Improvement of the Diagnostic Performance of Facial Neuritis Using Contrast-Enhanced 3D T1 Black-Blood Imaging: Comparison with Contrast-Enhanced 3D T1-Spoiled Gradient-Echo Imaging
Abstract
:1. Introduction
2. Materials and Method
2.1. Patients
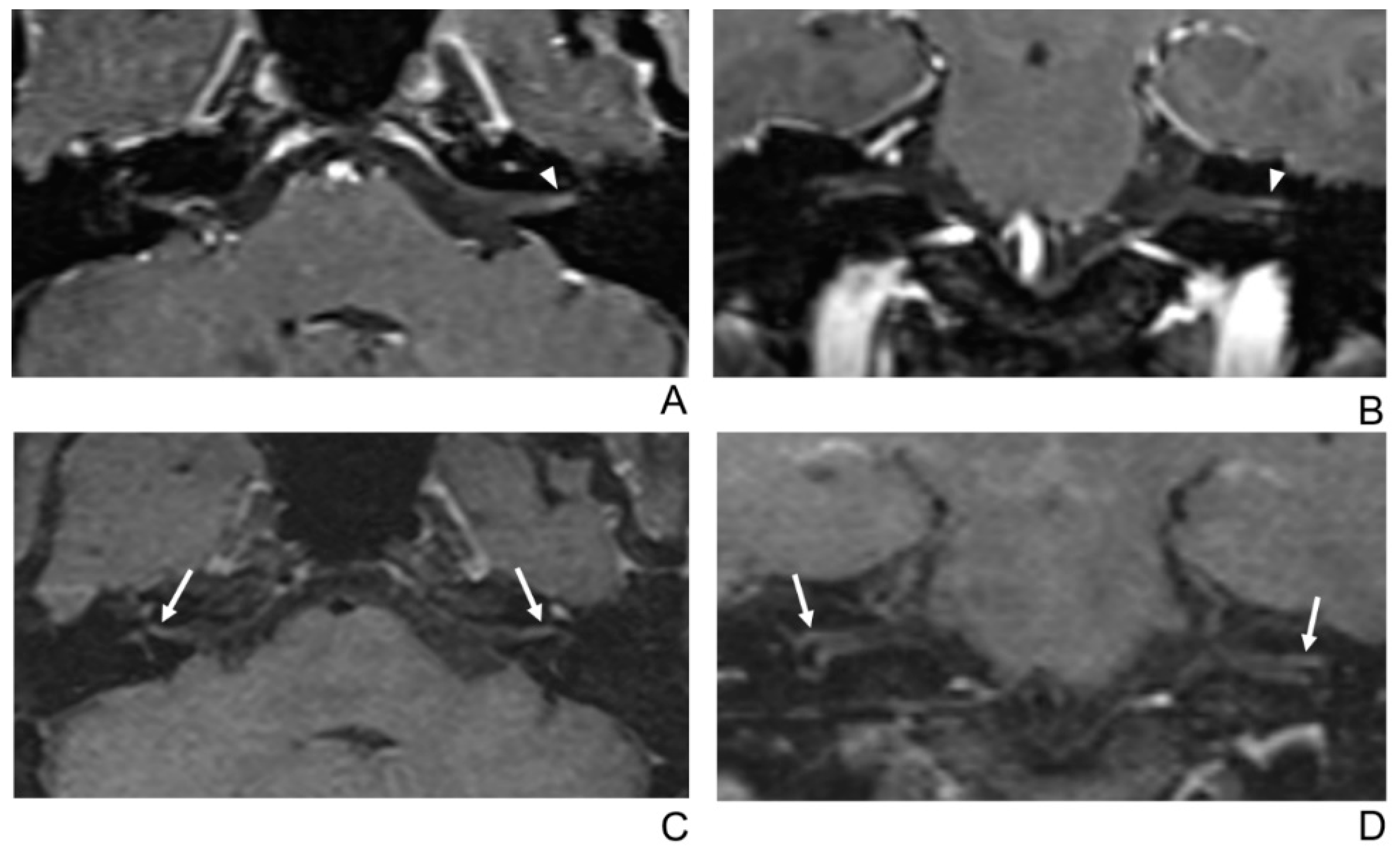
2.2. MRI Protocol
2.3. MR Image Analysis
2.4. Quantitative Analysis
2.5. Statistical Analysis
3. Results
3.1. Diagnostic Performance of the MR Imaging Sequences
3.2. Qualitative Analysis of the Facial Neuritis
3.3. Quantitative Analysis of the LEC and rSI of Facial Nerves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T1 BB-FSE | contrast-enhanced 3D T1 black-blood fast spin-echo |
| CE-GRE | contrast-enhanced 3D T1-spoiled gradient-echo |
| MRI | magnetic resonance imaging |
| CNR | contrast-to-noise ratio |
| SNR | signal-to-noise ratio |
| SPGR | 3D T1-spoiled gradient-echo |
| IR-FSPGR | inversion recovery prepared fast spoiled gradient-echo |
| SE | spin echo |
| FSE | fast spin echo |
| TSE | turbo spin echo |
| FLAIR | fluid attenuated inversion recovery |
| MSDE | motion-sensitized driven-equilibrium |
| PACS | picture archiving and communication system |
| SI | signal intensity |
| rSI | affected side-to-normal signal intensity ratio |
| LEC | contrast-enhancing lesion extent of the canalicular segment of affected facial nerve. |
References
- Peitersen, E. Bell’s palsy: The spontaneous course of 2500 peripheral facial nerve palsies of different etiologies. Acta Oto-LarynGologica Suppl. 2002, 549, 4–30. [Google Scholar] [CrossRef]
- Baugh, R.F.; Basura, G.J.; Ishii, L.E.; Schwartz, S.R.; Drumheller, C.M.; Burkholder, R.; Deckard, N.A.; Dawson, C.; Driscoll, C.; Gillespie, M.B.; et al. Clinical Practice Guideline. Otolaryngol. Neck Surg. 2013, 149, S1–S27. [Google Scholar] [CrossRef]
- Adour, K.K.; Byl, F.M.; Hilsinger, R.L.; Kahn, Z.M.; Sheldon, M.I. The True Nature of Bell’s Palsy: Analysis af 1000 Consecutive Patients. Laryngoscope 1978, 88, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mizobuchi, M.; Nakashiro, Y.; Doi, T.; Hato, N.; Yanagihara, N. Bell Palsy and Herpes Simplex Virus: Identification of Viral DNA in Endoneurial Fluid and Muscle. Ann. Intern. Med. 1996, 124, 27. [Google Scholar] [CrossRef] [PubMed]
- Owusu, J.A.; Stewart, C.M.; Boahene, K. Facial Nerve Paralysis. Med. Clin. North Am. 2018, 102, 1135–1143. [Google Scholar] [CrossRef]
- Proctor, B.; Corgill, D.A.; Proud, G. The pathology of Bell’s palsy. Trans. Sect. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1976, 82, 70–80. [Google Scholar]
