Current State of Analgesia and Sedation in the Pediatric Intensive Care Unit
Abstract
1. Introduction
1.1. National and International Analgesia and Sedation Guidelines
1.2. Pharmacokinetics and Pharmacodynamics in Critically Ill Children
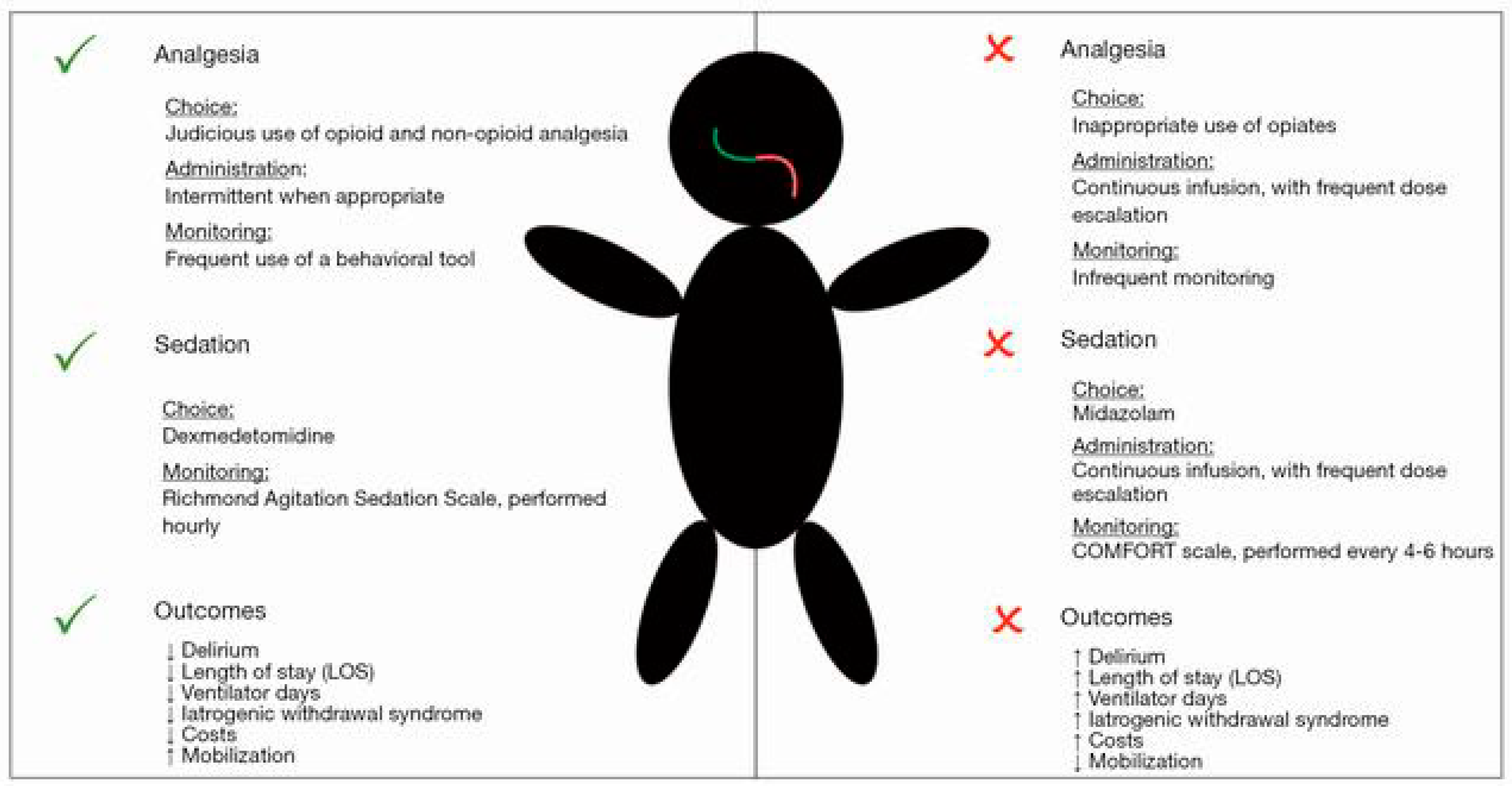
2. Analgesia
2.1. Pain Assessment
2.2. Systemic Analgesia: Opiates and Non-Opiates
2.2.1. Opioids
Morphine
Fentanyl
Remifentanil
Hydromorphone
Methadone
2.2.2. Patient-Controlled Analgesia (PCA) and Parent/Nursing-Controlled Analgesia (PNCA)
2.2.3. Non-Opioids
Acetaminophen
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Gabapentin
Ketamine
Alpha 2 Agonists
2.2.4. Multimodal Pain Management
2.3. Neuraxials and Peripheral Nerve Catheters
3. Sedation
3.1. Sedation Assessment
3.2. Sedatives
3.2.1. Benzodiazepines
Midazolam
Lorazepam
Diazepam
3.2.2. Barbiturates
Sodium Thiopental
Pentobarbital
3.2.3. Alpha Agonists
Clonidine
Dexmedetomidine
3.2.4. Propofol
3.2.5. Ketamine
3.2.6. Antihistamines and Antipsychotics in the PICU
3.3. Sedation Protocols
3.4. Daily Sedation Interruption (DSI)
3.5. Drug Cycling
3.6. Delirium in the PICU
Delirium Monitoring and Treatment
3.7. Withdrawal
Withdrawal Monitoring and Treatment
4. Neurodevelopmental Outcomes
5. Early Mobilization
6. Neuromuscular Blockade
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choong, K. PICU-acquired complications: The new marker of the quality of care. ICU Manag. Pract. 2019, 19, 85–88. [Google Scholar]
- Burns, J.P.; Sellers, D.E.; Meyer, E.C.; Lewis-Newby, M.; Truog, R.D. Epidemiology of death in the PICU at five U.S. teaching hospitals*. Crit. Care Med. 2014, 42, 2101–2108. [Google Scholar] [CrossRef]
- Doha, N.; El-Henawy, T.; Mohammed, M. Analgesia and sedation for patients in the intensive care unit: A systematic review. Menoufia Med. J. 2020, 33, 339–345. [Google Scholar] [CrossRef]
- Curley, M.A.; Wypij, D.; Watson, R.S.; Grant, M.J.; Asaro, L.A.; Cheifetz, I.M.; Dodson, B.L.; Franck, L.S.; Gedeit, R.G.; Angus, D.C.; et al. Protocolized sedation vs. usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA 2015, 313, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Yaster, M.; Kudchadkar, S.R. Pediatric sedation management. Pediatr. Rev. 2016, 37, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.O.; Choong, K.; Zebuhr, C.A.; Kudchadkar, S.R. Transforming PICU culture to facilitate early rehabilitation. J. Pediatr. Intensive Care 2015, 4, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Playfor, S.; Jenkins, I.; Boyles, C.; Choonara, I.; Davies, G.; Haywood, T.; Hinson, G.; Mayer, A.; Morton, N.; Ralph, T.; et al. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med. 2006, 32, 1125–1136. [Google Scholar] [CrossRef]
- Playfor, S.; Jenkins, I.; Boyles, C.; Choonara, I.; Davies, G.; Haywood, T.; Hinson, G.; Mayer, A.; Morton, N.; Ralph, T.; et al. Consensus guidelines for sustained neuromuscular blockade in critically ill children. Paediatr. Anaesth. 2007, 17, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Ramelet, A.S.; van Dijk, M.; Pokorna, P.; Wielenga, J.; Tume, L.; Tibboel, D.; Ista, E. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: An ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016, 42, 972–986. [Google Scholar] [CrossRef]
- Patel, A.K.; Trujillo-Rivera, E.; Faruqe, F.; Heneghan, J.A.; Workman, T.E.; Zeng-Treitler, Q.; Chamberlain, J.; Morizono, H.; Kim, D.; Bost, J.E.; et al. Sedation, analgesia, and neuromuscular blockade: An assessment of practices from 2009 to 2016 in a national sample of 66,443 pediatric patients cared for in the ICU. Pediatr. Crit. Care Med. 2020, 21, e599–e609. [Google Scholar] [CrossRef]
- Jenkins, I.A.; Playfor, S.D.; Bevan, C.; Davies, G.; Wolf, A.R. Current United Kingdom sedation practice in pediatric intensive care. Paediatr. Anaesth. 2007, 17, 675–683. [Google Scholar] [CrossRef]
- Anand, K.J.; Willson, D.F.; Berger, J.; Harrison, R.; Meert, K.L.; Zimmerman, J.; Carcillo, J.; Newth, C.J.; Prodhan, P.; Dean, J.M.; et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010, 125, e1208–e1225. [Google Scholar] [CrossRef]
- Mehta, S.; Burry, L.; Fischer, S.; Martinez-Motta, J.C.; Hallett, D.; Bowman, D.; Wong, C.; Meade, M.O.; Stewart, T.E.; Cook, D.J. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit. Care Med. 2006, 34, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.J. An audit of sedation, analgesia and muscle relaxation in paediatric intensive care in the United Kingdom. Pediatr. Anesth. 1993, 3, 107–115. [Google Scholar] [CrossRef]
- Hughes, C.G.; McGrane, S.; Pandharipande, P.P. Sedation in the intensive care setting. Clin. Pharmacol. 2012, 4, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.M.; Roberts, J.A.; Lipman, J. Pharmacokinetics and pharmacodynamics in critically ill patients. Curr. Opin. Anaesthesiol. 2010, 23, 472–478. [Google Scholar] [CrossRef]
- Lu, H.; Rosenbaum, S. Developmental pharmacokinetics in pediatric populations. J. Pediatr. Pharmacol. Ther. 2014, 19, 262–276. [Google Scholar] [CrossRef]
- Fernandez, E.; Perez, R.; Hernandez, A.; Tejada, P.; Arteta, M.; Ramos, J.T. Factors and mechanisms for pharmacokinetic differences between Pediatric population and adults. Pharmaceutics 2011, 3, 53–72. [Google Scholar] [CrossRef]
- Dzierba, A.L.; Abrams, D.; Brodie, D. Medicating patients during extracorporeal membrane oxygenation: The evidence is building. Crit. Care 2017, 21, 66. [Google Scholar] [CrossRef]
- Morgan, E.T. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin. Pharmacol. Ther. 2009, 85, 434–438. [Google Scholar] [CrossRef]
- Vet, N.J.; Kleiber, N.; Ista, E.; de Hoog, M.; de Wildt, S.N. Sedation in Critically Ill Children with Respiratory Failure. Front. Pediatr. 2016, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Levcovich, B.; Mojtahedzadeh, M. A systematic review on pharmacokinetic changes in critically ill patients: Role of extracorporeal membrane oxygenation. Daru 2011, 19, 312–321. [Google Scholar] [PubMed]
- Schetz, M. Drug dosing in continuous renal replacement therapy: General rules. Curr. Opin. Crit. Care 2007, 13, 645–651. [Google Scholar] [CrossRef]
- Jang, S.M.; Infante, S.; Abdi Pour, A. Drug dosing considerations in critically ill patients receiving continuous renal replacement therapy. Pharmacy 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Poloyac, S.M. The effect of therapeutic hypothermia on drug metabolism and response: Cellular mechanisms to organ function. Expert Opin. Drug Metab. Toxicol. 2011, 7, 803–816. [Google Scholar] [CrossRef]
