Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Material
2.2. Measurement of ACPA, IL-8, TRAP5b, Cathepsin K and CTX-I
2.3. Statistical Analyses
3. Results
3.1. Presence of ACPA in Bone Marrow of RA Patients
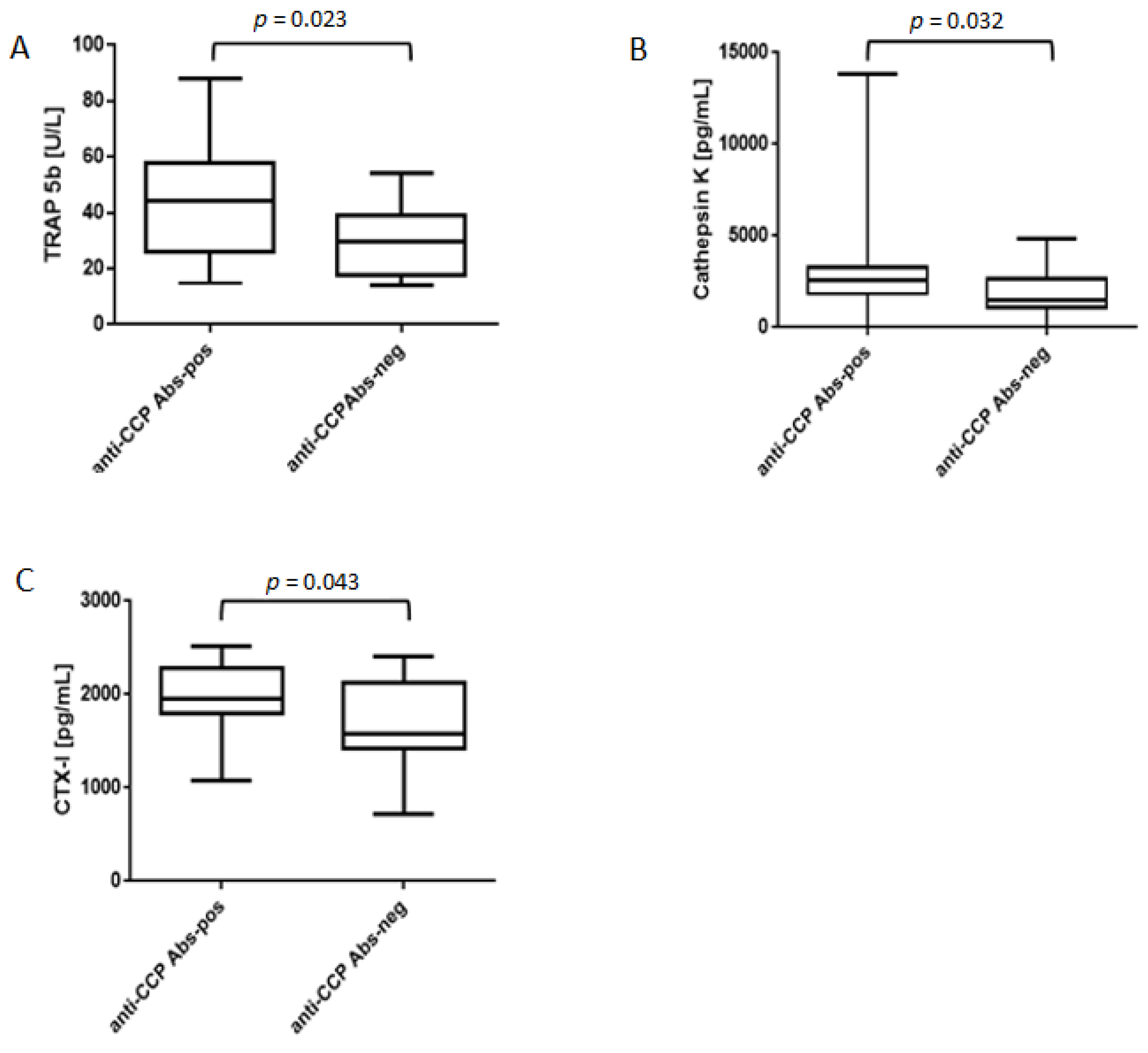
3.2. Elevated Levels of, TRAP5b, Cathepsin K and CTX-I in ACPA-Positive Bone Marrow
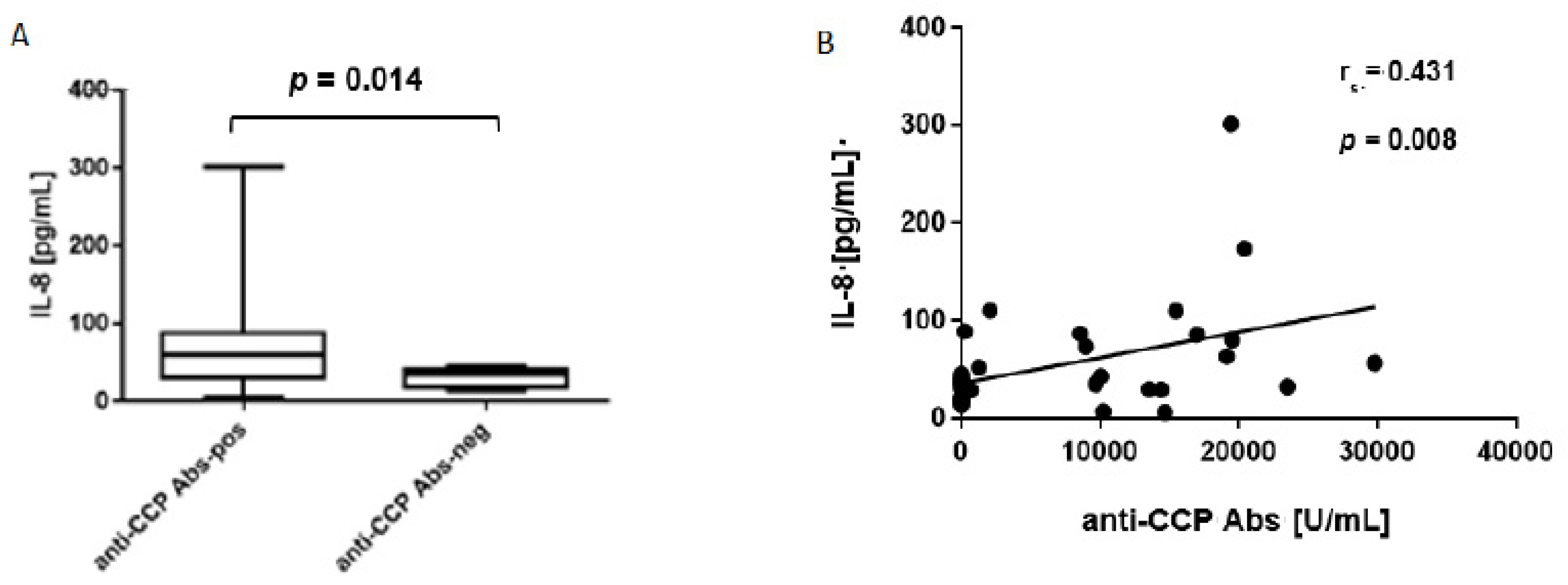
3.3. Elevated Levels of IL-8 in ACPA-Positive Bone Marrow
3.4. Levels of IL-8 Associate Positively with TRAP5b, Cathepsin K and CTX-I Concentrations in ACPA-Positive Bone Marrow
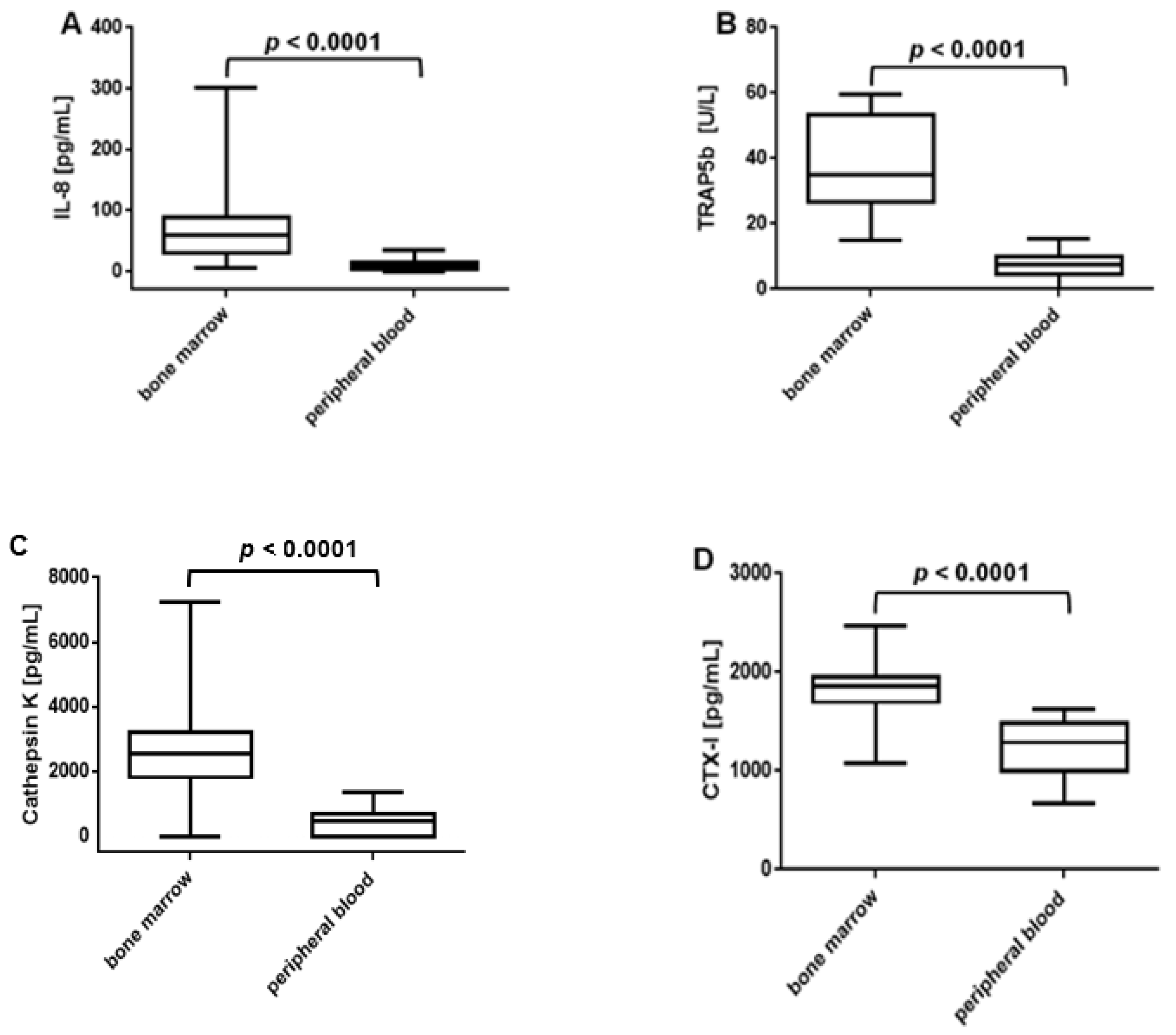
3.5. Higher Levels of IL-8, TRAP5b, Cathepsin K and CTX-I in Bone Marrow Than in Peripheral Blood od ACPA-Positive RA Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Van der Linden, M.P.; Boja, R.; Klarenbeek, N.B.; Huizinga, T.W.J.; van der Heijde, D.M.; van der Helm-van Mil, A.H.M. Repair of joint erosions in rheumatoid arthritis: Prevalence and patient characteristics in a large inception cohort. Ann. Rheum. Dis. 2010, 69, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Finzel, S.; Rech, J.; Schmidt, S.; Engelke, K.; Englbrecht, M.; Schett, G. Interleukin-6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: A micro CT study. Ann. Rheum. Dis. 2013, 72, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Gravallese, E.M. Mechanisms of bone loss in inflammatory arthritis: Diagnosis and therapeutic implications. Arthritis Res. 2000, 2, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proulx, S.T.; Kwok, E.; You, Z.; Papuga, M.O.; Beck, C.A.; Shealy, D.J.; Calvi, L.M.; Ritchlin, C.T.; Awad, H.A.; Boyce, B.F.; et al. Elucidating bone marrow edema and myelopoiesis in murine arthritis using contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2008, 58, 2019–2029. [Google Scholar] [CrossRef]
- Wang, F.; Luo, A.; Xuan, W.; Qi, L.; Wu, Q.; Gan, K.; Zhang, Q.; Zhang, M.; Tan, W. The bone marrow edema links to an osteoclastic environment and precedes synovitis during the development of collagen induced arthritis. Front. Immunol. 2019. [CrossRef]
