Idiopathic Peripheral Retinal Telangiectasia in Adults: A Case Series and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
- Category 1: IPT without peripheral exudates and without macular involvement (patient 1)
- Category 2: IPT with peripheral exudates and without macular involvement (patient 2)
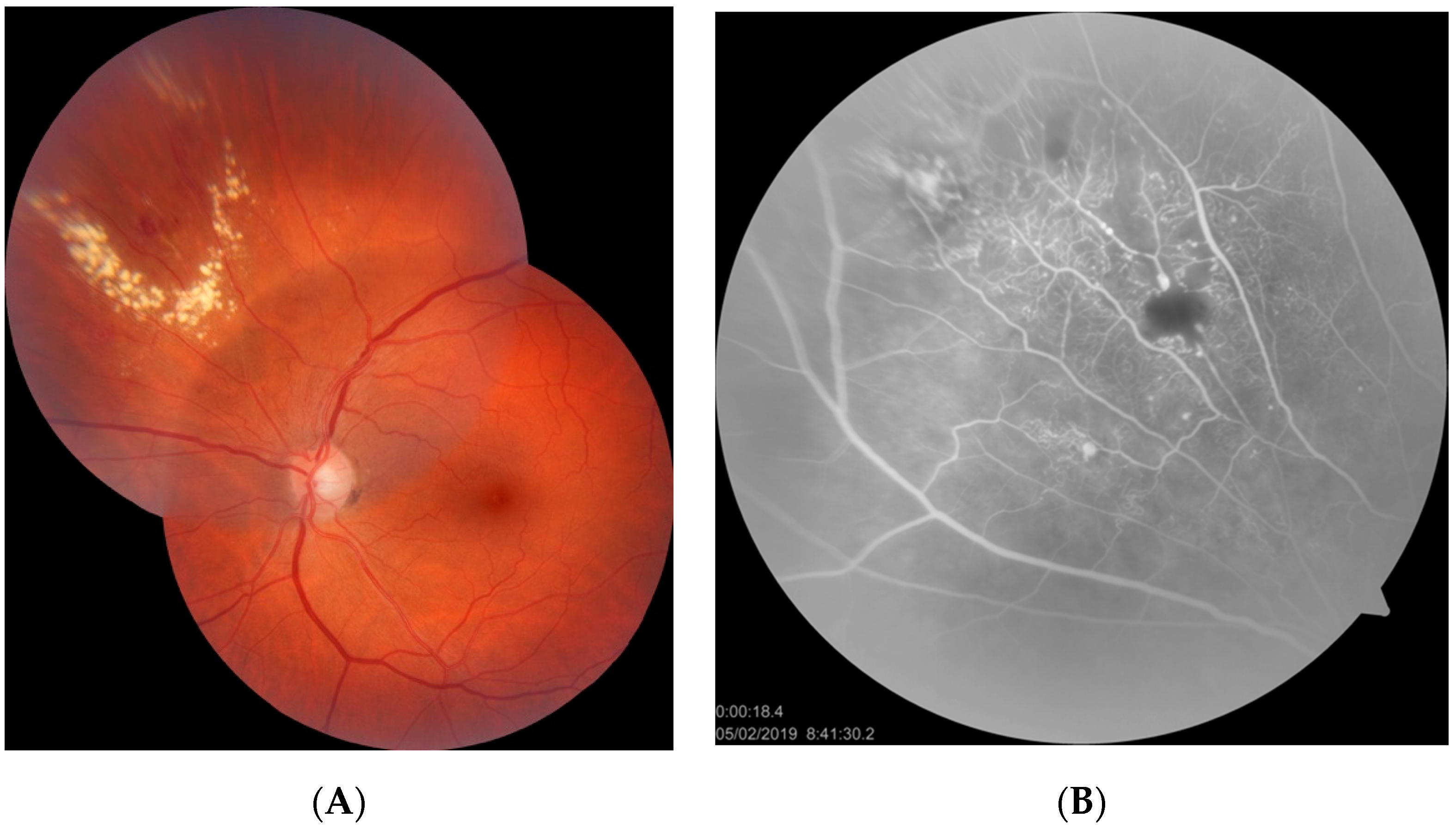
- Category 3: IPT with peripheral exudates and cystoid macular edema without exudates (patients 3,4,5)
- Category 4: IPT with peripheral exudates and macular hard exudates and edema (patient 6)
3.1. Categories 1 and 2
3.2. Category 3
3.3. Category 4
4. Literature Review
5. Discussion
5.1. Definitions, Classification, and Presentation
5.2. Diagnostics
5.3. Treatment
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coats, G. Forms of retinal diseases with massive exudation. R. Lond. Ophthalmol. Hosp. Rep. 1908, 17, 440–525. [Google Scholar]
- Shields, J.A.; Shields, C.L.; Honavar, S.G.; Demirci, H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am. J. Ophthalmol. 2001, 131, 561–571. [Google Scholar] [CrossRef]
- Egbert, P.R.; Chan, C.C.; Winter, F.C. Flat preparations of the retinal vessels in Coats’ disease. J. Pediatr. Ophthalmol. 1976, 13, 336–339. [Google Scholar]
- Fernandes, B.F.; Odashiro, A.N.; Maloney, S.; Zajdenweber, M.E.; Lopes, A.G.; Burnier, M.N., Jr. Clini-cal-histopathological correlation in a case of Coats’ disease. Diagn. Pathol. 2006, 1, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, C.L.; Sen, M.; Honavar, S.G.; Shields, J.A. Coats disease: An overview of classification, management and outcomes. Indian J. Ophthalmol. 2019, 67, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.H.; Kroll, A.J.; Lou, P.L.; Ryan, E.A. Coats’ disease. Int. Ophthalmol. Clin. 2001, 41, 189–198. [Google Scholar] [CrossRef]
- Gawecki, M. Tetniaki prosówkowate Lebera—Opis przypadku [Leber miliary aneurysms—A case report. Klin Oczna. 2009, 111, 131–133. [Google Scholar]
- Reese, A.B. Telangiectasis of the Retina and Coats’ Disease. Am. J. Ophthalmol. 1956, 42, 1–8. [Google Scholar] [CrossRef]
- Alturkistany, W.; Waheeb, S. Leber’s miliary aneurysms. Oman J. Ophthalmol. 2013, 6, 119–121. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L.; Honavar, S.G.; Demirci, H.; Cater, J. Classification and management of Coats disease: The 2000 Proctor Lecture. Am. J. Ophthalmol. 2001, 131, 572–583. [Google Scholar] [CrossRef]
- Yanuzzi, A.L. The Retinal Atlas; Elsevier Ltd.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Cahill, M.; O’Keefe, M.; Acheson, R.; Mulvihill, A.; Wallace, D.; Mooney, D. Classification of the spectrum of Coats’ disease as subtypes of idiopathic retinal telangiectasis with exudation. Acta Ophthalmol. Scand. 2001, 79, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Bardal, A.M.; Freund, K.B.; Chen, K.J.; Eandi, C.M.; Blodi, B. Idiopathic macular telangiectasia. Arch. Ophthalmol. 