Intraocular Foreign Body: Diagnostic Protocols and Treatment Strategies in Ocular Trauma Patients
Abstract
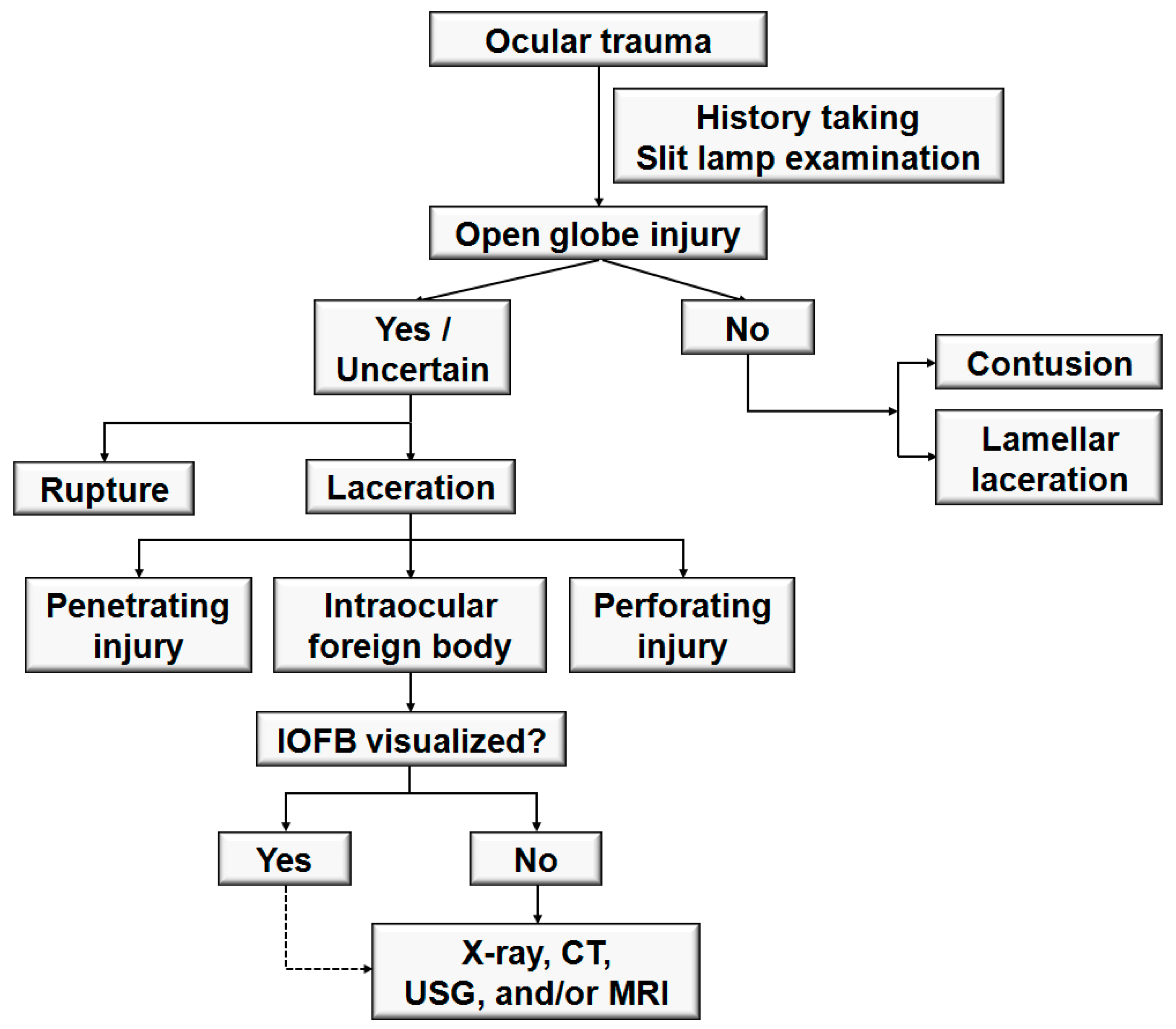
:1. Introduction
2. Materials and Methods
2.1. Participants and Clinical Assessment
2.2. Treatment Protocol
2.3. Statistical Analysis
3. Results
3.1. Demographics and Mechanisms of Injury
3.2. Characteristics of IOFB and Clinical Presentation
3.3. Management and Treatment Outcomes
3.4. Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, S.; Colyer, M.H.; Weichel, E.D. Current trends in the management of intraocular foreign bodies. Curr. Opin. Ophthalmol. 2008, 19, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Erakgun, T.; Egrilmez, S. Prognostic factors in vitrectomy for posterior segment intraocular foreign bodies. J. Trauma 2008, 64, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Tong, J.M.; Li, P.S.; Li, K.K. Epidemiology and clinical outcome of intraocular foreign bodies in Hong Kong: A 13-year review. Int. Ophthalmol. 2017, 37, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Langer, P.D.; Zarbin, M.A.; Bhagat, N. Diagnostic value of clinical examination and radiographic imaging in identification of intraocular foreign bodies in open globe injury. Eur. J. Ophthalmol. 2012, 22, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Imrie, F.R.; Cox, A.; Foot, B.; Macewen, C.J. Surveillance of intraocular foreign bodies in the UK. Eye 2008, 22, 1141–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, K.Y.; Cali, J.M. Epidemiology of lifetime work-related eye injuries in the U.S. population associated with one or more lost days of work. Ophthalmic Epidemiol. 2009, 16, 156–162. [Google Scholar] [CrossRef]