- Fowler, E.P. The pathologic findings in a case of facial paralysis. Trans. Am. Acad. Ophthalmol. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1963, 67, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Fisch, U.; Felix, H. On the Pathogenesls of Bell’s Palsy. Acta Oto-Laryngologica 1983, 95, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.; Podvinec, M.; Hofer, H. Histological and Ultrastructural Changes in Idiopathic Facial Palsy. ORL 1978, 40, 303–311. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Volk, G.F.; Olsen, K.D.; Mäkitie, A.A.; Silver, C.E.; Zafereo, M.E.; Rinaldo, A.; Randolph, G.W.; Simo, R.; Shaha, A.R.; et al. Facial nerve electrodiagnostics for patients with facial palsy: A clinical practice guideline. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1855–1874. [Google Scholar] [CrossRef]
- Lee, J.D.; Cho, Y.-S.; Jang, K.H.; Lee, H.K.; Kwon, K.H. Acute Inflammatory Facial Nerve Paralysis. Korean J. Otorhinolaryngol. Neck Surg. 2011, 54, 386–391. [Google Scholar] [CrossRef]
- Kress, B.P.J.; Griesbeck, F.; Efinger, K.; Solbach, T.; Gottschalk, A.; Kornhuber, A.W.; Bähren, W. Bell’s palsy: What is the prognostic value of measurements of signal intensity increases with contrast enhancement on MRI? Neuroradiology 2002, 44, 428–433. [Google Scholar] [CrossRef]
- Burmeister, H.P.; Baltzer, P.A.T.; Volk, G.F.; Klingner, C.M.; Kraft, A.; Dietzel, M.; Witte, O.W.; Kaiser, W.A.; Guntinas-Lichius, O. Evaluation of the early phase of Bell’s palsy using 3 T MRI. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1493–1500. [Google Scholar] [CrossRef]
- Engström, M.; Berg, T.; Stjernquist-Desatnik, A.; Axelsson, S.; Pitkäranta, A.; Hultcrantz, M.; Kanerva, M.; Hanner, P.; Jonsson, L. Prednisolone and valaciclovir in Bell’s palsy: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2008, 7, 993–1000. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Swan, I.R.C.; Donnan, P.T.; Morrison, J.M.; Smith, B.H.; McKinstry, B.; Davenport, R.J.; Vale, L.D.; Clarkson, J.E.; Hammersley, V.; et al. Early Treatment with Prednisolone or Acyclovir in Bell’s Palsy. N. Engl. J. Med. 2007, 357, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Tien, R.; Dillon, W.P.; Jackler, R.K. Contrast-enhanced MR imaging of the facial nerve in 11 patients with Bell’s palsy. AJNR Am. J. Neuroradiol. 1990, 11, 735–741. [Google Scholar] [CrossRef]
- Sartoretti-Schefer, S.; Wichmann, W.; Valavanis, A. Idiopathic, herpetic, and HIV-associated facial nerve palsies: Abnormal MR enhancement patterns. Am. J. Neuroradiol. 1994, 15, 479–485. [Google Scholar] [PubMed]
- Kress, B.; Griesbeck, F.; Stippich, C.; Bähren, W.; Sartor, K. Bell Palsy: Quantitative Analysis of MR Imaging Data as a Method of Predicting Outcome. Radiology 2004, 230, 504–509. [Google Scholar] [CrossRef]
- Krainik, A.; Casselman, J.W.; Hodler, J.; Kubik-Huch, R.A.; Von Schulthess, G.K. Imaging Evaluation of Patients with Cranial Nerve Disorders. In Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–161. [Google Scholar] [CrossRef]