- Jacobi, J.; Fraser, G.L.; Coursin, D.B.; Riker, R.R.; Fontaine, D.; Wittbrodt, E.T.; Chalfin, D.B.; Masica, M.F.; Bjerke, H.S.; Coplin, W.M.; et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit. Care Med. 2002, 30, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Playfor, S.D. Analgesia and sedation in critically ill children. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 90–94. [Google Scholar] [CrossRef]
- Keefe, F.J.; Williams, D.A.; Smith, S.J. Assessment of pain behaviors. In Handbook of Pain Assessment, 2nd ed.; The Guilford Press: New York, NY, USA, 2001; pp. 170–187. [Google Scholar]
- Freund, D.; Bolick, B.N. CE: Assessing a child’s pain. Am. J. Nurs. 2019, 119, 34–41. [Google Scholar] [CrossRef]
- Beckman, E.J. Analgesia and sedation in hospitalized children. In PedSAP 2017 Book 3: Sedation and Analgesia; The American College of Clinical Pharmacy: Lenexa, KS, USA, 2017; pp. 7–30. [Google Scholar]
- McGrath, P.J.; Unruh, A.M. Measurement and assessment of pediatric pai. In Wall and Melzack’s Textbook of Pain, 6th ed.; McMahon, S.B., Kolzenburg, M., Tracey, I., Turk, D., Eds.; Saunders: Philadelphia, PA, USA, 2013; pp. 320–327. [Google Scholar]
- Wells, N.; Pasero, C.; McCaffery, M. Improving the quality of care through pain assessment and management. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Hughes, R.G., Ed.; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Gommers, D.; Bakker, J. Medications for analgesia and sedation in the intensive care unit: An overview. Crit. Care 2008, 12 (Suppl. 3), S4. [Google Scholar] [CrossRef]
- Izrailtyan, I.; Qiu, J.; Overdyk, F.J.; Erslon, M.; Gan, T.J. Risk factors for cardiopulmonary and respiratory arrest in medical and surgical hospital patients on opioid analgesics and sedatives. PLoS ONE 2018, 13, e0194553. [Google Scholar] [CrossRef]
- Anand, K.J.; Clark, A.E.; Willson, D.F.; Berger, J.; Meert, K.L.; Zimmerman, J.J.; Harrison, R.; Carcillo, J.A.; Newth, C.J.; Bisping, S.; et al. Opioid analgesia in mechanically ventilated children: Results from the multicenter measuring opioid tolerance induced by fentanyl study. Pediatr. Crit. Care Med. 2013, 14, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T. Neuroexcitatory effects of morphine and hydromorphone: Evidence implicating the 3-glucuronide metabolites. Clin. Exp. Pharmacol. Physiol. 2000, 27, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Due, M.R.; Piekarz, A.D.; Wilson, N.; Feldman, P.; Ripsch, M.S.; Chavez, S.; Yin, H.; Khanna, R.; White, F.A. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J. Neuroinflamm. 2012, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Standifer, K.M.; Inturrisi, C.E.; Pasternak, G.W. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J. Pharmacol. Exp. Ther. 1989, 251, 477–483. [Google Scholar]
- Pasternak, G.W.; Bodnar, R.J.; Clark, J.A.; Inturrisi, C.E. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987, 41, 2845–2849. [Google Scholar] [CrossRef]
- Francés, B.; Gout, R.; Campistron, G.; Panconi, E.; Cros, J. Morphine-6-glucuronide is more mu-selective and potent in analgesic tests than morphine. Prog. Clin. Biol. Res. 1990, 328, 477–480. [Google Scholar]
- Krekels, E.H.; Tibboel, D.; de Wildt, S.N.; Ceelie, I.; Dahan, A.; van Dijk, M.; Danhof, M.; Knibbe, C.A. Evidence-based morphine dosing for postoperative neonates and infants. Clin. Pharmacokinet. 2014, 53, 553–563. [Google Scholar] [CrossRef]
- Dahlgren, N.; Messeter, K. Treatment of stress response to laryngoscopy and intubation with fentanyl. Anaesthesia 1981, 36, 1022–1026. [Google Scholar] [CrossRef]
- Martin, D.E.; Rosenberg, H.; Aukburg, S.J.; Bartkowski, R.R.; Edwards, M.W., Jr.; Greenhow, D.E.; Klineberg, P.L. Low-dose fentanyl blunts circulatory responses to tracheal intubation. Anesth. Analg. 1982, 61, 680–684. [Google Scholar] [CrossRef]
- Kautto, U.M. Attenuation of the circulatory response to laryngoscopy and intubation by fentanyl. Acta Anaesthesiol. Scand. 1982, 26, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, D.C.; Han, Y.J.; Song, H.S. Small-dose fentanyl: Optimal time of injection for blunting the circulatory responses to tracheal intubation. Anesth. Analg. 1998, 86, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.S.; Liu, K.P.; Liu, Y.; Xu, Y.C.; Liao, X.; Zhang, G.H.; Li, C.W.; Yang, Q.Y.; Sun, H.T. Assessment of small-dose fentanyl and sufentanil blunting the cardiovascular responses to laryngoscopy and intubation in children. Paediatr. Anaesth. 2007, 17, 568–574. [Google Scholar] [CrossRef]
- Çoruh, B.; Tonelli, M.R.; Park, D.R. Fentanyl-induced chest wall rigidity. Chest 2013, 143, 1145–1146. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, C.; Noto, A.; Crimi, C.; Sanfilippo, F. Remifentanil-induced postoperative hyperalgesia: Current perspectives on mechanisms and therapeutic strategies. Local Reg. Anesth. 2018, 11, 15–23. [Google Scholar] [CrossRef]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef]
- Welzing, L.; Link, F.; Junghaenel, S.; Oberthuer, A.; Harnischmacher, U.; Stuetzer, H.; Roth, B. Remifentanil-induced tolerance, withdrawal or hyperalgesia in infants: A randomized controlled trial. RAPIP trial: Remifentanil-based analgesia and sedation of paediatric intensive care patients. Neonatology 2013, 104, 34–41. [Google Scholar] [CrossRef]
- Quigley, C. Hydromorphone for acute and chronic pain. Cochrane Database Syst. Rev. 2002, Cd003447. [Google Scholar] [CrossRef]
- Reiter, P.D.; Ng, J.; Dobyns, E.L. Continuous hydromorphone for pain and sedation in mechanically ventilated infants and children. J. Opioid Manag. 2012, 8, 99–104. [Google Scholar] [CrossRef]
- Jeffries, S.A.; McGloin, R.; Pitfield, A.F.; Carr, R.R. Use of methadone for prevention of opioid withdrawal in critically ill children. Can. J. Hosp. Pharm. 2012, 65, 12–18. [Google Scholar] [CrossRef]
- Lugo, R.A.; MacLaren, R.; Cash, J.; Pribble, C.G.; Vernon, D.D. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy 2001, 21, 1566–1573. [Google Scholar] [CrossRef]
- Solodiuk, J.C.; Greco, C.D.; O’Donnell, K.A.; Morrill, D.R.; Curley, M.A.Q. Effect of a sedation weaning protocol on safety and medication use among hospitalized children post critical illness. J. Pediatr. Nurs. 2019, 49, 18–23. [Google Scholar] [CrossRef]
- Siddappa, R.; Fletcher, J.E.; Heard, A.M.; Kielma, D.; Cimino, M.; Heard, C.M. Methadone dosage for prevention of opioid withdrawal in children. Paediatr. Anaesth. 2003, 13, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gammaitoni, A.R.; Fine, P.; Alvarez, N.; McPherson, M.L.; Bergmark, S. Clinical application of opioid equianalgesic data. Clin. J. Pain 2003, 19, 286–297. [Google Scholar] [CrossRef]
- Ripamonti, C.; Groff, L.; Brunelli, C.; Polastri, D.; Stavrakis, A.; De Conno, F. Switching from morphine to oral methadone in treating cancer pain: What is the equianalgesic dose ratio? J. Clin. Oncol. 1998, 16, 3216–3221. [Google Scholar] [CrossRef]