- Punnonen, E.; Laatikainen, L. Prognosis of perforating eye injuries with intraocular foreign bodies. Acta Ophthalmol. 1989, 67, 483–491. [Google Scholar] [CrossRef]
- Greven, C.M.; Engelbrecht, N.E.; Slusher, M.M.; Nagy, S.S. Intraocular foreign bodies: Management, prognostic factors, and visual outcomes. Ophthalmology 2000, 107, 608–612. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Knorr, H.L.; Budde, W.M. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology 2000, 107, 823–828. [Google Scholar] [CrossRef]
- Szijarto, Z.; Gaal, V.; Kovacs, B.; Kuhn, F. Prognosis of penetrating eye injuries with posterior segment intraocular foreign body. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 161–165. [Google Scholar] [CrossRef]
- Bai, H.Q.; Yao, L.; Meng, X.X.; Wang, Y.X.; Wang, D.B. Visual outcome following intraocular foreign bodies: A retrospective review of 5-year clinical experience. Eur. J. Ophthalmol. 2011, 21, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Thoongsuwan, S.; Rodanant, N.; Namatra, C.; Trinavarat, A.; Tantaterdtum, J.; Singalavanija, A.; Rojananin, S. Visual outcome and prognostic factors in posterior segment intraocular foreign bodies. J. Med. Assoc. Thai. 2012, 95 (Suppl. 4), S82–S86. [Google Scholar] [PubMed]
- Woodcock, M.G.; Scott, R.A.; Huntbach, J.; Kirkby, G.R. Mass and shape as factors in intraocular foreign body injuries. Ophthalmology 2006, 113, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, C.; Zech, J.C.; Denis, P.; Adeleine, P.; Trepsat, C. Intraocular foreign bodies. Factors influencing final visual outcome. Acta Ophthalmol. Scand. 1999, 77, 321–325. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ali, N.; Rahman, N.A.; Joshi, N. Spectrum of intra-ocular foreign bodies and the outcome of their management in Brunei Darussalam. Int. Ophthalmol. 2013, 33, 277–284. [Google Scholar] [CrossRef]