2006, 124, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbel Issa, P.; Gillies, M.C.; Chew, E.Y.; Bird, A.C.; Heeren, T.F.; Peto, T.; Holz, F.G.; Scholl, H.P. Macular telangiectasia type 2. Prog. Retin. Eye Res. 2013, 34, 49–77. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Udyaver, S.; Dalvin, L.A.; Lim, L.S.; Atalay, H.T.; Khoo, C.T.L.; Mazloumi, M.; Shields, J.A. Coats disease in 351 eyes: Analysis of features and outcomes over 45 years (by decade) at a single center. Indian J. Ophthalmol. 2019, 67, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Smithen, L.M.; Brown, G.C.; Brucker, A.J.; Yannuzzi, L.A.; Klais, C.M.; Spaide, R.F. Coats’ Disease Diagnosed in Adulthood. Ophthalmology 2005, 112, 1072–1078. [Google Scholar] [CrossRef]
- Goel, N.; Kumar, V.; Seth, A.; Raina, U.K.; Ghosh, B. Role of intravitreal bevacizumab in adult onset Coats’ disease. Int. Ophthalmol. 2011, 31, 183–190. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Cheng, C.-K. A Combination of Intravitreal Bevacizumab Injection with Tunable Argon Yellow Laser Photocoagulation as a Treatment for Adult-Onset Coats’ Disease. J. Ocul. Pharmacol. Ther. 2011, 27, 525–530. [Google Scholar] [CrossRef]
- Zheng, X.X.; Jiang, Y.R. The effect of intravitreal bevacizumab injection as the initial treatment for Coats’ disease. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 35–42. [Google Scholar] [CrossRef]
- Park, S.; Cho, H.J.; Lee, D.W.; Kim, C.G.; Kim, J.W. Intravitreal bevacizumab injections combined with laser photocoagulation for adult-onset Coats’ disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 254, 1511–1517. [Google Scholar] [CrossRef]
- Rishi, P.; Rishi, E.; Appukuttan, B.; Uparkar, M.; Sharma, T.; Gopal, L. Coats′ disease of adult-onset in 48 eyes. Indian J. Ophthalmol. 2016, 64, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ke, Y.; Wang, W.; Shi, X.; Hei, K.; Li, X. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats’ disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Metelitsina, T.I.; Sheth, V.S.; Patel, S.B.; Grassi, M.A. Peripheral retinopathy associated with aplastic anemia. Retin. Cases Brief. Rep. 2017, 11, 108–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klifto, M.R.; Balaratnasingam, C.; Weissman, H.H.; Yannuzzi, L.A. Bilateral coats reaction in bannayan–zonana syndrome: A single case report. Retin. Cases Brief. Rep. 2017, 11, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Taleb, E.A.; Nagpal, M.P.; Mehrotra, N.S.; Bhatt, K. Retinal findings in a case of presumed cutis marmorata telangiectatica congenita. Retin. Cases Brief. Rep. 2018, 12, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Soohoo, J.R.; McCourt, E.A.; Lenahan, D.S.; Oliver, S.C. Fluorescein angiogram findings in a case of cutis marmorata telangiectatica congenita. Ophthalmic Surg. Lasers Imaging Retina 2013, 44, 398–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Massougnes, S.; Borruat, F.X.; Ambresin, A. Peripheral Bilateral Telangiectasiae in Multiple Sclerosis Patients Treated with Interferon B1a. Klin. Monbl. Augenheilkd. 2016, 233, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Batliwala, S.Y.; Perez, M.; Aston, W.; Chavala, S.H. Peripheral Retinal Telangiectasia and Ischemia in Takayasu Arteritis. Arthritis Rheumatol. 2016, 68, 2350. [Google Scholar] [CrossRef] [Green Version]