- Anand, K.J.; Arnold, J.H. Opioid tolerance and dependence in infants and children. Crit. Care Med. 1994, 22, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Fife, A.; Postier, A.; Flood, A.; Friedrichsdorf, S.J. Methadone conversion in infants and children: Retrospective cohort study of 199 pediatric inpatients. J. Opioid Manag. 2016, 12, 123–130. [Google Scholar] [CrossRef]
- Franck, L.S.; Harris, S.K.; Soetenga, D.J.; Amling, J.K.; Curley, M.A. The Withdrawal Assessment Tool-1 (WAT-1): An assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr. Crit. Care Med. 2008, 9, 573–580. [Google Scholar] [CrossRef]
- Curley, M.A.; Harris, S.K.; Fraser, K.A.; Johnson, R.A.; Arnold, J.H. State behavioral scale: A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr. Crit. Care Med. 2006, 7, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.D.; Kovach, J.R.; Thompson, N.E. Methadone’s effect on cardiac repolarization: Safety in the PICU. Pediatr. Crit. Care Med. 2020, 21, e747–e751. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.L.; Stapleton, J.V.; Mather, L.E. Relationship between blood meperidine concentrations and analgesic response: A preliminary report. Anesthesiology 1980, 53, 460–466. [Google Scholar] [CrossRef]
- Grass, J.A. Patient-controlled analgesia. Anesth. Analg. 2005, 101, S44–S61. [Google Scholar] [CrossRef] [PubMed]
- Morlion, B.; Schäfer, M.; Betteridge, N.; Kalso, E. Non-invasive patient-controlled analgesia in the management of acute postoperative pain in the hospital setting. Curr. Med. Res. Opin. 2018, 34, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Berde, C.B.; Lehn, B.M.; Yee, J.D.; Sethna, N.F.; Russo, D. Patient-controlled analgesia in children and adolescents: A randomized, prospective comparison with intramuscular administration of morphine for postoperative analgesia. J. Pediatr. 1991, 118, 460–466. [Google Scholar] [CrossRef]
- Nelson, K.L.; Yaster, M.; Kost-Byerly, S.; Monitto, C.L. A national survey of American Pediatric Anesthesiologists: Patient-controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesth. Analg. 2010, 110, 754–760. [Google Scholar] [CrossRef]
- Anghelescu, D.L.; Burgoyne, L.L.; Oakes, L.L.; Wallace, D.A. The safety of patient-controlled analgesia by proxy in pediatric oncology patients. Anesth. Analg. 2005, 101, 1623–1627. [Google Scholar] [CrossRef]
- Voepel-Lewis, T.; Marinkovic, A.; Kostrzewa, A.; Tait, A.R.; Malviya, S. The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth. Analg. 2008, 107, 70–75. [Google Scholar] [CrossRef]
- Czarnecki, M.L.; Salamon, K.S.; Jastrowski Mano, K.E.; Ferrise, A.S.; Sharp, M.; Weisman, S.J. A preliminary report of parent/nurse-controlled analgesia (PNCA) in infants and preschoolers. Clin. J. Pain 2011, 27, 102–107. [Google Scholar] [CrossRef]
- Anghelescu, D.L.; Faughnan, L.G.; Oakes, L.L.; Windsor, K.B.; Pei, D.; Burgoyne, L.L. Parent-controlled PCA for pain management in pediatric oncology: Is it safe? J. Pediatr. Hematol. Oncol. 2012, 34, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Jahr, J.S.; Lee, V.K. Intravenous acetaminophen. Anesthesiol. Clin. 2010, 28, 619–645. [Google Scholar] [CrossRef]
- Nishimoto, R.N. OFIRMEV: An old drug becomes new again. Anesth. Prog. 2014, 61, 99–102. [Google Scholar] [CrossRef]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New vistas of an old drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef]
- Malaise, O.; Bruyere, O.; Reginster, J.-Y. Intravenous paracetamol: A review of efficacy and safety in therapeutic use. Future Neurol. 2007, 2, 673–688. [Google Scholar] [CrossRef]
- Jarde, O.; Boccard, E. Parenteral versus oral route increases paracetamol efficacy. Clin. Drug Investig. 1997, 14, 474–481. [Google Scholar] [CrossRef]
- Holmér Pettersson, P.; Owall, A.; Jakobsson, J. Early bioavailability of paracetamol after oral or intravenous administration. Acta Anaesthesiol. Scand. 2004, 48, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.A.; Ruggles, C.A.; Thompson, M.; Metzger, K.Z.; Christopher, J.A.; Bigham, M.T. Using quality improvement to reduce IV Acetaminophen use in a PICU. Pediatr. Crit. Care Med. 2020, 21, 550–556. [Google Scholar] [CrossRef]
- Vincent, W.R., 3rd; Huiras, P.; Empfield, J.; Horbowicz, K.J.; Lewis, K.; McAneny, D.; Twitchell, D. Controlling postoperative use of i.v. acetaminophen at an academic medical center. Am. J. Health Syst. Pharm. 2018, 75, 548–555. [Google Scholar] [CrossRef]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Adelizzi, R.A. COX-1 and COX-2 in health and disease. J. Am. Osteopath. Assoc. 1999, 99, S7–S12. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- de Martino, M.; Chiarugi, A.; Boner, A.; Montini, G.; De’Angelis, G.L. Working towards an appropriate use of ibuprofen in children: An evidence-based appraisal. Drugs 2017, 77, 1295–1311. [Google Scholar] [CrossRef]
- McNicol, E.D.; Rowe, E.; Cooper, T.E. Ketorolac for postoperative pain in children. Cochrane Database Syst. Rev. 2018, 7, Cd012294. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.N.; Christians, U.; Henthorn, T.; Vu Tran, Z.; Moll, V.; Zuk, J.; Galinkin, J. Pharmacokinetics of single-dose intravenous ketorolac in infants aged 2-11 months. Anesth. Analg. 2011, 112, 655–660. [Google Scholar] [CrossRef]
- Jitpakdee, T.; Mandee, S. Strategies for preventing side effects of systemic opioid in postoperative pediatric patients. Paediatr. Anaesth. 2014, 24, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.B.; Heitlinger, E.L.; Revell, S. Ketorolac for postoperative pain management in children. Drug Saf. 1997, 16, 309–329. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ibuprofen pathways. Pharm. Genom. 2015, 25, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Gaid, E.; Mehta, R.; Waller, J. The routine use of post-operative NSAIDs in the pediatric patient is effective and safe. Pediatrics 2020, 146, 165–166. [Google Scholar] [CrossRef]
- Kaye, A.D.; Urman, R.D.; Rappaport, Y.; Siddaiah, H.; Cornett, E.M.; Belani, K.; Salinas, O.J.; Fox, C.J. Multimodal analgesia as an essential part of enhanced recovery protocols in the ambulatory settings. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S40–S45. [Google Scholar] [CrossRef]
- Tiippana, E.M.; Hamunen, K.; Kontinen, V.K.; Kalso, E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth. Analg. 2007, 104, 1545–1556. [Google Scholar] [CrossRef]
- Laskowski, K.; Stirling, A.; McKay, W.P.; Lim, H.J. A systematic review of intravenous ketamine for postoperative analgesia. Can. J. Anaesth. 2011, 58, 911–923. [Google Scholar] [CrossRef]
