New “Wrinkle Method” for Intracorporeal Anterior Vaginal Wall Plication during Sacrocolpopexy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
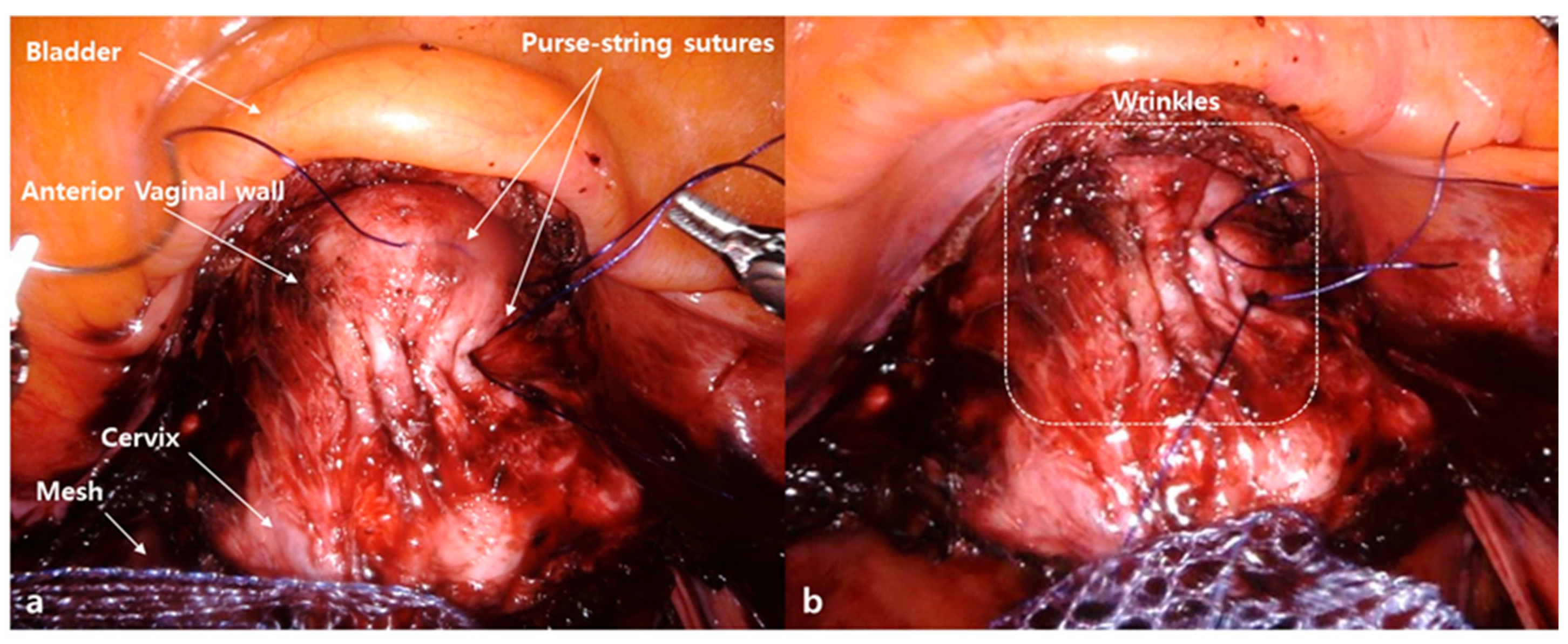
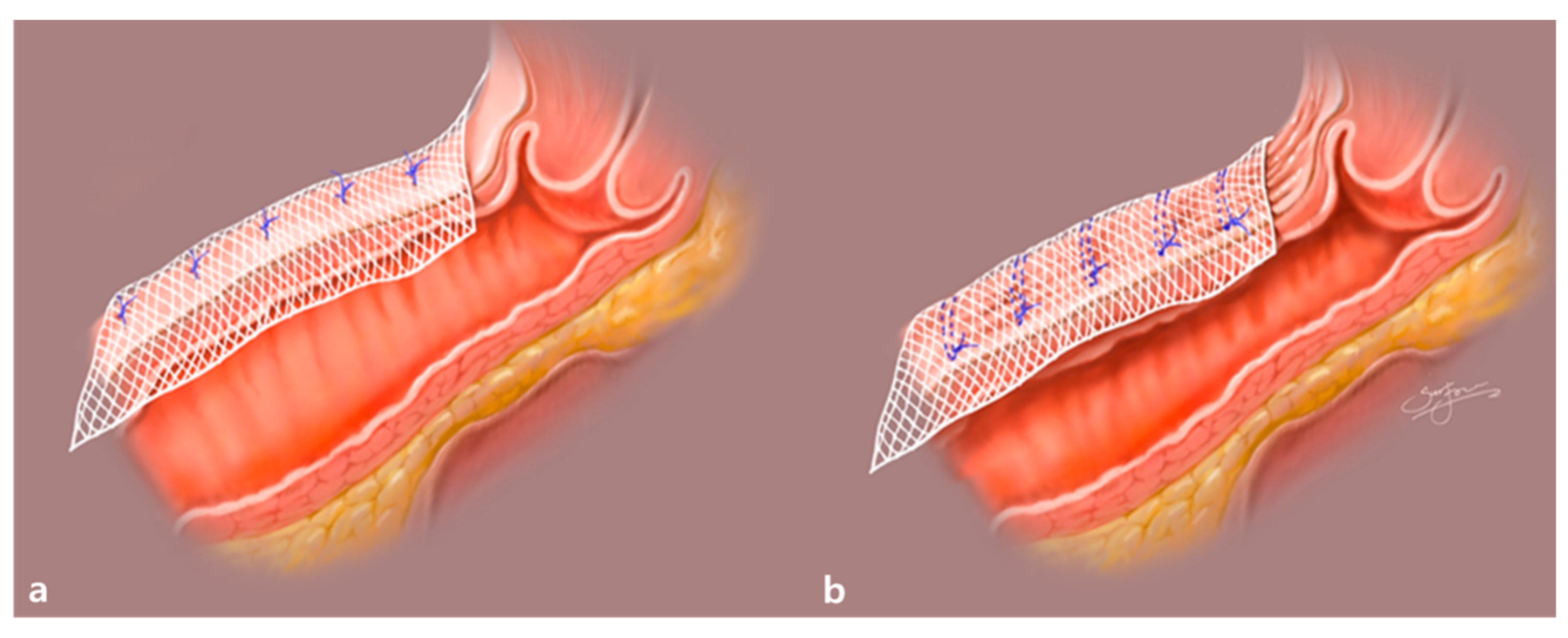
2.2. Surgical Procedures
2.3. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Comparison of Surgical Outcomes between the Wrinkle and Non-Wrinkle Groups
3.3. Comparison of Intraoperative and Postoperative Adverse Surgical Outcomes
4. Discussion
4.1. Feasibility of the Wrinkle Method during RSC
4.2. Previous Reports on POP Recurrence and Mesh Erosion Rates after SC
4.3. Previous Reports on Prevention of Recurrent POP after SC
4.4. Comparison of Total OT in the Wrinkle and Non-Wrinkle Groups
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Løwenstein, E.P.; Ottesen, B.; Gimbel, H. Incidence and lifetime risk of pelvic organ prolapse surgery in Denmark from 1977 to 2009. Int. Urogynecol. J. 2015, 26, 49–55. [Google Scholar] [CrossRef]
- Olsen, A.L.; Smith, V.J.; Bergstrom, J.; Colling, J.C.; Clark, A.L. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef]
- Wu, J.M.; Matthews, C.A.; Conover, M.M.; Pate, V.; Funk, M.J. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstet. Gynecol. 2014, 123, 1201–1206. [Google Scholar] [CrossRef]
- Smith, F.J.; Holman, C.D.J.; Moorin, R.E.; Tsokos, N. Lifetime Risk of Undergoing Surgery for Pelvic Organ Prolapse. Obstet. Gynecol. 2010, 116, 1096–1100. [Google Scholar] [CrossRef]
- Barber, M.D.; Maher, C. Epidemiology and outcome assessment of pelvic organ prolapse. Int. Urogynecol. J. 2013, 24, 1783–1790. [Google Scholar] [CrossRef]
- Maher, C.; Baessler, K.; Glazener, C.; Adams, E.; Hagen, S. Surgical management of pelvic organ prolapse in women: A short version Cochrane review. Neurourol. Urodyn. 2008, 27, 3–12. [Google Scholar] [CrossRef]
- Nygaard, I.E.; McCreery, R.; Brubaker, L.; Connolly, A.; Cundiff, G.; Weber, A.M.; Zyczynski, H. Abdominal Sacrocolpopexy: A Comprehensive Review. Obstet. Gynecol. 2004, 104, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Hilger, W.S.; Poulson, M.; Norton, P. Long-term results of abdominal sacrocolpopexy. Am. J. Obstet. Gynecol. 2003, 189, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Trowers, O.; Stewart, J.R.; Meriwether, K.V.; Francis, S.L.; Gupta, A. Perioperative Outcomes of Minimally Invasive Sacrocolpopexy Based on Route of Concurrent Hysterectomy: A Secondary Analysis of the National Surgical Quality Improvement Program Database. J. Minim. Invasive Gynecol. 2020, 27, 953–958. [Google Scholar] [CrossRef]