- Saremi, F.; Helmy, M.; Farzin, S.; Zee, C.S.; Go, J.L. MRI of Cranial Nerve Enhancement. Am. J. Roentgenol. 2005, 185, 1487–1497. [Google Scholar] [CrossRef]
- Qiao, Y.; Steinman, D.A.; Qin, Q.; Etesami, M.; Schär, M.; Astor, B.C.; Wasserman, B.A. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J. Magn. Reson. Imaging 2011, 34, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Choi, S.; Lee, E.; Shin, D.; Jo, S.; Yoo, R.-E.; Kang, K.; Yun, T.; Kim, J.-H.; Sohn, C.-H. Application of 3D Fast Spin-Echo T1 Black-Blood Imaging in the Diagnosis and Prognostic Prediction of Patients with Leptomeningeal Carcinomatosis. Am. J. Neuroradiol. 2018, 39, 1453–1459. [Google Scholar] [CrossRef]
- Sommer, N.N.; Lucas, R.P.; Coppenrath, E.; Kooijman, H.; Galiè, F.; Hesse, N.; Sommer, W.H.; Treitl, K.M.; Saam, T.; Froelich, M.F. Contrast-enhanced modified 3D T1-weighted TSE black-blood imaging can improve detection of infectious and neoplastic meningitis. Eur. Radiol. 2020, 30, 866–876. [Google Scholar] [CrossRef]
- Thaler, C.; Schneider, T.; Sedlacik, J.; Kutzner, D.; Stellmann, J.-P.; Heesen, C.; Fiehler, J.; Siemonsen, S. T1w dark blood imaging improves detection of contrast enhancing lesions in multiple sclerosis. PLoS ONE 2017, 12, e0183099. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yoo, E.; Lee, H.; Chang, J.-H.; Kim, E.Y. Detection of Small Metastatic Brain Tumors. Investig. Radiol. 2012, 47, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, E.Y. Contrast-enhanced, three-dimensional, whole-brain, black-blood imaging: Application to small brain metastases. Magn. Reson. Med. 2010, 63, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kammer, N.N.; Coppenrath, E.; Treitl, K.M.; Kooijman, H.; Dietrich, O.; Saam, T. Comparison of contrast-enhanced modified T1-weighted 3D TSE black-blood and 3D MP-RAGE sequences for the detection of cerebral metastases and brain tumours. Eur. Radiol. 2016, 26, 1818–1825. [Google Scholar] [CrossRef]
- Riederer, I.; Sollmann, N.; Mühlau, M.; Zimmer, C.; Kirschke, J. Gadolinium-Enhanced 3D T1-Weighted Black-Blood MR Imaging for the Detection of Acute Optic Neuritis. Am. J. Neuroradiol. 2020, 41, 2333–2338. [Google Scholar] [CrossRef]
- Hong, H.S.; Yi, B.-H.; Cha, J.-G.; Park, S.-J.; Kim, D.H.; Lee, H.K.; Lee, J.-D. Enhancement pattern of the normal facial nerve at 3.0 T temporal MRI. Br. J. Radiol. 2010, 83, 118–121. [Google Scholar] [CrossRef]
- Byun, J.S.; Kim, H.-J.; Yim, Y.J.; Kim, S.T.; Jeon, P.; Kim, K.H.; Kim, S.S.; Jeon, Y.H.; Lee, J. MR Imaging of the Internal Auditory Canal and Inner Ear at 3T: Comparison between 3D Driven Equilibrium and 3D Balanced Fast Field Echo Sequences. Korean J. Radiol. 2008, 9, 212–218. [Google Scholar] [CrossRef]