- Nicoara, S.D.; Irimescu, I.; Calinici, T.; Cristian, C. Intraocular foreign bodies extracted by pars plana vitrectomy: Clinical characteristics, management, outcomes and prognostic factors. BMC Ophthalmol. 2015, 15, 151. [Google Scholar] [CrossRef]
- El-Asrar, A.M.; Al-Amro, S.A.; Khan, N.M.; Kangave, D. Visual outcome and prognostic factors after vitrectomy for posterior segment foreign bodies. Eur. J. Ophthalmol. 2000, 10, 304–311. [Google Scholar] [CrossRef]
- Wickham, L.; Xing, W.; Bunce, C.; Sullivan, P. Outcomes of surgery for posterior segment intraocular foreign bodies—A retrospective review of 17 years of clinical experience. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1620–1626. [Google Scholar] [CrossRef]
- Benson, W.E.; Machemer, R. Severe perforating injuries treated with pars plana vitrectomy. Am. J. Ophthalmol. 1976, 81, 728–732. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Sajjadi, H.; Azarmina, M.; Soheilian, M.; Baharivand, N. Surgical management of intraretinal foreign bodies. Retina 1994, 14, 397–403. [Google Scholar] [CrossRef]
- Moisseiev, J.; Segev, F.; Harizman, N.; Arazi, T.; Rotenstreich, Y.; Assia, E.I. Primary cataract extraction and intraocular lens implantation in penetrating ocular trauma. Ophthalmology 2001, 108, 1099–1103. [Google Scholar] [CrossRef]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef]