- Linder, B.J.; Occhino, J.A.; Habermann, E.B.; Glasgow, A.E.; Bews, K.A.; Gershman, B. A National Contemporary Analysis of Perioperative Outcomes of Open versus Minimally Invasive Sacrocolpopexy. J. Urol. 2018, 200, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Nemirovsky, A.; Bs, A.S.H.; Gorman, E.F.; Malik, R.D. A systematic review of best practices for the perioperative management of abdominal sacrocolpopexy. Neurourol. Urodyn. 2020, 39, 1264–1275. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Safety Communication: Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse. 2011. Available online: http://www.fda.gov/downloads/MedicalDevices/Safety/AlettsandNotices/UCM262760.pdf (accessed on 9 October 2020).
- Serati, M.; Bogani, G.; Sorice, P.; Braga, A.; Torella, M.; Salvatore, S.; Uccella, S.; Cromi, A.; Ghezzi, F. Robot-assisted Sacrocolpopexy for Pelvic Organ Prolapse: A Systematic Review and Meta-analysis of Comparative Studies. Eur. Urol. 2014, 66, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.; For the Systematic Review Group of the Society of Gynecologic Surgeons; Rahn, D.D.; Lowenstein, L.; Balk, E.M.; Clemons, J.L.; Rogers, R.G. Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: A systematic review. Int. Urogynecol. J. 2011, 22, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Jeon, M.J. Risk factors for vaginal mesh erosion after sacrocolpopexy in Korean women. PLoS ONE 2020, 15, e0228566. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.; Morris, S.; McKinnie, V.; Freeman, R.; Petri, E.; Scotti, R.J.; Dwyer, P. Validation of a simplified technique for using the POPQ pelvic organ prolapse classification system. Int. Urogynecol. J. 2006, 17, 615–620. [Google Scholar] [CrossRef]
- Haylen, B.T.; Freeman, R.M.; Swift, S.E.; Cosson, M.; Davila, G.W.; Deprest, J.; Dwyer, P.L.; Fatton, B.; Kocjancic, E.; Lee, J.; et al. An International Urogynecological Association (IUGA) / International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int. Urogynecol. J. 2011, 22, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Utomo, E.; Korfage, I.J.; Wildhagen, M.F.; Steensma, A.B.; Bangma, C.H.; Blok, B.F. Validation of the urogenital distress inventory (UDI-6) and incontinence impact questionnaire (IIQ-7) in a Dutch population. Neurourol. Urodyn. 2015, 34, 24–31. [Google Scholar] [CrossRef]