- Kuca-Warnawin, E.; Burakowski, T.; Kurowska, W.; Prochorec-Sobieszek, M.; Radzikowska, A.; Chorazy-Massalska, M.; Maldyk, P.; Kontny, E.; Maslinski, W. Elevated number of recently activated T cells in bone marrow of patients with rheumatoid arthritis: A role for interleukin 15? Ann. Rheum. Dis. 2011, 70, 227–233. [Google Scholar] [CrossRef]
- Bugatti, S.; Caporali, R.; Manzo, A.; Vitolo, B.; Pitzalis, C.; Montecucco, C. Involvement of subchondral bone marrow in rheumatoid arthritis: Lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum. 2005, 52, 3448–3459. [Google Scholar]
- Radzikowska, A.; Burakowski, T.; Maldyk, P.; Rudnicka, W.; Maslinski, W. Soluble and cell-surface expressed rankl and osteoprotegerin in bone marrow from rheumatoid arthritis patients. Ann. Rheum. Dis. 2007, 66, S297. [Google Scholar]
- Lisbona, M.P.; Pàmies, A.; Ares, J.; Almirall, M.; Navallas, M.; Solano, A.; Maymó, J. Association of bone edema with the progression of bone erosions quantified by hand magnetic resonance imaging in patients with rheumatoid arthritis in remission. J. Rheumatol. 2014, 41, 1623–1629. [Google Scholar] [CrossRef]
- Hetland, M.L.; Ejbjerg, B.; Hørslev-Petersen, K.; Jacobsen, S.; Vestergaard, A.; Jurik, A.G.; Stengaard-Pedersen, K.; Junker, P.; Lottenburger, T.; Hansen, I.; et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann. Rheum. Dis. 2009, 68, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, W.P.; van Steenbergen, H.W.; Stomp, W.; Stijnen, T.; Huizinga, T.W.J.; Bloem, J.L.; van der Heijde, D.; Reijnierse, M.; van der Helm-van Mil, A.H.M. The course of bone marrow edema in early undifferentiated arthritis and rheumatoid arthritis: A longitudinal magnetic resonance imaging study at bone level. Arthritis Rheumatol. 2016, 68, 1080–1088. [Google Scholar] [PubMed] [Green Version]
- Krishnamurthy, A.; Joshua, V.; Haj Hensvold, A.; Tao, J.; Meng, S.; Vivar, N.; Ytterberg, A.J.; Engström, M.; Fernandes-Cerqueira, C.; Amara, K.; et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 2016, 75, 721–729. [Google Scholar]