- Fulcher, T.P.; McNab, A.A.; Sullivan, T.J. Clinical features and management of intraorbital foreign bodies. Ophthalmology 2002, 109, 494–500. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Kunimoto, D.Y.; Ittoop, S.; Maguire, J.I.; Ho, A.C.; Regillo, C.D. Metallic intraocular foreign bodies: Characteristics, interventions, and prognostic factors for visual outcome and globe survival. Am. J. Ophthalmol. 2008, 146, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.; Pieramici, D.J. Intraocular foreign bodies. In Ocular Trauma: Principles and Practice; Thieme: New York, NY, USA, 2002; pp. 235–263. [Google Scholar]
- Irvine, A.R. Old and new techniques combined in the management of intraocular foreign bodies. Ann. Ophthalmol. 1981, 13, 41–47. [Google Scholar] [PubMed]
- Kuhn, F.; Halda, T.; Witherspoon, C.D.; Morris, R.; Mester, V. Intraocular foreign bodies: Myths and truths. Eur. J. Ophthalmol. 1996, 6, 464–471. [Google Scholar] [CrossRef]
- Arora, R.; Gupta, A.; Mazumdar, S.; Gupta, A.K. A retained intraretinal foreign body. Ophthalmic Surg. Lasers 1996, 27, 885–887. [Google Scholar] [CrossRef]
- Kuhn, F.; Morris, R.; Witherspoon, C.D.; Heimann, K.; Jeffers, J.B.; Treister, G. A standardized classification of ocular trauma. Ophthalmology 1996, 103, 240–243. [Google Scholar] [CrossRef]
- Kuhn, F.; Morris, R.; Witherspoon, C.D.; Mester, V. The Birmingham Eye Trauma Terminology system (BETT). J. Fr. Ophtalmol. 2004, 27, 206–210. [Google Scholar] [CrossRef]
- Kuhn, F.; Pieramici, D.J. BETT: The Terminology of Ocular Trauma. In Ocular Trauma: Principles and Practice; Thieme: New York, NY, USA, 2002; pp. 3–5. [Google Scholar]
- Kuhn, F.; Pieramici, D.J. Management of patients with polytrauma. In Ocular Trauma: Principles and Practice; Thieme: New York, NY, USA, 2002; pp. 73–76. [Google Scholar]