- Utomo, E.; Blok, B.F.; Steensma, A.B.; Korfage, I.J. Validation of the Pelvic Floor Distress Inventory (PFDI-20) and Pelvic Floor Impact Questionnaire (PFIQ-7) in a Dutch population. Int. Urogynecol. J. 2014, 25, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.M.; Pizarro-Berdichevsky, J.; Goldman, H.B. Robotic Female Pelvic Floor Reconstruction: A Review. Urology 2016, 91, 33–40. [Google Scholar] [CrossRef]
- Chan, S.S.C.; Pang, S.M.W.; Cheung, T.H.; Cheung, R.Y.K.; Chung, T.K.H. Laparoscopic sacrocolpopexy for the treatment of vaginal vault prolapse: With or without robotic assistance. Hong Kong Med. J. 2011, 17, 54–60. [Google Scholar]
- Noé, G.K. Genital Prolapse Surgery: What Options Do We Have in the Age of Mesh Issues? J. Clin. Med. 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Dandolu, V.; Akiyama, M.; Allenback, G.; Pathak, P. Mesh complications and failure rates after transvaginal mesh repair compared with abdominal or laparoscopic sacrocolpopexy and to native tissue repair in treating apical prolapse. Int. Urogynecol. J. 2017, 28, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bako, A.; Dhar, R. Review of synthetic mesh-related complications in pelvic floor reconstructive surgery. Int. Urogynecol. J. 2009, 20, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gutman, R.; Maher, C. Uterine-preserving POP surgery. Int. Urogynecol. J. 2013, 24, 1803–1813. [Google Scholar] [CrossRef]
- Trilling, B.; Martin, G.; Faucheron, J.-L. Mesh erosion after laparoscopic rectopexy: A benign complication? Color. Dis. 2014, 16, 832–833. [Google Scholar] [CrossRef]
- Deffieux, X.; Letouzey, V.; Savary, D.; Sentilhes, L.; Agostini, A.; Mares, P.; Pierre, F. Prevention of complications related to the use of prosthetic meshes in prolapse surgery: Guidelines for clinical practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 170–180. [Google Scholar] [CrossRef]
- Denman, M.A.; Gregory, W.T.; Boyles, S.H.; Smith, V.; Edwards, S.R.; Clark, A.L. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am. J. Obstet. Gynecol. 2008, 198, 555.e1–555.e5. [Google Scholar] [CrossRef]
