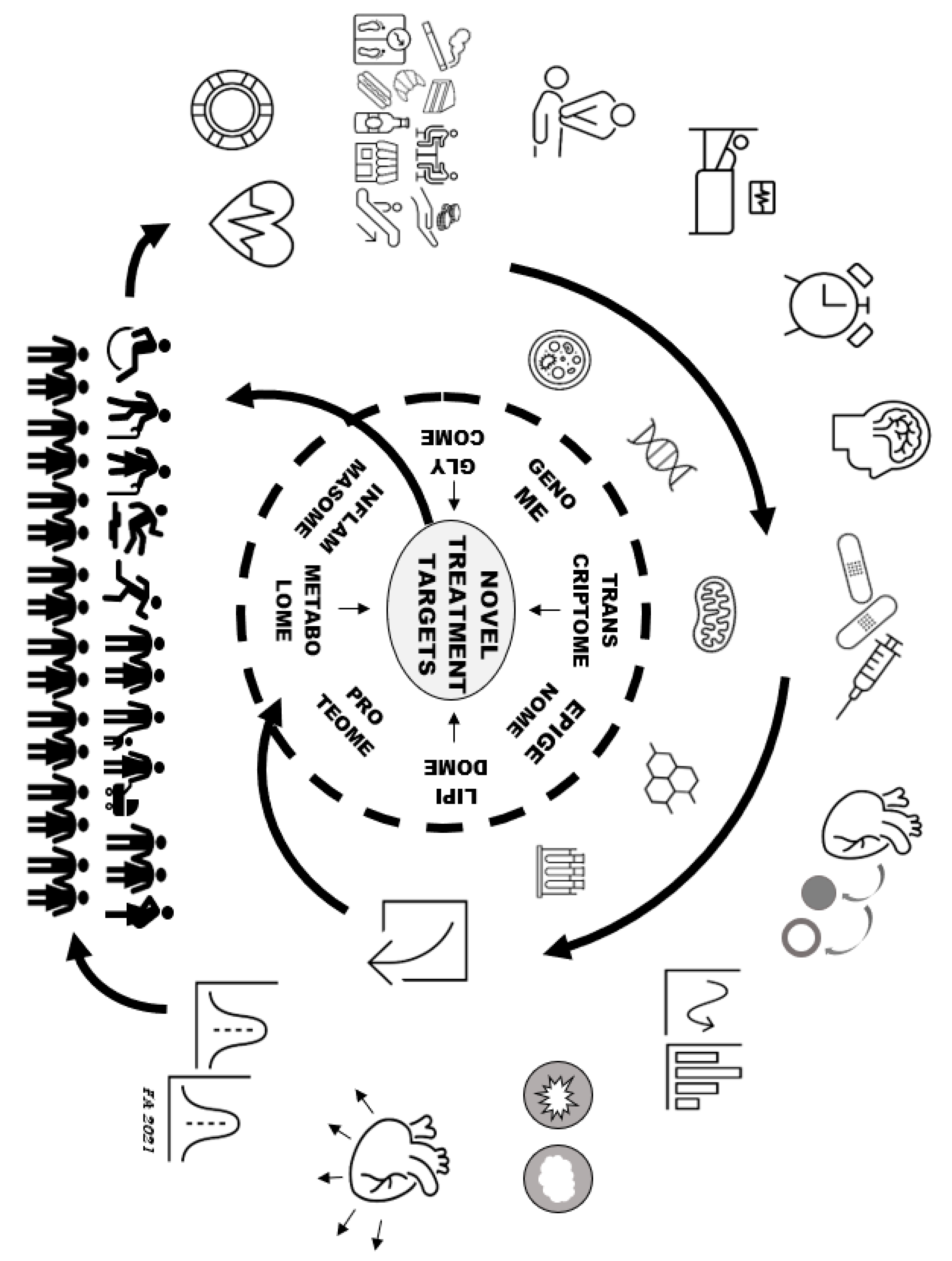
| Non-Omic Characteristics | Genomic and Extra-Genomic Mapping Tools | Future Challenges |
|---|---|---|
| ● Demographics ● Presentation ● History ● Environment ● Diet ● Psychosocial ● Other risk factors ● Outcomes ● Medications ● Medical adherence ● Adverse events ● Imaging ● Traditional laboratory ● Histology ● Cytometry ● Immunophenotype | ● Genome ● Epigenome ● Transcriptome ● Next-generation sequencing ● Metabolome ● Lipidome ● Glycome ● Proteome ● Inflammasome ● Thrombosome ● Fibrosome ● Immunophenome ● Metagenome/microbiome ● Interactome ● Radiomics | ● Standardisation and quality control ● Accounting for big data multiple testing ● Identification of:
|
| Criterion | Precision Treatment | |
|---|---|---|
| Established Treatments |
| |
| ||
| ||
| ||
| Investigational Treatments |
| |
| ||
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreotti, F.; Iervolino, A.; Navarese, E.P.; Maggioni, A.P.; Crea, F.; Scambia, G. Precision Phenomapping of Acute Coronary Syndromes to Improve Patient Outcomes. J. Clin. Med. 2021, 10, 1755. https://doi.org/10.3390/jcm10081755
Andreotti F, Iervolino A, Navarese EP, Maggioni AP, Crea F, Scambia G. Precision Phenomapping of Acute Coronary Syndromes to Improve Patient Outcomes. Journal of Clinical Medicine. 2021; 10(8):1755. https://doi.org/10.3390/jcm10081755
Chicago/Turabian StyleAndreotti, Felicita, Adelaide Iervolino, Eliano Pio Navarese, Aldo Pietro Maggioni, Filippo Crea, and Giovanni Scambia. 2021. "Precision Phenomapping of Acute Coronary Syndromes to Improve Patient Outcomes" Journal of Clinical Medicine 10, no. 8: 1755. https://doi.org/10.3390/jcm10081755