- Wigerblad, G.; Bas, D.B.; Fernades-Cerqueira, C.; Krishnamurthy, A.; Nandakumar, K.S.; Rogoz, K.; Kato, J.; Sandor, K.; Su, J.; Jimenez-Andrade, J.M.; et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann. Rheum. Dis. 2016, 75, 730–738. [Google Scholar]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.-J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, A.; Finzel, S.; Rech, J.; Manger, B.; Krieter, M.; Faustini, F.; Araujo, E.; Axel, J.H.; Harre, U.; Engelke, K.; et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann. Rheum. Dis. 2014, 73, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Voskuyl, A.E.; Dijkmans, B.A. Remission and radiographic progression in rheumatoid arthritis. Clin. Exp. Rheumatol. 2006, 24, S37–S40. [Google Scholar]
- Rönnelid, J.; Wick, M.C.; Lampa, J.; Lindblad, S.; Nordmark, B.; Klareskog, L.; van Vollenhoven, R.F. Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: Anti-CP status predicts worse disease activity and greater radiological progression. Ann. Rheum. Dis. 2005, 64, 1744–1749. [Google Scholar] [CrossRef]
- Van der Helm-van Mil, A.H.; Verpoort, K.N.; Breedveld, F.C.; Toes, R.E.; Huizinga, T.W. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R949–R958. [Google Scholar] [CrossRef] [Green Version]
- Syversen, S.W.; Gaarder, P.I.; Goll, G.L.; Ødegård, S.; Haavardsholm, E.A.; Mowinckel, P.; van der Heijde, D.; Landewé, R.; Kvien, T.K. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: Results from a 10-year longitudinal study. Ann. Rheum. Dis. 2008, 67, 212–217. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Machold, K.P.; Stamm, T.A.; Nell, V.P.K.; Pflugbeil, S.; Aletaha, D.; Steiner, G.; Uffmann, M.; Smolen, J.S. Very recent onset rheumatoid arthritis: Clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology 2007, 46, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Di Matteo, A.; Mankia, K.; Duquenne, L.; Cipolletta, E.; Wakefield, R.J.; Garcia-Montoya, L.; Nam, J.L.; Emery, P. Ultrasound erosions in the feet best predict progression to inflammatory arthritis in anti-CCP positive at-risk individuals without clinical synovitis. Ann. Rheum. Dis. 2020, 79, 901–907. [Google Scholar] [CrossRef]