- Stacey, A.W.; Sparagna, C.; Borri, M.; Rizzo, S.; Hadjistilianou, T. A 6-year-old boy with Cornelia de Lange syndrome and Coats disease: Case report and review of the literature. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, A.; Yolcu, U.; Gundogan, F.C.; Akay, F. An association between subclinical familial exudative vitreoretinopathy and rod-cone dystrophy. Arq. Bras. Oftalmol. 2014, 77. [Google Scholar] [CrossRef]
- Kashani, A.H.; Brown, K.T.; Chang, E.; Drenser, K.A.; Capone, A.; Trese, M.T. Diversity of Retinal Vascular Anomalies in Patients with Familial Exudative Vitreoretinopathy. Ophthalmology 2014, 121, 2220–2227. [Google Scholar] [CrossRef]
- Sacconi, S.; Baillif-Gostoli, S.; Desnuelle, C. Atteinte rétinienne et myopathies génétiques [Retinal involvement and genetic myopathy]. Rev. Neurol. 2010, 166, 998–1009. [Google Scholar] [CrossRef]
- Johnson, C.A.; Hatfield, M.; Pulido, J.S. Retinal vasculopathy in a family with autosomal dominant dyskeratosis congenita. Ophthalmic Genet. 2009, 30, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Yilmaz, T.; Aydemir, O.; Güler, M.; Kurt, J. Coats-like retinitis pigmentosa: Reports of three cases. Clin. Ophthalmol. 2007, 1, 193–198. [Google Scholar] [PubMed]
- Char, D.H. Coats’ syndrome: Long term follow up. Br. J. Ophthalmol. 2000, 84, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Chandra, P.; Kumar, A. Ultra-wide field imaging in the diagnosis and management of adult-onset Coats’ disease. Clin. Exp. Optom. 2017, 100, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joussen, A.M.; Brockmann, C.; Urban, J.; Seibel, I.; Winterhalter, S.; Zeitz, O.; Müller, B. Ultraweitwinkel-Fundusfotografie und -angiografie in der Differenzialdiagnose und zur Therapieplanung bei peripheren vaskulären Netzhauterkrankungen [Ultra-Wide Field Retinal Imaging and Angiography in the Differential Diagnosis and Therapeutic Decisions in Vascular Diseases of the Peripheral Retina]. Klin. Monbl. Augenheilkd. 2018, 235, 980–993. [Google Scholar]
- Sakurada, Y.; Freund, K.B.; Yannuzzi, L.A. Multimodal Imaging in Adult-Onset Coats’ Disease. Ophthalmology 2018, 125, 485. [Google Scholar] [CrossRef]
- Temkar, S.; Azad, S.V.; Chawla, R.; Damodaran, S.; Garg, G.; Regani, H.; Nawazish, S.; Raj, N.; Venkatraman, V. Ultra-widefield fundus fluorescein angiography in pediatric retinal vascular diseases. Indian J. Ophthalmol. 2019, 67, 788–794. [Google Scholar] [CrossRef]
- Turczyńska, M.; Brydak-Godowska, J. The role of peripheral retinal angiography in the diagnosis of adult Coats’ disease based on a case report. Klin. Ocz. 2020, 122, 171–176. [Google Scholar] [CrossRef]