- Fialkow, M.F.; Newton, K.M.; Weiss, N.S. Incidence of recurrent pelvic organ prolapse 10 years following primary surgical management: A retrospective cohort study. Int. Urogynecol. J. 2008, 19, 1483–1487. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.R.; Lee, E.S.; Kim, S.H.; Chae, H.D. Robot-Assisted Laparoscopic Surgery for Pelvic Organ Prolapse among Peri- and Post-Menopausal Women. J. Menopausal Med. 2020, 26, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Roh, A.-M.; Jeong, K.; Kim, S.H.; Chae, H.D.; Moon, H.-S. First report comparing the two types of single-incision robotic sacrocolpopexy: Single site using the da Vinci Xi or Si system and single port using the da Vinci SP system. Taiwan. J. Obstet. Gynecol. 2021, 60, 60–65. [Google Scholar] [CrossRef]
- Lee, R.K.; Mottrie, A.; Payne, C.K.; Waltregny, D. A Review of the Current Status of Laparoscopic and Robot-assisted Sacrocolpopexy for Pelvic Organ Prolapse. Eur. Urol. 2014, 65, 1128–1137. [Google Scholar] [CrossRef]
- Tan-Kim, J.; Menefee, S.A.; Luber, K.M.; Nager, C.W.; Lukacz, E.S. Robotic-Assisted and Laparoscopic Sacrocolpopexy. Female Pelvic Med. Reconstr. Surg. 2011, 17, 44–49. [Google Scholar] [CrossRef]
- Van Zanten, F.; Koops, S.S.; O’Sullivan, O.; Lenters, E.; Broeders, I.; O’Reilly, B. Robot-assisted surgery for the management of apical prolapse: A bi-centre prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Jong, K.; Klein, T.; Zimmern, P.E. Long-term outcomes of robotic mesh sacrocolpopexy. J. Robot. Surg. 2018, 12, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hoke, T.P.; Goldstein, H.; Saks, E.K.; Vakili, B. Surgical Outcomes of Paravaginal Repair After Robotic Sacrocolpopexy. J. Minim. Invasive Gynecol. 2018, 25, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Shippey, S.H.; Quiroz, L.H.; Sanses, T.V.D.; Knoepp, L.R.; Cundiff, G.W.; Handa, V.L. Anatomic outcomes of abdominal sacrocolpopexy with or without paravaginal repair. Int. Urogynecol. J. 2010, 21, 279–283. [Google Scholar] [CrossRef]
- Noé, G.K.; Schiermeier, S.; Anapolski, M. Defect Oriented Strategy Reducing mesh in pelvic floor surgery by laparoscopic approach. Thetrocar 2020, 1, 6–9. [Google Scholar] [CrossRef]
- Lee, S.R. Robotic Single-Site® Sacrocolpopexy: First Report and Technique Using the Single-Site® Wristed Needle Driver. Yonsei Med. J. 2016, 57, 1029–1033. [Google Scholar] [CrossRef]
- Liu, J.; Bardawil, E.; Zurawin, R.K.; Wu, J.; Fu, H.; Orejuela, F.; Guan, X. Robotic Single-Site Sacrocolpopexy with Retroperitoneal Tunneling. JSLS J. Soc. Laparoendosc. Surg. 2018, 22, e2018.00009. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 19 September 2020).