- Courbon, G.; Lamarque, R.; Gerbaix, M.; Caire, R.; Linossier, M.-T.; Laroche, N.; Thomas, M.; Thomas, T.; Vico, L.; Marotte, H. Early sclerostin expression explains bone formation inhibition before arthritis onset in the rat adjuvant-induced arthritis model. Sci. Rep. 2018, 8, 3492. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maslinski, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef]
- Rantapää-Dahlqvist, S.; de Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.T.; Wiik, A.; Pedersen, M.; Hedegaard, C.J.; Vestergaard, B.F.; Gislefoss, R.E.; Kvien, T.K.; Wohlfahrt, J.; Bendtzen, K.; Frisch, M. Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: Case-control study nested in a cohort of Norwegian blood donors. Ann. Rheum. Dis. 2008, 67, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Bøyesen, P.; Haavardsholm, E.A.; Ostergaard, M.; van der Heijde, D.; Sesseng, S.; Kvien, T.K. MRI in early rheumatoid arthritis: Synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann. Rheum. Dis. 2011, 70, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Tamai, M.; Kawakami, A.; Uetani, M.; Takao, S.; Arima, K.; Iwamoto, N.; Fujikawa, K.; Aramaki, T.; Kawashiri, S.-Y.; Ichinose, K.; et al. A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis Rheum. 2009, 61, 772–778. [Google Scholar] [CrossRef]
- Snir, O.; Widhe, M.; Hermansson, M.; von Spee, C.; Lindberg, J.; Hensen, S.; Lundberg, K.; Engström, A.; Venables, P.J.; Toes, R.E.; et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010, 62, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Mankia, K.; Emery, P. Is localized autoimmunity the trigger for rheumatoid arthritis? Unravelling new targets for prevention. Discov. Med. 2015, 20, 129–135. [Google Scholar]