- Saeed, A.; Cassidy, L.; Malone, D.E.; Beatty, S. Plain X-ray and computed tomography of the orbit in cases and suspected cases of intraocular foreign body. Eye 2008, 22, 1373–1377. [Google Scholar] [CrossRef] [Green Version]
- Parke, D.W., 3rd; Flynn, H.W., Jr.; Fisher, Y.L. Management of intraocular foreign bodies: A clinical flight plan. Can. J. Ophthalmol. 2013, 48, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lit, E.S.; Young, L.H. Anterior and posterior segment intraocular foreign bodies. Int. Ophthalmol. Clin. 2002, 42, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A.; Lipman, A.T.; Gupta, S.K.; Nacey, N.C. Undetected intraocular metallic foreign body causing hyphema in a patient undergoing MRI: A rare occurrence demonstrating the limitations of pre-MRI safety screening. Magn. Reson. Imaging 2015, 33, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Ta, C.N.; Bowman, R.W. Hyphema caused by a metallic intraocular foreign body during magnetic resonance imaging. Am. J. Ophthalmol. 2000, 129, 533–534. [Google Scholar] [CrossRef]
- Loporchio, D.; Mukkamala, L.; Gorukanti, K.; Zarbin, M.; Langer, P.; Bhagat, N. Intraocular foreign bodies: A review. Surv. Ophthalmol. 2016, 61, 582–596. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Locci, E.; Fossarello, M.; d’Aloja, E. Morphological analysis of corneal findings modifications after death: A preliminary OCT study on an animal model. Exp. Eye Res. 2018, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.M.; Andreoli, M.T.; Kloek, C.E.; Ahuero, A.E.; Vavvas, D.; Durand, M.L. Low rate of endophthalmitis in a large series of open globe injuries. Am. J. Ophthalmol. 2009, 147, 601–608 e602. [Google Scholar] [CrossRef]