- Yang, L.-H.; Shi, X.-H.; Tian, B.; Zhou, D.; Wei, W.-B. Clinical analysis of macular disease involved by Coats disease. [Zhonghua yan ke za zhi] Chin. J. Ophthalmol. 2009, 45. [Google Scholar]
- Khoo, C.T.L.; Dalvin, L.A.; Lim, L.S.; Mazloumi, M.; Atalay, H.T.; Udyaver, S.; Shields, J.A.; Shields, C.L. Fac-tors Predictive of Subretinal Fluid Resolution in Coats Disease: Analysis of 177 Eyes in 177 Pa-tients at a Single Center. Asia Pac. J. Ophthalmol. 2019, 8, 290–297. [Google Scholar] [CrossRef]
- Zhao, Q.; Peng, X.-Y.; Chen, F.-H.; Zhang, Y.-P.; Wang, L.; You, Q.-S.; Jonas, J.B. Vascular endothelial growth factor in Coats’ disease. Acta Ophthalmol. 2013, 92, e225–e228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Ruan, L.; Huang, X. Associations of cytokine concentrations in aqueous humour with retinal vascular abnormalities and exudation in Coats’ disease. Acta Ophthalmol. 2019, 97, 319–324. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, W.; Shi, X. Successful use of intravitreal ranibizumab injection and combined treatment in the management of Coats’ disease. Acta Ophthalmol. 2016, 94, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, A.S.; Villegas, V.M.; Murray, T.G.; Berrocal, A.M. Advanced Coats’ disease treated with intravitreal bevacizumab combined with laser vascular ablation. Clin. Ophthalmol. 2014, 8, 973–976. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Shields, C.L. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br. J. Ophthalmol. 2011, 96, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Tran, H.V.; Gaillard, M.-C.; Munier, F.L. Extramacular fibrosis in coats’ disease. Retina 2016, 36, 2022–2028. [Google Scholar] [CrossRef]
- Schefler, A.C.; Berrocal, A.M.; Murray, T.G. Advanced Coats’ disease. Management with repetitive aggressive laser ablation therapy. Retina 2008, 28 (Suppl. 3), S38–S41. [Google Scholar] [CrossRef]
- Georgakopoulos, C.D.; Foteini, T.; Makri, O.E.; Vavvas, D. Two-year results of intravitreal injections of aflibercept in Coats’ Disease; a case report. Retin. Cases Brief. Rep. 2020. [Google Scholar] [CrossRef]
- Beselga, D.; Campos, A.; Mendes, S.; Carvalheira, F.; Castro, M.; Castanheira, D. Refractory Coats’ Disease of Adult Onset. Case Rep. Ophthalmol. 2012, 3, 118–122. [Google Scholar] [CrossRef]
- Namba, M.; Shiode, Y.; Morizane, Y.; Kimura, S.; Hosokawa, M.; Doi, S.; Toshima, S.; Takahashi, K.; Hosogi, M.; Fujiwara, A.; et al. Successful resolution of coats disease by photodynamic therapy: A case report. BMC Ophthalmol. 2018, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, K.H.; Woo, S.J. Combined Photodynamic Therapy and Intravitreal Bevacizumab Injection for the Treatment of Adult Coats’ Disease: A Case Report. Korean J. Ophthalmol. 2010, 24, 374–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Term | Characteristics |
|---|---|