- Amara, K.; Hensvold, A.; Thyagarajan, R.; Israelsson, L.; Steen, J.; Wähämaa, H.; Hansson, M.; Engström, M.; van Vollenhoven, A.; Catrina, A.; et al. Exploring the RA bone marrow niche by single-cell technology to identify long lived ACPA+ plasma cells. Arthritis Rheumatol. 2020, 72 (Suppl. 10), 1456. [Google Scholar]
- Lv, Y.; Wang, G.; Xu, W.; Tao, P.; Lv, X.; Wang, Y. Tartrate-resistant acid phosphatase 5b is a marker of osteoclast number and volume in RAW 264.7 cells treated with receptor-activated nuclear κB ligand. Exp. Ther. Med. 2015, 9, 143–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Yamaza, T.; Tanaka, T. Cathepsins in the osteoclast. J. Electron Microsc. 2003, 52, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaissé, J.M. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.; Krege, J.; Lane, N.; Leary, E.; Libanati, C.; Miller, P.; Myers, G.; Silverman, S.; Vesper, H.W.; Lee, D. et at. National bone health alliance bone turnover marker project: Current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. 2012, 23, 2425–2433. [Google Scholar] [CrossRef]
- Asquith, D.L.; Miller, A.M.; McInnes, I.B.; Liew, F.Y. Animal models of rheumatoid arthritis. Eur. J. Immunol. 2009, 8, 2040–2044. [Google Scholar] [CrossRef]
- Redlich, K.; Gortz, B.; Hayer, S.; Zwerina, J.; Doerr, N.; Kostenuik, P.; Bergmeister, H.; Kollias, G.; Steiner, G.; Smolen, J.; et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am. J. Patho. 2004, 16, 543–555. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.H.; Kay, J.; Duryea, J.; Lu, B.; Bolster, M.B.; Yood, R.A.; Han, R.; Ball, S.; Coleman, C.; Lo, E.; et al. Effects of teriparatide on joint erosions in rheumatoid arthritis: A randomized controlled trial. Arthritis Rheumatol. 2017, 69, 1741–1750. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Kikuta, J.; Sudo, T.; Matsuura, Y.; Matsui, T.; Simmons, S.; Ebina, K.; Hirao, M.; Okuzaki, D.; Yoshida, Y.; et al. Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat. Immunol. 2019, 20, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Cantley, M.D.; Smith, M.D. Pathogenic bone loss in rheumatoid arthritis: Mechanisms and therapeutic approaches. Int. J. Clin. Rheumatol. 2009, 4, 561–582. [Google Scholar] [CrossRef]