- Noé, G.K.; Alkatout, I.; Schiermeier, S.; Soltécz, S.; Anapolski, M. Laparoscopic anterior and posterior native tissue repair: A new pelvic floor approach. Minim. Invasive Ther. Allied Technol. 2018, 28, 241–246. [Google Scholar] [CrossRef]
- Noé, G.K.; Schiermeier, S.; Anapolski, M. Laparoscopic Rectocele Repair with Native Tissue. Videourology 2019. [Google Scholar] [CrossRef]
- Unger, C.A.; Paraiso, M.F.R.; Jelovsek, J.E.; Barber, M.D.; Ridgeway, B. Perioperative adverse events after minimally invasive abdominal sacrocolpopexy. Am. J. Obstet. Gynecol. 2014, 211, 547.e1–547.e8. [Google Scholar] [CrossRef] [PubMed]
- Sa, M.D.G.D.; Claydon, L.S.; Whitlow, B.; Artahona, M.A.D. Laparoscopic versus open sacrocolpopexy for treatment of prolapse of the apical segment of the vagina: A systematic review and meta-analysis. Int. Urogynecol. J. 2016, 27, 3–17. [Google Scholar] [CrossRef]
- Thomas, T.N.; Davidson, E.R.W.; Lampert, E.J.; Paraiso, M.F.R.; Ferrando, C.A. Long-term pelvic organ prolapse recurrence and mesh exposure following sacrocolpopexy. Int. Urogynecol. J. 2020, 31, 1763–1770. [Google Scholar] [CrossRef]
- Cronje, H.; Prollius, A.; De Beer, J. Stage IV cystocele treated by sacrocolpopexy. Int. J. Gynecol. Obstet. 2006, 92, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, I.; Brubaker, L.; Zyczynski, H.M.; Cundiff, G.; Richter, H.; Gantz, M.; Fine, P.; Menefee, S.; Ridgeway, B.; Visco, A.; et al. Long-term Outcomes Following Abdominal Sacrocolpopexy for Pelvic Organ Prolapse. JAMA 2013, 309, 2016–2024. [Google Scholar] [CrossRef]
- Lallemant, M.; Tresch, C.; Puyraveau, M.; Delplanque, S.; Cosson, M.; Ramanah, R. Evaluating the morbidity and long-term efficacy of laparoscopic sacrocolpopexy with and without robotic assistance for pelvic organ prolapse. J. Robot. Surg. 2020. [Google Scholar] [CrossRef]
- Hendrix, S.L.; Clark, A.; Nygaard, I.; Aragaki, A.; Barnabei, V.; McTiernan, A. Pelvic organ prolapse in the women’s health initiative: Gravity and gravidity. Am. J. Obstet. Gynecol. 2002, 186, 1160–1166. [Google Scholar] [CrossRef]
- Daneshgari, F.; Kefer, J.C.; Moore, C.; Kaouk, J. Robotic abdominal sacrocolpopexy/sacrouteropexy repair of advanced female pelvic organ prolaspe (POP): Utilizing POP-quantification-based staging and outcomes. BJU Int. 2007, 100, 875–879. [Google Scholar] [CrossRef]
- Illiano, E.; Ditonno, P.; Giannitsas, K.; De Rienzo, G.; Bini, V.; Costantini, E. Robot-assisted Vs Laparoscopic Sacrocolpopexy for High-stage Pelvic Organ Prolapse: A Prospective, Randomized, Single-center Study. Urology 2019, 134, 116–123. [Google Scholar] [CrossRef]
- Seror, J.; Yates, D.R.; Seringe, E.; Vaessen, C.; Bitker, M.-O.; Chartier-Kastler, E.; Rouprêt, M. Prospective comparison of short-term functional outcomes obtained after pure laparoscopic and robot-assisted laparoscopic sacrocolpopexy. World J. Urol. 2012, 30, 393–398. [Google Scholar] [CrossRef] [PubMed]