- Haneda, J.; Ishikawa, K.; Okamoto, K. Better continuity of the facial nerve demonstrated in the temporal bone on three-dimensional T1-weighted imaging with volume isotropic turbo spin echo acquisition than that with fast field echo at 3.0 tesla MRI. J. Med. Imaging Radiat. Oncol. 2019, 63, 745–750. [Google Scholar] [CrossRef]
- Dehkharghani, S.; Lubarsky, M.; Aiken, A.H.; Kang, J.; Hudgins, P.A.; Saindane, A.M. Redefining Normal Facial Nerve Enhancement: Healthy Subject Comparison of Typical Enhancement Patterns—Unenhanced and Contrast-Enhanced Spin-Echo Versus 3D Inversion Recovery–Prepared Fast Spoiled Gradient-Echo Imaging. Am. J. Roentgenol. 2014, 202, 1108–1113. [Google Scholar] [CrossRef]
- Yun, S.J.; Ryu, C.-W.; Jahng, G.-H.; Kim, E.J.; Choi, W.S.; Lee, K.M.; Kim, S.M. Usefulness of contrast-enhanced 3-dimensional T1-VISTA in the diagnosis of facial neuritis: Comparison with contrast-enhanced T1-TSE. J. Neuroradiol. 2015, 42, 93–98. [Google Scholar] [CrossRef]
- Lim, H.; Lee, J.; Hyun, D.; Park, J.; Kim, J.; Lee, H.; Park, S.; Ahn, J.; Baek, J.; Choi, C. MR Diagnosis of Facial Neuritis: Diagnostic Performance of Contrast-Enhanced 3D-FLAIR Technique Compared with Contrast-Enhanced 3D-T1-Fast-Field Echo with Fat Suppression. Am. J. Neuroradiol. 2011, 33, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; You, S.K.; Lee, I.H.; Lee, J.E.; Lee, S.M.; Cho, H.-H. Quantitative Analysis of the Facial Nerve Using Contrast-Enhanced Three Dimensional FLAIR-VISTA Imaging in Pediatric Bell’s Palsy. Investig. Magn. Reson. Imaging 2015, 19, 162–167. [Google Scholar] [CrossRef]
- Brant-Zawadzki, M.; Gillan, G.D.; Nitz, W.R. MP RAGE: A three-dimensional, T1-weighted, gradient-echo sequence--initial experience in the brain. Radiology 1992, 182, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Mugler, J.P.; Brookeman, J.R. Theoretical analysis of gadopentetate dimeglumine enhancement in T1-weighted imaging of the brain: Comparison of two-dimensional spin-echo and three-dimensional gradient-echo sequences. J. Magn. Reson. Imaging 1993, 3, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Nagao, E.; Yoshiura, T.; Hiwatashi, A.; Obara, M.; Yamashita, K.; Kamano, H.; Takayama, Y.; Kobayashi, K.; Honda, H. 3D Turbo Spin-Echo Sequence with Motion-Sensitized Driven-Equilibrium Preparation for Detection of Brain Metastases on 3T MR Imaging. Am. J. Neuroradiol. 2011, 32, 664–670. [Google Scholar] [CrossRef]
- Sakurai, K.; Miura, T.; Sagisaka, T.; Hattori, M.; Matsukawa, N.; Mase, M.; Kasai, H.; Arai, N.; Kawai, T.; Shimohira, M.; et al. Evaluation of luminal and vessel wall abnormalities in subacute and other stages of intracranial vertebrobasilar artery dissections using the volume isotropic turbo-spin-echo acquisition (VISTA) sequence: A preliminary study. J. Neuroradiol. 2013, 40, 19–28. [Google Scholar] [CrossRef]