- Neunhoeffer, F.; Hanser, A.; Esslinger, M.; Icheva, V.; Kumpf, M.; Gerbig, I.; Hofbeck, M.; Michel, J. Ketamine infusion as a counter measure for opioid tolerance in mechanically ventilated children: A pilot study. Paediatr. Drugs 2017, 19, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Blaudszun, G.; Lysakowski, C.; Elia, N.; Tramèr, M.R. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: Systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2012, 116, 1312–1322. [Google Scholar] [CrossRef]
- Tryba, M.; Gehling, M. Clonidine—A potent analgesic adjuvant. Curr. Opin. Anaesthesiol. 2002, 15, 511–517. [Google Scholar] [CrossRef]
- Basker, S.; Singh, G.; Jacob, R. Clonidine in paediatrics—A review. Indian J. Anaesth. 2009, 53, 270–280. [Google Scholar]
- Reimer, E.J.; Dunn, G.S.; Montgomery, C.J.; Sanderson, P.M.; Scheepers, L.D.; Merrick, P.M. The effectiveness of clonidine as an analgesic in paediatric adenotonsillectomy. Can. J. Anaesth. 1998, 45, 1162–1167. [Google Scholar] [CrossRef]
- Nishina, K.; Mikawa, K.; Shiga, M.; Takao, Y.; Maekawa, N.; Obara, H. Diclofenac and flurbiprofen with or without clonidine for postoperative analgesia in children undergoing elective ophthalmological surgery. Paediatr. Anaesth. 2000, 10, 645–651. [Google Scholar] [CrossRef]
- Reddy, S.K.; Jones, J.J.; Gordish-Dressman, H.; Pestieau, S.R. Dexmedetomidine as an opioid-sparing agent in pediatric craniofacial surgery. Children 2020, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Olutoye, O.A.; Glover, C.D.; Diefenderfer, J.W.; McGilberry, M.; Wyatt, M.M.; Larrier, D.R.; Friedman, E.M.; Watcha, M.F. The effect of intraoperative dexmedetomidine on postoperative analgesia and sedation in pediatric patients undergoing tonsillectomy and adenoidectomy. Anesth. Analg. 2010, 111, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Verghese, S.T.; Hannallah, R.S. Acute pain management in children. J. Pain Res. 2010, 3, 105–123. [Google Scholar] [CrossRef]
- Jin, F.; Chung, F. Multimodal analgesia for postoperative pain control. J. Clin. Anesth. 2001, 13, 524–539. [Google Scholar] [CrossRef]
- Hong, J.Y.; Won Han, S.; Kim, W.O.; Kil, H.K. Fentanyl sparing effects of combined ketorolac and acetaminophen for outpatient inguinal hernia repair in children. J. Urol. 2010, 183, 1551–1555. [Google Scholar] [CrossRef]
- Ong, C.K.; Seymour, R.A.; Lirk, P.; Merry, A.F. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: A qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth. Analg. 2010, 110, 1170–1179. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N.; Ayuk, A.C.; Ndu, I.K.; Eneh, C.I.; Mbanefo, N.R.; Ezenwosu, O.U. Vaso-occlusive crisis in sickle cell disease: Current paradigm on pain management. J. Pain Res. 2018, 11, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Dadure, C.; Veyckemans, F.; Bringuier, S.; Habre, W. Epidemiology of regional anesthesia in children: Lessons learned from the European Multi-Institutional Study APRICOT. Paediatr. Anaesth. 2019, 29, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Ivani, G.; Mosseti, V. Pediatric regional anesthesia. Minerva Anestesiol. 2009, 75, 577–583. [Google Scholar]
- Walker, B.J.; Long, J.B.; De Oliveira, G.S.; Szmuk, P.; Setiawan, C.; Polaner, D.M.; Suresh, S. Peripheral nerve catheters in children: An analysis of safety and practice patterns from the pediatric regional anesthesia network (PRAN). Br. J. Anaesth. 2015, 115, 457–462. [Google Scholar] [CrossRef] [PubMed]
- De Pinto, M.; Dagal, A.; O’Donnell, B.; Stogicza, A.; Chiu, S.; Edwards, W.T. Regional anesthesia for management of acute pain in the intensive care unit. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 138–143. [Google Scholar] [CrossRef]
- Yuki, K.; Matsunami, E.; Tazawa, K.; Wang, W.; DiNardo, J.A.; Koutsogiannaki, S. Pediatric perioperative stress responses and anesthesia. Transl. Perioper. Pain Med. 2017, 2, 1–12. [Google Scholar]
- Wolf, A.R. Effects of regional analgesia on stress responses to pediatric surgery. Paediatr. Anaesth. 2012, 22, 19–24. [Google Scholar] [CrossRef]
- Craven, P.D.; Badawi, N.; Henderson-Smart, D.J.; O’Brien, M. Regional (spinal, epidural, caudal) versus general anaesthesia in preterm infants undergoing inguinal herniorrhaphy in early infancy. Cochrane Database Syst. Rev. 2003, Cd003669. [Google Scholar] [CrossRef]
- Arms, D.M.; Smith, J.T.; Osteyee, J.; Gartrell, A. Postoperative epidural analgesia for pediatric spine surgery. Orthopedics 1998, 21, 539–544. [Google Scholar] [PubMed]
- Fonsmark, L.; Rasmussen, Y.H.; Carl, P. Occurrence of withdrawal in critically ill sedated children. Crit. Care Med. 1999, 27, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Ista, E.; van Dijk, M.; Gamel, C.; Tibboel, D.; de Hoog, M. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: A first evaluation. Crit. Care Med. 2008, 36, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Garcia Guerra, G.; Joffe, A.R.; Cave, D.; Duff, J.; Duncan, S.; Sheppard, C.; Tawfik, G.; Hartling, L.; Jou, H.; Vohra, S. Survey of sedation and analgesia practice among canadian pediatric critical care physicians. Pediatr. Crit. Care Med. 2016, 17, 823–830. [Google Scholar] [CrossRef]
- Cunningham, M.E.; Vogel, A.M. Analgesia, sedation, and delirium in pediatric surgical critical care. Semin. Pediatr. Surg. 2019, 28, 33–42. [Google Scholar] [CrossRef]
- Saliski, M.; Kudchadkar, S.R. Optimizing Sedation management to promote early mobilization for critically ill children. J. Pediatr. Intensive Care 2015, 4, 188–193. [Google Scholar] [CrossRef]
- Ambuel, B.; Hamlett, K.W.; Marx, C.M.; Blumer, J.L. Assessing distress in pediatric intensive care environments: The COMFORT scale. J. Pediatr. Psychol. 1992, 17, 95–109. [Google Scholar] [CrossRef]
- Curley, M.; McDermott, B.; Berry, P.; Hurley, J.; MacKey, C.; McAleer, D.; Alsip, C. Nurses’ decision making regarding the use of sedatives and analgesics in pediatric ICU. Heart Lung 1992, 21, 296. [Google Scholar]
- Carnevale, F.A.; Razack, S. An item analysis of the COMFORT scale in a pediatric intensive care unit. Pediatr. Crit. Care Med. 2002, 3, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ista, E.; van Dijk, M.; Tibboel, D.; de Hoog, M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT “behavior” scale. Pediatr. Crit. Care Med. 2005, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Kerson, A.G.; DeMaria, R.; Mauer, E.; Joyce, C.; Gerber, L.M.; Greenwald, B.M.; Silver, G.; Traube, C. Validity of the Richmond Agitation-Sedation Scale (RASS) in critically ill children. J. Intensive Care 2016, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Kihlstrom, M.J.; Edge, A.P.; Cherry, K.M.; Zarick, P.J.; Beck, S.D.; Boyd, J.M. Multi-modal educational curriculum to improve richmond agitation-sedation scale inter-rater reliability in pediatric patients. Pediatr. Qual. Saf. 2018, 3, e096. [Google Scholar] [CrossRef]