Short Biography of Author
| Characteristics | Wrinkle (n = 57) | Non-Wrinkle (n = 66) | p Value |
|---|---|---|---|
| Age (years, mean ± SD) | 59.05 ± 9.59 | 63.29 ± 10.97 | 0.02 |
| BMI (kg/m2, mean ± SD) | 24.02 ± 2.56 | 23.55 ± 2.97 | 0.38 |
| Menopause, n (%) | 43 (75.4) | 59 (89.4) | 0.07 |
| Vaginal delivery, (median (range)) | 2 (0–8) | 2 (0–9) | 0.12 |
| Previous pelvic surgery, n (%) | 17 (29.8) | 30 (45.5) | 0.11 |
| Vault prolapse, n (%) | 9 (15.8) | 11 (16.7) | 1 |
| POP-Q stage, n (%) | 1 | ||
| Stage III | 43 (75.4) | 53 (80.3) | |
| Stage IV | 14 (24.6) | 13 (19.7) |
| Wrinkle (n = 57) | Non-Wrinkle (n = 66) | p Value | |
|---|---|---|---|
| Total OT, (min, mean ± SD) | 109.71 ± 26.45 | 125.35 ± 28.24 | 0.003 |
| Concomitant surgery, n (%) | 0.26 | ||
| Supracervical hysterectomy | 49 (86) | 55 (83.3) | 0.88 |
| Adnexectomy | 14 (71.9) | 59 (89.4) | 0.02 |
| Adhesiolysis | 2 (3.5) | 21 (31.8) | <0.001 |
| Posterior colpoperineorrhaphy | 15 (26.3) | 6 (9.09) | 0.02 |
| TOT | 17 (29.8) | 12 (18.2) | 0.19 |
| Estimated blood loss (mL, mean ± SD) | 64.42 ± 50.3 | 70.08 ± 67.8 | 0.61 |
| Wrinkle (n = 57) | Non-Wrinkle (n = 66) | p Value | |
|---|---|---|---|
| Intraoperative AEs, n (%) | |||
| Bladder injury | 2 (3.5) | 2 (3.03) | 1 |
| Bowel injury | 0 | 0 | 1 |
| Postoperative AEs, n (%) | |||
| Bowel obstruction | 0 | 0 | 1 |
| Urinary tract infection | 0 | 0 | 1 |
| Retroperitoneal abscess and fever | 1 (1.75) | 0 | 1 |
| Umbilical incisional hernia | 0 | 1 (1.51) | 0.98 |
| De novo stress urinary incontinence | 0 | 1 (1.51) | 0.98 |
| Posterior vaginal wall—mesh erosion | 0 | 2 (3.03) | 0.97 |
| Recurrence of anterior compartment POP | 0 | 2 (3.03) | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.R.; Kim, J.H.; Kim, S.H.; Chae, H.D. New “Wrinkle Method” for Intracorporeal Anterior Vaginal Wall Plication during Sacrocolpopexy. J. Clin. Med. 2021, 10, 1822. https://doi.org/10.3390/jcm10091822
Lee SR, Kim JH, Kim SH, Chae HD. New “Wrinkle Method” for Intracorporeal Anterior Vaginal Wall Plication during Sacrocolpopexy. Journal of Clinical Medicine. 2021; 10(9):1822. https://doi.org/10.3390/jcm10091822
Chicago/Turabian StyleLee, Sa Ra, Ju Hee Kim, Sung Hoon Kim, and Hee Dong Chae. 2021. "New “Wrinkle Method” for Intracorporeal Anterior Vaginal Wall Plication during Sacrocolpopexy" Journal of Clinical Medicine 10, no. 9: 1822. https://doi.org/10.3390/jcm10091822
APA StyleLee, S. R., Kim, J. H., Kim, S. H., & Chae, H. D. (2021). New “Wrinkle Method” for Intracorporeal Anterior Vaginal Wall Plication during Sacrocolpopexy. Journal of Clinical Medicine, 10(9), 1822. https://doi.org/10.3390/jcm10091822