- Soheilian, M.; Rafati, N.; Mohebbi, M.R.; Yazdani, S.; Habibabadi, H.F.; Feghhi, M.; Shahriary, H.A.; Eslamipour, J.; Piri, N.; Peyman, G.A. Prophylaxis of acute posttraumatic bacterial endophthalmitis: A multicenter, randomized clinical trial of intraocular antibiotic injection, report 2. Arch. Ophthalmol. 2007, 125, 460–465. [Google Scholar] [CrossRef]
- Peyman, G.A.; Daun, M. Prophylaxis of endophthalmitis. Ophthalmic Surg. 1994, 25, 671–674. [Google Scholar]
- Narang, S.; Gupta, V.; Gupta, A.; Dogra, M.R.; Pandav, S.S.; Das, S. Role of prophylactic intravitreal antibiotics in open globe injuries. Indian J. Ophthalmol. 2003, 51, 39–44. [Google Scholar]
- Meredith, T.A.; Aguilar, H.E.; Shaarawy, A.; Kincaid, M.; Dick, J.; Niesman, M.R. Vancomycin levels in the vitreous cavity after intravenous administration. Am. J. Ophthalmol. 1995, 119, 774–778. [Google Scholar] [CrossRef]
- Davis, J.L.; Koidou-Tsiligianni, A.; Pflugfelder, S.C.; Miller, D.; Flynn, H.W., Jr.; Forster, R.K. Coagulase-negative staphylococcal endophthalmitis. Increase in antimicrobial resistance. Ophthalmology 1988, 95, 1404–1410. [Google Scholar] [CrossRef]
- Donahue, S.P.; Kowalski, R.P.; Eller, A.W.; DeVaro, J.M.; Jewart, B.H. Empiric treatment of endophthalmitis. Are aminoglycosides necessary? Arch. Ophthalmol. 1994, 112, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.E.; Meredith, T.A.; Shaarawy, A.; Kincaid, M.; Dick, J. Vitreous cavity penetration of ceftazidime after intravenous administration. Retina 1995, 15, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.; Pieramici, D.J. Endophthalmitis. In Ocular Trauma: Principles and Practice; Thieme: New York, NY, USA, 2002; pp. 293–300. [Google Scholar]
- Azad, R.; Sharma, Y.R.; Mitra, S.; Pai, A. Triple procedure in posterior segment intraocular foreign body. Indian J. Ophthalmol. 1998, 46, 91–92. [Google Scholar]