| Coats disease | Idiopathic, nonhereditary aneurysmal retinal telangiectasia associated with intraretinal exudation and frequent exudative retinal detachment, occurring in patients aged a few months to seven decades with the peak in the first decade of life [2] |
| Leber miliary aneurysms | A milder variant of Coats disease, usually occurring in young adults and located at the retinal periphery [7,8,9] |
| Idiopathic retinal telangiectasia | A descriptive name for a Coats disease |
| MACTEL type 1 | Macular telangiectasia type 1—aneurysmal type of macular telangiectasia, considered a central variant of Coats disease [13] |
| MACTEL type 2 | Macular telangiectasia type 2—non-aneurysmal perifoveal capillary telangiectasia, associated with atrophy of neurosensory retina, presenting in the non-proliferative or proliferative form [13,14] |
| Case No/Gender | Proposed Category | Age (Years) | Eye, BCVA, Presentation of the Macula | Treatment | Follow-Up | Final BCVA and Disease State |
|---|---|---|---|---|---|---|
| 1. M | 1 | 20 | RE, 20/20; no exudates, macular area normal | Observation | 12 months | 20/20; no progression |
| 2. F | 2 | 60 | LE, 20/25; peripheral hard exudates, macular area normal | LPC in the periphery | 12 months | 20/25; no progression |
| 3. M | 3 | 20 | LE, 20/60; peripheral hard exudates, CME without exudates | LPC in the periphery, intravitreal anti-VEGF | 12 months | 20/30; remission of CME |
| 4. M | 3 | 44 | RE, 20/25; demarcated peripheral hard exudates, mild CME without exudates | LPC in the periphery, intravitreal anti-VEGF | 20 months | 20/80; progression of CME despite treatment |
| 5. M | 3 | 55 | RE, 20/100; asteroid hyalosis, localized hard exudates outside the fovea, CME without exudates; risk of macular hole formation | LPC in the periphery | 6 months | 20/100; no improvement, intravitreal anti-VEGF scheduled, possible surgical treatment |
| 6. M | 4 | 21 | LE, 6/200; peripheral and central hard exudates, CME | LPC in the periphery and GRID in the macula | 12 months | 20/20; remission of ME |
| Study | Population | Treatment | Mean Follow-Up | Main Outcome |
|---|---|---|---|---|
| Smithen et al., 2005 [16] | 13 adults >35 years | LPC in 11 cases; 2 cases observed (short follow-up) | 5.8 years (range: 0–17) | Average loss of 2.1 lines. BCVA improvement in 2 cases, stability in 3 cases, and decline in 6 cases. At the final follow-up, BCVA ≥ 20/40 in 5 cases and BCVA < 20/200 in 3 cases. |
| Goel et al., 2011 [17] | 3 adults | Single intravitreal bevacizumab followed by LPC | 9 months | Significant improvement of BCVA in all cases of from counting fingers to 20/300, counting fingers to 20/240, and 20/240 to 20/120; regression of hard exudates from the macula in all cases |
| Wang et al., 2011 [18] | 3 adults | 2 injections of bevacizumab followed by LPC | 0.5–2 years (2, 0.5, 1 years, respectively) | Significant improvement in BCVA, reduction of CRT, and regression of telangiectasias.