- Kuca-Warnawin, E.H.; Kurowska, W.J.; Radzikowska, A.; Massalska, M.A.; Burakowski, T.; Kontny, E.; Slowinska, I.; Gasik, R.; Maslinski, W. Different expression of chemokines in rheumatoid arthritis and osteoarthritis bone marrow. Reumatologia 2016, 54, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, W.; Burakowski, T.; Warnawin, E.; Jastrzebska, M.; Bik, M.; Chorazy-Massalska, M.; Radzikowska, A.; Buler, M.; Maldyk, P.; Maslinski, W. Functional TLR9 modulates bone marrow B cells from rheumatoid arthritis patients. Eur. J. Immunol. 2009, 39, 1211–1220. [Google Scholar] [CrossRef]

| ACPA-Positive 1 (n = 22) | ACPA-Negative 1 (n = 20) | |
|---|---|---|
| Age (years), median (range) | 59 (49–69) | 60 (45–70) |
| Sex, female/male | 19/3 | 17/3 |
| ESR 2 (mm/h), mean ± SD 3 | 32.5 ± 16.7 | 21.0 ± 16.9 |
| CRP 4 (mg/L), median (range) | 10.5 (1–65) | 8 (1–56) |
| DAS28 5 | 3.76 ± 0.24 | 3.62 ± 0.51 |
| Methotrexate/Sulfasalazine/Leflunomide/Azathioprine | 21 | 17 |
| Steroids | 17 | 11 |
| Biologics 6 | 7 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowska, W.; Slowinska, I.; Krogulec, Z.; Syrowka, P.; Maslinski, W. Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 1778. https://doi.org/10.3390/jcm10081778
Kurowska W, Slowinska I, Krogulec Z, Syrowka P, Maslinski W. Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis. Journal of Clinical Medicine. 2021; 10(8):1778. https://doi.org/10.3390/jcm10081778
Chicago/Turabian StyleKurowska, Weronika, Iwona Slowinska, Zbigniew Krogulec, Piotr Syrowka, and Wlodzimierz Maslinski. 2021. "Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis" Journal of Clinical Medicine 10, no. 8: 1778. https://doi.org/10.3390/jcm10081778
APA StyleKurowska, W., Slowinska, I., Krogulec, Z., Syrowka, P., & Maslinski, W. (2021). Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis. Journal of Clinical Medicine, 10(8), 1778. https://doi.org/10.3390/jcm10081778