- Triltsch, A.E.; Nestmann, G.; Orawa, H.; Moshirzadeh, M.; Sander, M.; Grosse, J.; Genähr, A.; Konertz, W.; Spies, C.D. Bispectral index versus COMFORT score to determine the level of sedation in paediatric intensive care unit patients: A prospective study. Crit. Care 2005, 9, R9–R17. [Google Scholar] [CrossRef] [PubMed]
- Playfor, S.D. The use of bispectral index monitors in paediatric intensive care. Crit. Care 2005, 9, 25–26. [Google Scholar] [CrossRef][Green Version]
- Kudchadkar, S.R.; Yaster, M.; Punjabi, N.M. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: A wake-up call for the pediatric critical care community*. Crit. Care Med. 2014, 42, 1592–1600. [Google Scholar] [CrossRef]
- Fox, C.; Liu, H.; Kaye, A. Antianxiety Agents. In Clinical Aspects of Pain Medicine and Interventional Pain Management: A Comprehensive Review; ASIP Publishing: Paducah, KY, USA, 2011; pp. 543–552. [Google Scholar]
- Griffin, C.E., 3rd; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- Mihic, S.J.; Harris, R.A. Hypnotics and sedatives. In Goodman & Gilman’s Pharmacological Basis of Therapeutics, 12th ed.; Goodman, L.S., Brunton, L.L., Chabner, B., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Anand, K.J.; Hall, R.W.; Desai, N.; Shephard, B.; Bergqvist, L.L.; Young, T.E.; Boyle, E.M.; Carbajal, R.; Bhutani, V.K.; Moore, M.B.; et al. Effects of morphine analgesia in ventilated preterm neonates: Primary outcomes from the NEOPAIN randomised trial. Lancet 2004, 363, 1673–1682. [Google Scholar] [CrossRef]
- Boylan, G.B.; Rennie, J.M.; Chorley, G.; Pressler, R.M.; Fox, G.F.; Farrer, K.; Morton, M.; Binnie, C.D. Second-line anticonvulsant treatment of neonatal seizures: A video-EEG monitoring study. Neurology 2004, 62, 486–488. [Google Scholar] [CrossRef]
- Castro Conde, J.R.; Hernández Borges, A.A.; Doménech Martínez, E.; González Campo, C.; Perera Soler, R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology 2005, 64, 876–879. [Google Scholar] [CrossRef]
- Bauer, T.M.; Ritz, R.; Haberthür, C.; Ha, H.R.; Hunkeler, W.; Sleight, A.J.; Scollo-Lavizzari, G.; Haefeli, W.E. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995, 346, 145–147. [Google Scholar] [CrossRef]
- Yahwak, J.A.; Riker, R.R.; Fraser, G.L.; Subak-Sharpe, S. Determination of a lorazepam dose threshold for using the osmol gap to monitor for propylene glycol toxicity. Pharmacotherapy 2008, 28, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Chicella, M.; Jansen, P.; Parthiban, A.; Marlowe, K.F.; Bencsath, F.A.; Krueger, K.P.; Boerth, R. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit. Care Med. 2002, 30, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- van der Vossen, A.C.; van Nuland, M.; Ista, E.G.; de Wildt, S.N.; Hanff, L.M. Oral lorazepam can be substituted for intravenous midazolam when weaning paediatric intensive care patients off sedation. Acta Paediatr. 2018, 107, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Krauss, B.; Green, S.M. Procedural sedation and analgesia in children. Lancet 2006, 367, 766–780. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Harmatz, J.S.; Friedman, H.; Locniskar, A.; Shader, R.I. A large-sample study of diazepam pharmacokinetics. Ther. Drug Monit. 1989, 11, 652–657. [Google Scholar] [CrossRef]
- Lamson, M.J.; Sitki-Green, D.; Wannarka, G.L.; Mesa, M.; Andrews, P.; Pellock, J. Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: A phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin. Drug Investig. 2011, 31, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Damhoff, H.; McCune, C.L. Barbiturates in the Pediatric ICU. In Sedation and Analgesia for the Pediatric Intensivist; Kamat, P.P., Berkenbosch, J.W., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 85–94. [Google Scholar]
- Löscher, W.; Rogawski, M.A. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia 2012, 53 (Suppl. 8), 12–25. [Google Scholar] [CrossRef]
- Wada, D.R.; Björkman, S.; Ebling, W.F.; Harashima, H.; Harapat, S.R.; Stanski, D.R. Computer simulation of the effects of alterations in blood flows and body composition on thiopental pharmacokinetics in humans. Anesthesiology 1997, 87, 884–899. [Google Scholar] [CrossRef]
- Westrin, P.; Jonmarker, C.; Werner, O. Thiopental requirements for induction of anesthesia in neonates and in infants one to six months of age. Anesthesiology 1989, 71, 344–346. [Google Scholar] [CrossRef]
- Jonmarker, C.; Westrin, P.; Larsson, S.; Werner, O. Thiopental requirements for induction of anesthesia in children. Anesthesiology 1987, 67, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Russo, H.; Bressolle, F. Pharmacodynamics and pharmacokinetics of thiopental. Clin. Pharmacokinet. 1998, 35, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Mellion, S.A.; Bennett, K.S.; Ellsworth, G.L.; Moore, K.; Riva-Cambrin, J.; Metzger, R.R.; Bratton, S.L. High-dose barbiturates for refractory intracranial hypertension in children with severe traumatic brain injury. Pediatr. Crit. Care Med. 2013, 14, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.T.; James, R.F.; Landman, M.P.; O’Neill, P.J.; Cotton, B.A.; Hansen, E.N.; Morris, J.A., Jr.; May, A.K. Pentobarbital coma for refractory intra-cranial hypertension after severe traumatic brain injury: Mortality predictions and one-year outcomes in 55 patients. J. Trauma 2010, 69, 275–283. [Google Scholar] [CrossRef]
- Giovannitti, J.A., Jr.; Thoms, S.M.; Crawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–39. [Google Scholar] [CrossRef]
- Walker, J.; Maccallum, M.; Fischer, C.; Kopcha, R.; Saylors, R.; McCall, J. Sedation using dexmedetomidine in pediatric burn patients. J. Burn Care Res. 2006, 27, 206–210. [Google Scholar] [CrossRef]
- Ebert, T.J.; Hall, J.E.; Barney, J.A.; Uhrich, T.D.; Colinco, M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000, 93, 382–394. [Google Scholar] [CrossRef]
- Pichot, C.; Ghignone, M.; Quintin, L. Dexmedetomidine and clonidine: From second- to first-line sedative agents in the critical care setting? J. Intensive Care Med. 2012, 27, 219–237. [Google Scholar] [CrossRef]
- Wolf, A.; McKay, A.; Spowart, C.; Granville, H.; Boland, A.; Petrou, S.; Sutherland, A.; Gamble, C. Prospective multicentre randomised, double-blind, equivalence study comparing clonidine and midazolam as intravenous sedative agents in critically ill children: The SLEEPS (Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation) study. Health Technol. Assess. 2014, 18, 1–212. [Google Scholar] [CrossRef]
- Almenrader, N.; Passariello, M.; Coccetti, B.; Haiberger, R.; Pietropaoli, P. Steal-induction after clonidine premedication: A comparison of the oral and nasal route. Paediatr. Anaesth. 2007, 17, 230–234. [Google Scholar] [CrossRef]