- Hennig, J.; Weigel, M.; Scheffler, K. Multiecho sequences with variable refocusing flip angles: Optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS). Magn. Reson. Med. 2003, 49, 527–535. [Google Scholar] [CrossRef]
- Gold, G.E.; Busse, R.F.; Beehler, C.; Han, E.; Brau, A.C.S.; Beatty, P.J.; Beaulieu, C.F. Isotropic MRI of the Knee with 3D Fast Spin-Echo Extended Echo-Train Acquisition (XETA): Initial Experience. Am. J. Roentgenol. 2007, 188, 1287–1293. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ishii, K.; Okitsu, T.; Okudera, T.; Ogawa, T. Facial Nerve Palsy: Evaluation by Contrast-enhanced MR Imaging. Clin. Radiol. 2001, 56, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Czervionke, L.F.; Daniels, D.L.; Wehrli, F.W.; Mark, L.P.; Hendrix, L.E.; Strandt, J.A.; Williams, A.L.; Haughton, V.M. Magnetic susceptibility artifacts in gradient-recalled echo MR imaging. AJNR Am. J. Neuroradiol. 1988, 9, 1149–1155. [Google Scholar] [PubMed]
- Furutani, K.; Harada, M.; Mawlan, M.; Nishitani, H. Difference in Enhancement Between Spin Echo and 3-Dimensional Fast Spoiled Gradient Recalled Acquisition in Steady State Magnetic Resonance Imaging of Brain Metastasis at 3-T Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2008, 32, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Proctor, B.; Nager, G.T. The facial canal: Normal anatomy, variations and anomalies. I. Normal anatomy of the facial canal. Ann. Otol. Rhinol. Laryngol. Suppl. 1982, 97, 33–44. [Google Scholar] [PubMed]




| Clinical Characteristics | Facial Neuritis (n = 45) |
|---|---|
| Mean age (yrs.) | 49.6 (25–80) |
| Sex (Male/Female) | 26/19 |
| Affected side (Rt./Lt.) | 26/19 |
| Mean duration time (days) | 7.1 (0–29) |
| Reviewer 1 | pa | Reviewer 2 | pa | Total | pa | ||||
|---|---|---|---|---|---|---|---|---|---|
| T1-BB-FSE | CE-GRE | T1-BB-FSE | CE-GRE | T1-BB-FSE | CE-GRE | ||||
| Sensitivity (%) (TP/Disease) | 97.8 (44/45) | 86.7 (39/45) | 0.063 | 97.8 (44/45) | 88.9 (40/45) | 0.125 | 97.8 (88/90) | 87.8 (79/90) | 0.004 |
| Specificity (%) (TN/Normal) | 95 (19/20) | 75 (15/20) | 0.219 | 90 (18/20) | 75 (15/20) | 0.375 | 92.5 (37/40) | 75 (30/40) | 0.065 |
| Accuracy (%) | 96.9 (63/65) | 83.1 (54/65) | 0.012 | 95.4 (62/65) | 84.6 (55/65) | 0.039 | 96.2 (125/130) | 83.8 (109/130) | <0.001 |
| PPV (%) | 97.8 | 88.6 | 95.7 | 88.9 | |||||
| NPV (%) | 95 | 71.4 | 94.7 | 75 | |||||
| AUC | 0.964 (0.885–0.994) | 0.808 (0.692–0.895) | 0.014 | 0.939 (0.850–0.983) | 0.819 (0.704–0.904) | 0.042 | |||
| Assessment | T1 BB-FSE | Agreement, n (%) | CE-GRE | Agreement, n (%) | p (Intra-Reviewer Comparison) |
|---|---|---|---|---|---|
| Diagnostic confidence | 38 (84%) | 37 (82%) | |||
| Reviewer 1 | 1.87 0.06 (1.75–1.99) | 1.49 0.73 (1.27–1.71) | 0.002 | ||