|
| Zheng et al., 2014 [19] | 5 adults | Intravitreal bevacizumab followed by LPC (3 cases) or intravitreal triamcinolone (1 case) or subsequent intravitreal bevacizumab (average of 2 injections during follow-up) | 10.6 months | Resolution of subretinal fluid and telangiectasias without significant improvement in BCVA (range: 1.42–1.25 logMAR). Vitreoretinal fibrosis in two cases. |
| Park et al., 2016 [20] | 13 adults | LPC combined with intravitreal bevacizumab (mean no. of injections: 2.69 and mean no of laser sessions: 1.68) | 24.8 months | Mean BCVA change from 0.72 logMAR to 0.68 logMAR (statistically insignificant). BCVA improvement of more than 3 lines in 3 patients (23%) and stability in 7 patients (54%). Mean CRT was significantly decreased from 473 to 288 μm. Poor baseline BCVA and subfoveal hard exudates correlated with poor final BCVA result. |
| Rishi et al., 2016 [21] | 48 adults ≥ 35 years 32 cases observed > 6 months | LPC (60.4%), observation (27.08%), surgery (6.2%), cryotherapy (4%), LPC plus cryotherapy (2%) | 40 months (range: 1–122 months) | Patients with follow-up longer than 6 months (32 cases):
|
| Zhang et al., 2018 [22] | 12 adults | Intravitreal ranibizumab or conbercept followed by LPC | 23.10 ± 7.8 | Mean BCVA improvement significant from 1.27 ± 0.69 to 1.05 ± 0.73 logMAR; mean injection no. 2.33 ± 0.65, mean no. of laser treatments 2.5 ± 0.8 |
| Reference studies: children and adults reported in one cohort | ||||
| Shields et al., 2001 [10] | 124 eyes observed > 6 months Age 1 month to 63 years (average: 5 years) | Cryotherapy (42%), LPC (13%), observation (18%), surgery 17% and enucleation 11% | 55 months (range: 6–300 months) | Anatomic improvement and stability in 76%. BCVA ≥ 20/50 in 14%, 20/60 to 20/100 in 6%, 20/200 to finger counting in 24%, and hand motion to light perception in 40% |
| Shields et al., 2019 [15] | 351 cases, data from 45 years Age 0–79 years, median: 6 years | Overall (1973–2018): observation (21%), LPC (42%), cryotherapy (55%), sub-Tenon corticosteroids (12%), intravitreal corticosteroids (4%), anti-VEGF (10%), and primary enucleation (5%) Years 2010–2018: observation (11%), LPC (72%), cryotherapy (68%), sub-Tenon corticosteroids (29%), intravitreal corticosteroids (9%), anti-VEGF (18%), primary enucleation (1%) | 58 months (range: 0–466 months) | BCVA overall Verbal
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawęcki, M. Idiopathic Peripheral Retinal Telangiectasia in Adults: A Case Series and Literature Review. J. Clin. Med. 2021, 10, 1767. https://doi.org/10.3390/jcm10081767
Gawęcki M. Idiopathic Peripheral Retinal Telangiectasia in Adults: A Case Series and Literature Review. Journal of Clinical Medicine. 2021; 10(8):1767. https://doi.org/10.3390/jcm10081767
Chicago/Turabian StyleGawęcki, Maciej. 2021. "Idiopathic Peripheral Retinal Telangiectasia in Adults: A Case Series and Literature Review" Journal of Clinical Medicine 10, no. 8: 1767. https://doi.org/10.3390/jcm10081767
APA StyleGawęcki, M. (2021). Idiopathic Peripheral Retinal Telangiectasia in Adults: A Case Series and Literature Review. Journal of Clinical Medicine, 10(8), 1767. https://doi.org/10.3390/jcm10081767