- Schmidt, A.P.; Valinetti, E.A.; Bandeira, D.; Bertacchi, M.F.; Simões, C.M.; Auler, J.O., Jr. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr. Anaesth. 2007, 17, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.C.; De Kock, M.; Klimscha, W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995). Anesthesiology 1996, 85, 655–674. [Google Scholar] [CrossRef]
- Ambrose, C.; Sale, S.; Howells, R.; Bevan, C.; Jenkins, I.; Weir, P.; Murphy, P.; Wolf, A. Intravenous clonidine infusion in critically ill children: Dose-dependent sedative effects and cardiovascular stability. Br. J. Anaesth. 2000, 84, 794–796. [Google Scholar] [CrossRef] [PubMed]
- De Witte, J.; Sessler, D.I. Perioperative shivering: Physiology and pharmacology. Anesthesiology 2002, 96, 467–484. [Google Scholar] [CrossRef]
- Taketomo, C.K.; Hodding, J.H.; Kraus, D.M. Pediatric & Neonatal Dosage Handbook: An Extensive Resource for Clinicians Treating Pediatric and Neonatal Patients; Lexi-Comp Inc: Hudson, OH, USA, 2017. [Google Scholar]
- Capino, A.C.; Miller, J.L.; Johnson, P.N. Clonidine for sedation and analgesia and withdrawal in critically ill infants and children. Pharmacotherapy 2016, 36, 1290–1299. [Google Scholar] [CrossRef]
- Weerink, M.A.S.; Struys, M.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.P.; O’Mahony, E.; Zurakowski, D.; Libenson, M.H. Effects of dexmedetomidine sedation on the EEG in children. Paediatr. Anaesth. 2009, 19, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.P.; Park, R.S.; Sullivan, C.A.; Lukovits, K.; Halpin, E.M.; Imbrescia, S.T.; Cavanaugh, D.; Prescilla, R.; Fox, V.L. The synergistic effect of dexmedetomidine on propofol for paediatric deep sedation: A randomised trial. Eur. J. Anaesthesiol. 2020. [Google Scholar] [CrossRef]
- Erickson, S.J.; Millar, J.; Anderson, B.J.; Festa, M.S.; Straney, L.; Shehabi, Y.; Long, D.A. Dexmedetomidine sedation in mechanically ventilated critically ill children: A pilot randomized controlled trial. Pediatr. Crit. Care Med. 2020, 21, e731–e739. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, F.; Mondardini, M.C.; Dell’Oste, C.; Vitale, F.; Ferrario, S.; Lapi, M.; Ferrero, F.; Dusio, M.P.; Rossetti, E.; Daverio, M.; et al. Efficacy and safety of dexmedetomidine for prolonged sedation in the PICU: A Prospective Multicenter Study (PROSDEX). Pediatr. Crit. Care Med. 2020, 21, 625–636. [Google Scholar] [CrossRef]
- Sahinovic, M.M.; Struys, M.; Absalom, A.R. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef]
- Barr, J.; Egan, T.D.; Sandoval, N.F.; Zomorodi, K.; Cohane, C.; Gambus, P.L.; Shafer, S.L. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic-pharmacodynamic model. Anesthesiology 2001, 95, 324–333. [Google Scholar] [CrossRef]
- Hemphill, S.; McMenamin, L.; Bellamy, M.C.; Hopkins, P.M. Propofol infusion syndrome: A structured literature review and analysis of published case reports. Br. J. Anaesth. 2019, 122, 448–459. [Google Scholar] [CrossRef]
- Krajčová, A.; Waldauf, P.; Anděl, M.; Duška, F. Propofol infusion syndrome: A structured review of experimental studies and 153 published case reports. Crit. Care 2015, 19, 398. [Google Scholar] [CrossRef]
- Timpe, E.M.; Eichner, S.F.; Phelps, S.J. Propofol-related infusion syndrome in critically ill pediatric patients: Coincidence, association, or causation? J. Pediatr. Pharmacol. Ther. 2006, 11, 17–42. [Google Scholar] [CrossRef]
- Playfor, S.D.; Venkatesh, K. Current patterns of propofol use in PICU in the United Kingdom and North America. Paediatr. Anaesth. 2004, 14, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Villevieille, T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef]
- Green, S.M.; Krauss, B. Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann. Emerg. Med. 2004, 44, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Wathen, J.E.; Roback, M.G.; Mackenzie, T.; Bothner, J.P. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled, emergency department trial. Ann. Emerg. Med. 2000, 36, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, T.S.; Green, S.M.; Khan, A.; Chapman, D.S.; Dannenberg, B. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann. Emerg. Med. 2000, 35, 229–238. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism and metabolomics of ketamine: A toxicological approach. Forensic Sci. Res. 2017, 2, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Allotey, P.; Reidpath, D.D.; Elisha, D. “Social medication” and the control of children: A qualitative study of over-the-counter medication among Australian children. Pediatrics 2004, 114, e378–e383. [Google Scholar] [CrossRef] [PubMed]
- Kishk, O.A.; Simone, S.; Lardieri, A.B.; Graciano, A.L.; Tumulty, J.; Edwards, S. Antipsychotic treatment of delirium in critically ill children: A retrospective matched cohort study. J. Pediatr. Pharmacol. Ther. 2019, 24, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Turkel, S.B.; Hanft, A. The pharmacologic management of delirium in children and adolescents. Paediatr. Drugs 2014, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Turkel, S.B.; Jacobson, J.R.; Tavaré, C.J. The diagnosis and management of delirium in infancy. J. Child Adolesc. Psychopharmacol. 2013, 23, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Poh, Y.N.; Poh, P.F.; Buang, S.N.; Lee, J.H. Sedation guidelines, protocols, and algorithms in PICUs: A systematic review. Pediatr. Crit. Care Med. 2014, 15, 885–892. [Google Scholar] [CrossRef]
- Rosenberg, L.; Traube, C. Sedation strategies in children with pediatric acute respiratory distress syndrome (PARDS). Ann. Transl. Med. 2019, 7, 509. [Google Scholar] [CrossRef]
- Deeter, K.H.; King, M.A.; Ridling, D.; Irby, G.L.; Lynn, A.M.; Zimmerman, J.J. Successful implementation of a pediatric sedation protocol for mechanically ventilated patients. Crit. Care Med. 2011, 39, 683–688. [Google Scholar] [CrossRef]
- Gupta, K.; Gupta, V.K.; Jayashree, M.; Singhi, S. Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated children. Pediatr. Crit. Care Med. 2012, 13, 131–135. [Google Scholar] [CrossRef]
- Vet, N.J.; de Wildt, S.N.; Verlaat, C.W.; Knibbe, C.A.; Mooij, M.G.; van Woensel, J.B.; van Rosmalen, J.; Tibboel, D.; de Hoog, M. A randomized controlled trial of daily sedation interruption in critically ill children. Intensive Care Med. 2016, 42, 233–244. [Google Scholar] [CrossRef]
- Cunliffe, M.; McArthur, L.; Dooley, F. Managing sedation withdrawal in children who undergo prolonged PICU admission after discharge to the ward. Paediatr. Anaesth. 2004, 14, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Keogh, S.J.; Long, D.A.; Horn, D.V. Practice guidelines for sedation and analgesia management of critically ill children: A pilot study evaluating guideline impact and feasibility in the PICU. BMJ Open 2015, 5, e006428. [Google Scholar] [CrossRef]