| Reviewer 2 | 1.80 0.46 (1.66–1.94) | 1.49 0.69 (1.28–1.70) | 0.002 | ||
| Mean | 1.83 0.43 (1.74–1.92) | 1.49 0.71 (1.34–1.64) | <0.001 | ||
| Visual asymmetric enhancement | |||||
| Canalicular | 33 (73%) | 32 (71%) | |||
| Reviewer 1 | 2.47 0.76 (2.24–2.69) | 1.82 0.89 (1.56–2.09) | <0.001 | ||
| Reviewer 2 | 2.51 0.66 (2.31–2.71) | 2.02 0.66 (1.82–2.22) | <0.001 | ||
| Mean | 2.49 0.71 (2.34–2.64) | 1.92 0.78 (1.76–2.09) | <0.001 | ||
| Labyrinthine | 33 (73%) | 31 (69%) | |||
| Reviewer 1 | 1.60 0.75 (1.37–1.83) | 1.20 0.73 (0.98–1.42) | 0.005 | ||
| Reviewer 2 | 1.71 0.69 (1.50–1.92) | 1.04 0.88 (0.78–1.31) | <0.001 | ||
| Mean | 1.66 0.72 (1.50–1.81) | 1.12 0.80 (0.95–1.29) | <0.001 | ||
| Anterior genu | 37(82%) | 33 (73%) | |||
| Reviewer 1 | 0.84 0.52 (0.69–1.00) | 0.87 0.84 (0.61–1.12) | 0.979 | ||
| Reviewer 2 | 0.71 0.46 (0.57–0.85) | 0.71 0.97 (0.42–1.00) | 0.900 | ||
| Mean | 0.78 0.49 (0.67–0.88) | 0.79 0.91 (0.60–0.98) | 0.852 |
| T1-BB-FSE | CE-GRE | p-Value a | |
|---|---|---|---|
| Enhancing lesion extent | |||
| Length (median, (IQR)) | 4.08 mm, (3.31–4.67) | 2.23 mm, (1.80–3.44) | <0.001 |
| Width (median, (IQR)) | 1.60 mm, (1.41–1.87) | 1.42 mm, (1.17–1.72) | 0.026 |
| rSI of each segment | |||
| Canalicular (median, (IQR)) | 2.00 (1.68–2.31) | 1.73 (1.29–2.17) | 0.029 |
| Labyrinthine (median, (IQR)) | 1.84 (1.50–2.50) | 1.67 (1.40–2.12) | 0.193 |
| Anterior genu (median, (IQR)) | 1.38 (1.24–1.70) | 1.25 (1.13–1.85) | 0.480 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-A.; Jo, S.-W.; Chang, S.-K.; Kwon, K.-H. Improvement of the Diagnostic Performance of Facial Neuritis Using Contrast-Enhanced 3D T1 Black-Blood Imaging: Comparison with Contrast-Enhanced 3D T1-Spoiled Gradient-Echo Imaging. J. Clin. Med. 2021, 10, 1850. https://doi.org/10.3390/jcm10091850
Lee S-A, Jo S-W, Chang S-K, Kwon K-H. Improvement of the Diagnostic Performance of Facial Neuritis Using Contrast-Enhanced 3D T1 Black-Blood Imaging: Comparison with Contrast-Enhanced 3D T1-Spoiled Gradient-Echo Imaging. Journal of Clinical Medicine. 2021; 10(9):1850. https://doi.org/10.3390/jcm10091850
Chicago/Turabian StyleLee, Seun-Ah, Sang-Won Jo, Suk-Ki Chang, and Ki-Han Kwon. 2021. "Improvement of the Diagnostic Performance of Facial Neuritis Using Contrast-Enhanced 3D T1 Black-Blood Imaging: Comparison with Contrast-Enhanced 3D T1-Spoiled Gradient-Echo Imaging" Journal of Clinical Medicine 10, no. 9: 1850. https://doi.org/10.3390/jcm10091850
APA StyleLee, S.-A., Jo, S.-W., Chang, S.-K., & Kwon, K.-H. (2021). Improvement of the Diagnostic Performance of Facial Neuritis Using Contrast-Enhanced 3D T1 Black-Blood Imaging: Comparison with Contrast-Enhanced 3D T1-Spoiled Gradient-Echo Imaging. Journal of Clinical Medicine, 10(9), 1850. https://doi.org/10.3390/jcm10091850