- European Delirium Association; American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med 2014, 12, 141. [Google Scholar] [CrossRef]
- Smith, H.A.B.; Gangopadhyay, M.; Goben, C.M.; Jacobowski, N.L.; Chestnut, M.H.; Thompson, J.L.; Chandrasekhar, R.; Williams, S.R.; Griffith, K.; Ely, E.W.; et al. Delirium and benzodiazepines associated with prolonged ICU stay in critically ill infants and young children. Crit. Care Med. 2017, 45, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Traube, C.; Silver, G.; Reeder, R.W.; Doyle, H.; Hegel, E.; Wolfe, H.A.; Schneller, C.; Chung, M.G.; Dervan, L.A.; DiGennaro, J.L.; et al. Delirium in critically ill children: An international point prevalence study. Crit. Care Med. 2017, 45, 584–590. [Google Scholar] [CrossRef]
- Traube, C.; Silver, G.; Gerber, L.M.; Kaur, S.; Mauer, E.A.; Kerson, A.; Joyce, C.; Greenwald, B.M. Delirium and mortality in critically ill children: Epidemiology and outcomes of pediatric delirium. Crit. Care Med. 2017, 45, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Fuchs, D.C.; Pandharipande, P.P.; Barr, F.E.; Ely, E.W. Delirium: An emerging frontier in the management of critically ill children. Anesthesiol. Clin. 2011, 29, 729–750. [Google Scholar] [CrossRef]
- Silver, G.; Traube, C.; Gerber, L.M.; Sun, X.; Kearney, J.; Patel, A.; Greenwald, B. Pediatric delirium and associated risk factors: A single-center prospective observational study. Pediatr. Crit. Care Med. 2015, 16, 303–309. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, M.; Schoonhoven, L.; Evers, A.W.; van der Hoeven, J.G.; van Achterberg, T.; Pickkers, P. Delirium in critically ill patients: Impact on long-term health-related quality of life and cognitive functioning. Crit. Care Med. 2012, 40, 112–118. [Google Scholar] [CrossRef]
- Alvarez, R.V.; Palmer, C.; Czaja, A.S.; Peyton, C.; Silver, G.; Traube, C.; Mourani, P.M.; Kaufman, J. Delirium is a common and early finding in patients in the pediatric cardiac intensive care unit. J. Pediatr. 2018, 195, 206–212. [Google Scholar] [CrossRef]
- Mody, K.; Kaur, S.; Mauer, E.A.; Gerber, L.M.; Greenwald, B.M.; Silver, G.; Traube, C. Benzodiazepines and development of delirium in critically ill children: Estimating the causal effect. Crit. Care Med. 2018, 46, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Boyd, J.; Fuchs, D.C.; Melvin, K.; Berry, P.; Shintani, A.; Eden, S.K.; Terrell, M.K.; Boswell, T.; Wolfram, K.; et al. Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit. Care Med. 2011, 39, 150–157. [Google Scholar] [CrossRef]
- Schieveld, J.N.; Leroy, P.L.; van Os, J.; Nicolai, J.; Vos, G.D.; Leentjens, A.F. Pediatric delirium in critical illness: Phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 2007, 33, 1033–1040. [Google Scholar] [CrossRef]
- Kamdar, B.B.; Niessen, T.; Colantuoni, E.; King, L.M.; Neufeld, K.J.; Bienvenu, O.J.; Rowden, A.M.; Collop, N.A.; Needham, D.M. Delirium transitions in the medical ICU: Exploring the role of sleep quality and other factors. Crit. Care Med. 2015, 43, 135–141. [Google Scholar] [CrossRef]
- Traube, C.; Silver, G.; Kearney, J.; Patel, A.; Atkinson, T.M.; Yoon, M.J.; Halpert, S.; Augenstein, J.; Sickles, L.E.; Li, C.; et al. Cornell Assessment of Pediatric Delirium: A valid, rapid, observational tool for screening delirium in the PICU*. Crit. Care Med. 2014, 42, 656–663. [Google Scholar] [CrossRef]
- Kaur, S.; Silver, G.; Samuels, S.; Rosen, A.H.; Weiss, M.; Mauer, E.A.; Gerber, L.M.; Greenwald, B.M.; Traube, C. Delirium and developmental disability: Improving specificity of a pediatric delirium screen. Pediatr. Crit. Care Med. 2020, 21, 409–414. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; Knoester, H.; van Beusekom, B.S.; Ista, E. Screening pediatric delirium with an adapted version of the Sophia Observation withdrawal Symptoms scale (SOS). Intensive Care Med. 2012, 38, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Ista, E.; van Beusekom, B.; van Rosmalen, J.; Kneyber, M.C.J.; Lemson, J.; Brouwers, A.; Dieleman, G.C.; Dierckx, B.; de Hoog, M.; Tibboel, D.; et al. Validation of the SOS-PD scale for assessment of pediatric delirium: A multicenter study. Crit. Care 2018, 22, 309. [Google Scholar] [CrossRef]
- Simone, S.; Edwards, S.; Lardieri, A.; Walker, L.K.; Graciano, A.L.; Kishk, O.A.; Custer, J.W. Implementation of an ICU bundle: An interprofessional quality improvement project to enhance delirium management and monitor delirium prevalence in a single PICU. Pediatr. Crit. Care Med. 2017, 18, 531–540. [Google Scholar] [CrossRef]
- Best, K.M.; Wypij, D.; Asaro, L.A.; Curley, M.A. Patient, process, and system predictors of iatrogenic withdrawal syndrome in critically ill children. Crit. Care Med. 2017, 45, e7–e15. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Grap, M.J.; Younger, J.B.; Ameringer, S.; Elswick, R.K. Opioid withdrawal signs and symptoms in children: Frequency and determinants. Heart Lung 2013, 42, 407–413. [Google Scholar] [CrossRef]
- da Silva, P.S.; Reis, M.E.; Fonseca, T.S.; Fonseca, M.C. Opioid and benzodiazepine withdrawal syndrome in PICU patients: Which risk factors matter? J. Addict. Med. 2016, 10, 110–116. [Google Scholar] [CrossRef]
- Amigoni, A.; Vettore, E.; Brugnolaro, V.; Brugnaro, L.; Gaffo, D.; Masola, M.; Marzollo, A.; Pettenazzo, A. High doses of benzodiazepine predict analgesic and sedative drug withdrawal syndrome in paediatric intensive care patients. Acta Paediatr. 2014, 103, e538–e543. [Google Scholar] [CrossRef]
- Ávila-Alzate, J.A.; Gómez-Salgado, J.; Romero-Martín, M.; Martínez-Isasi, S.; Navarro-Abal, Y.; Fernández-García, D. Assessment and treatment of the withdrawal syndrome in paediatric intensive care units: Systematic review. Medicine 2020, 99, e18502. [Google Scholar] [CrossRef]
- Madden, K.; Burns, M.M.; Tasker, R.C. Differentiating delirium from sedative/hypnotic-related iatrogenic withdrawal syndrome: Lack of specificity in pediatric critical care assessment tools. Pediatr. Crit. Care Med. 2017, 18, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, K.D.; Lomako, D.M.; Katz, R.W.; Kelly, H.W. Opioid withdrawal in critically ill neonates. Ann. Pharmacother. 2003, 37, 473–477. [Google Scholar] [CrossRef]
- Tobias, J.D. Dexmedetomidine to treat opioid withdrawal in infants following prolonged sedation in the pediatric ICU. J. Opioid Manag. 2006, 2, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.O.; Zaccariello, M.J.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; Sprung, J.; et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology 2018, 129, 89–105. [Google Scholar] [CrossRef]
- McCann, M.E.; de Graaff, J.C.; Dorris, L.; Disma, N.; Withington, D.; Bell, G.; Grobler, A.; Stargatt, R.; Hunt, R.W.; Sheppard, S.J.; et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. Lancet 2019, 393, 664–677. [Google Scholar] [CrossRef]
- Ing, C.; Jackson, W.M.; Zaccariello, M.J.; Goldberg, T.E.; McCann, M.E.; Grobler, A.; Davidson, A.; Sun, L.; Li, G.; Warner, D.O. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: A systematic review and meta-analysis. Br. J. Anaesth. 2021, 126, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kocek, M.; Wilcox, R.; Crank, C.; Patra, K. Evaluation of the relationship between opioid exposure in extremely low birth weight infants in the neonatal intensive care unit and neurodevelopmental outcome at 2 years. Early Hum. Dev. 2016, 92, 29–32. [Google Scholar] [CrossRef]
- Ng, E.; Taddio, A.; Ohlsson, A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst. Rev. 2017, 1, Cd002052. [Google Scholar] [CrossRef]
- Guerra, G.G.; Robertson, C.M.; Alton, G.Y.; Joffe, A.R.; Cave, D.A.; Dinu, I.A.; Creighton, D.E.; Ross, D.B.; Rebeyka, I.M. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Paediatr. Anaesth. 2011, 21, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Garcia Guerra, G.; Robertson, C.M.; Alton, G.Y.; Joffe, A.R.; Cave, D.A.; Yasmin, F.; Dinu, I.A.; Creighton, D.E.; Ross, D.B.; Rebeyka, I.M. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Paediatr. Anaesth. 2014, 24, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Ely, E.W.; Pandharipande, P.P.; Patel, M.B. The ABCDEF bundle in critical care. Crit. Care Clin. 2017, 33, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.C.; Kudchadkar, S.R. Early mobilization in the pediatric intensive care unit. Transl. Pediatr. 2018, 7, 308–313. [Google Scholar] [CrossRef]
- Manning, J.C.; Pinto, N.P.; Rennick, J.E.; Colville, G.; Curley, M.A.Q. Conceptualizing post intensive care syndrome in children-the PICS-p framework. Pediatr. Crit. Care Med. 2018, 19, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Herrup, E.A.; Wieczorek, B.; Kudchadkar, S.R. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: A systematic review. World J. Crit. Care Med. 2017, 6, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Andelic, N.; Bautz-Holter, E.; Ronning, P.; Olafsen, K.; Sigurdardottir, S.; Schanke, A.K.; Sveen, U.; Tornas, S.; Sandhaug, M.; Roe, C. Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J. Neurotrauma 2012, 29, 66–74. [Google Scholar] [CrossRef]
- Jacobs, B.R.; Salman, B.A.; Cotton, R.T.; Lyons, K.; Brilli, R.J. Postoperative management of children after single-stage laryngotracheal reconstruction. Crit. Care Med. 2001, 29, 164–168. [Google Scholar] [CrossRef]
- Wieczorek, B.; Ascenzi, J.; Kim, Y.; Lenker, H.; Potter, C.; Shata, N.J.; Mitchell, L.; Haut, C.; Berkowitz, I.; Pidcock, F.; et al. PICU up!: Impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatr. Crit. Care Med. 2016, 17, e559–e566. [Google Scholar] [CrossRef] [PubMed]
- Renew, J.R.; Ratzlaff, R.; Hernandez-Torres, V.; Brull, S.J.; Prielipp, R.C. Neuromuscular blockade management in the critically Ill patient. J. Intensive Care 2020, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J. Early phase of lung-protective ventilation: A place for paralytics? Crit. Care Med. 2006, 34, 2851–2853. [Google Scholar] [CrossRef]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S. Neuromuscular blocking agents in ARDS. N. Engl. J. Med. 2010, 363, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- deBacker, J.; Hart, N.; Fan, E. Neuromuscular blockade in the 21st century management of the critically ill patient. Chest 2017, 151, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zuppa, A.F.; Curley, M.A.Q. Sedation analgesia and neuromuscular blockade in pediatric critical care: Overview and current landscape. Pediatr. Clin. N. Am. 2017, 64, 1103–1116. [Google Scholar] [CrossRef]
- Stevens, R.D.; Dowdy, D.W.; Michaels, R.K.; Mendez-Tellez, P.A.; Pronovost, P.J.; Needham, D.M. Neuromuscular dysfunction acquired in critical illness: A systematic review. Intensive Care Med. 2007, 33, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.T.; Papazian, L. Is cisatracurium the neuromuscular blocking agent of choice in acute respiratory distress syndrome? Am. J. Respir. Crit. Care Med. 2018, 197, 849–850. [Google Scholar] [CrossRef]
- Reich, D.L.; Hollinger, I.; Harrington, D.J.; Seiden, H.S.; Chakravorti, S.; Cook, D.R. Comparison of cisatracurium and vecuronium by infusion in neonates and small infants after congenital heart surgery. Anesthesiology 2004, 101, 1122–1127. [Google Scholar] [CrossRef]
- Isenstein, D.A.; Venner, D.S.; Duggan, J. Neuromuscular blockade in the intensive care unit. Chest 1992, 102, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; DeBlock, H.; Erstad, B.; Gray, A.; Jacobi, J.; Jordan, C.; McGee, W.; McManus, C.; Meade, M.; Nix, S.; et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit. Care Med. 2016, 44, 2079–2103. [Google Scholar] [CrossRef] [PubMed]
| DRS-88 | DRS-R-98 | PAED | pCAM-ICU | psCAM-ICU | CAPD | |
|---|---|---|---|---|---|---|
| PICU population (n) | Med/surg (154) | Med/surg (154) | Med/surg a (154) | Med/surg, cardiac (68) | Med/surg, cardiac (300) | General (111) |
| Age (year) | 1–17 | 1–17 | 1–17 | ≥5 | 0.5–5 | Birth–21 year |
| Include mechanical ventilation? | No | No | No | Yes | Yes | Yes |
| Include developmental delay? | No | No | No | No | No | Yes |
| Type of delirium | Hyperactive | Hyperactive | Hyperactive | Hyperactive Hypoactive | Hyperactive Hypoactive | Hyperactive Hypoactive |
| No. of questions or domains | 10 | 16 | 5 | 4 | 4 | 8 |
| Administering provider | Psychiatrist | Psychiatrist | Anesthesia | Bedside b | Bedside b | Bedside b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbuta, C.; Mason, K.P. Current State of Analgesia and Sedation in the Pediatric Intensive Care Unit. J. Clin. Med. 2021, 10, 1847. https://doi.org/10.3390/jcm10091847
Egbuta C, Mason KP. Current State of Analgesia and Sedation in the Pediatric Intensive Care Unit. Journal of Clinical Medicine. 2021; 10(9):1847. https://doi.org/10.3390/jcm10091847
Chicago/Turabian StyleEgbuta, Chinyere, and Keira P. Mason. 2021. "Current State of Analgesia and Sedation in the Pediatric Intensive Care Unit" Journal of Clinical Medicine 10, no. 9: 1847. https://doi.org/10.3390/jcm10091847
APA StyleEgbuta, C., & Mason, K. P. (2021). Current State of Analgesia and Sedation in the Pediatric Intensive Care Unit. Journal of Clinical Medicine, 10(9), 1847. https://doi.org/10.3390/jcm10091847