- Zigiotti, G.L.; Cavarretta, S.; Morara, M.; Nam, S.M.; Ranno, S.; Pichi, F.; Lembo, A.; Lupo, S.; Nucci, P.; Meduri, A. Standard enucleation with aluminium oxide implant (bioceramic) covered with patient’s sclera. Sci. World J. 2012, 2012, 481584. [Google Scholar] [CrossRef] [Green Version]
- Bowman, R.J.; Yorston, D.; Wood, M.; Gilbert, C.; Foster, A. Primary intraocular lens implantation for penetrating lens trauma in Africa. Ophthalmology 1998, 105, 1770–1774. [Google Scholar] [CrossRef]
- Rubsamen, P.E.; Irvin, W.D.; McCuen, B.W., 2nd; Smiddy, W.E.; Bowman, C.B. Primary intraocular lens implantation in the setting of penetrating ocular trauma. Ophthalmology 1995, 102, 101–107. [Google Scholar] [CrossRef]
- Pavlovic, S. Primary intraocular lens implantation during pars plana vitrectomy and intraretinal foreign body removal. Retina 1999, 19, 430–436. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Kheterpal, S.; Callear, A.B.; Kirkby, G.R.; Price, N.J. Simultaneous posterior chamber intraocular lens implant combined with vitreoretinal surgery for intraocular foreign body injuries. Eye 1998, 12 Pt 2, 230–233. [Google Scholar] [CrossRef]
- Chan, T.K.; Mackintosh, G.; Yeoh, R.; Lim, A.S. Primary posterior chamber IOL implantation in penetrating ocular trauma. Int. Ophthalmol. 1993, 17, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. The Bull’s Eye Pattern of the Tear Film in Humans during Visual Fixation on En-Face Optical Coherence Tomography. Sci. Rep. 2019, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
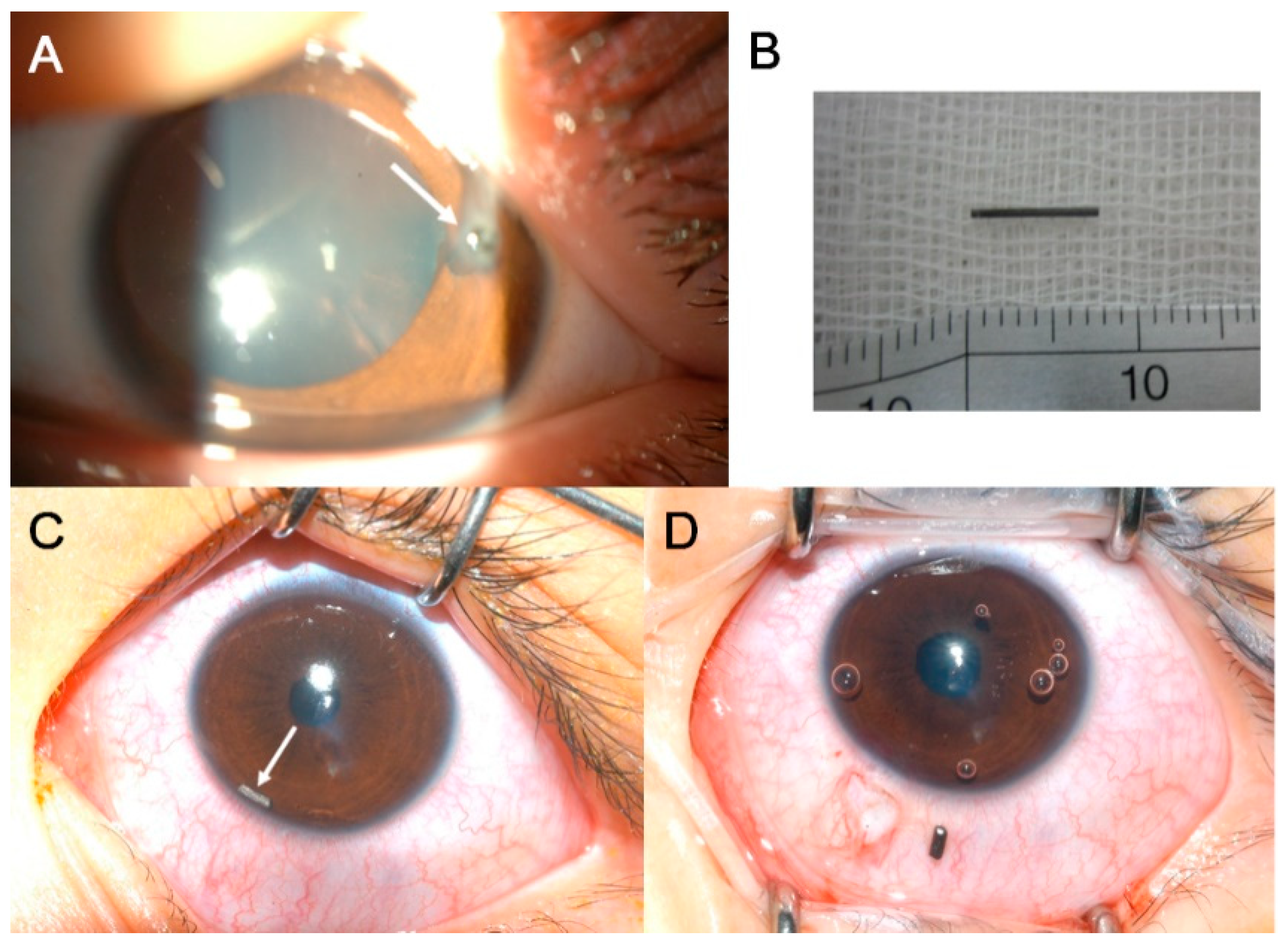
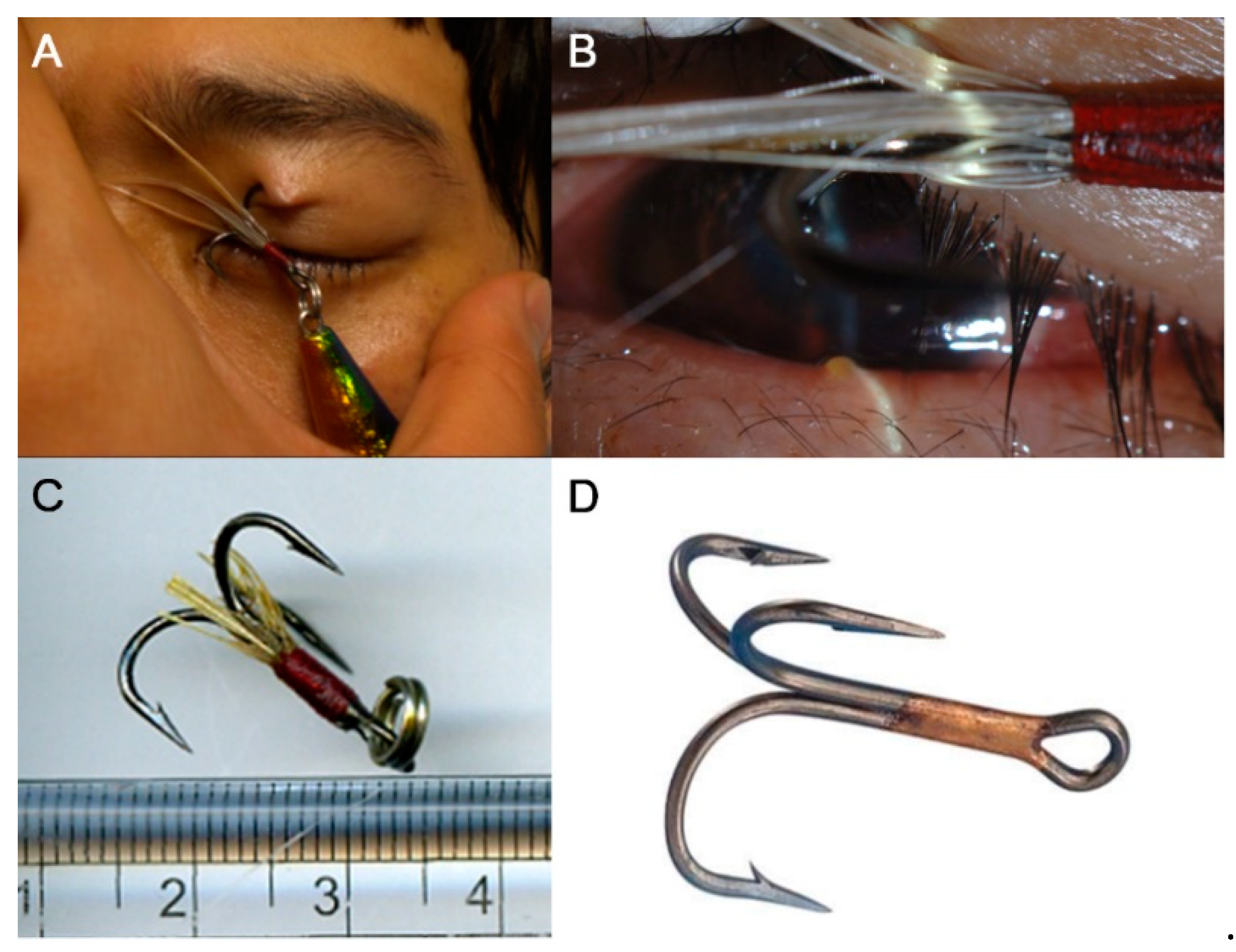
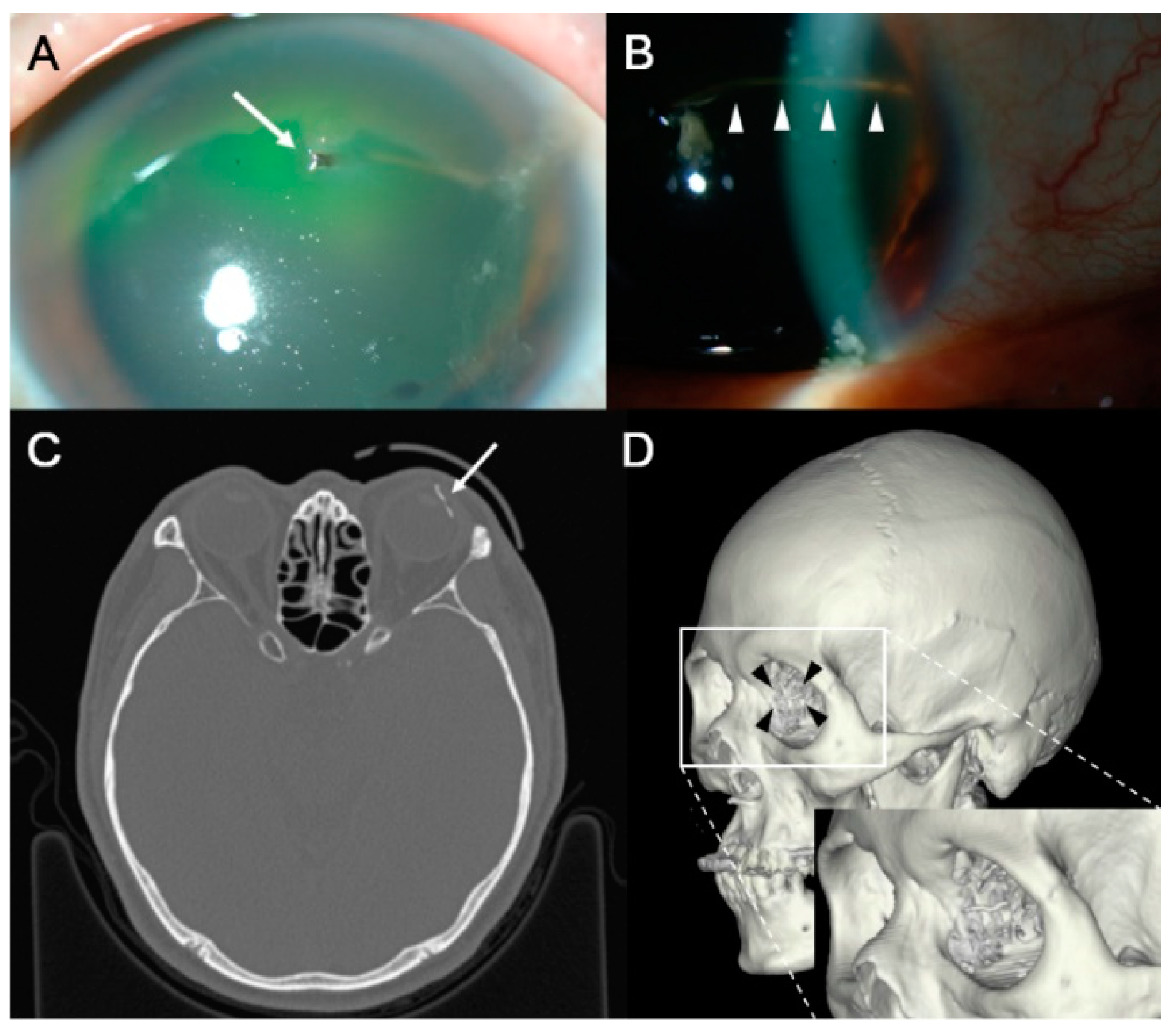


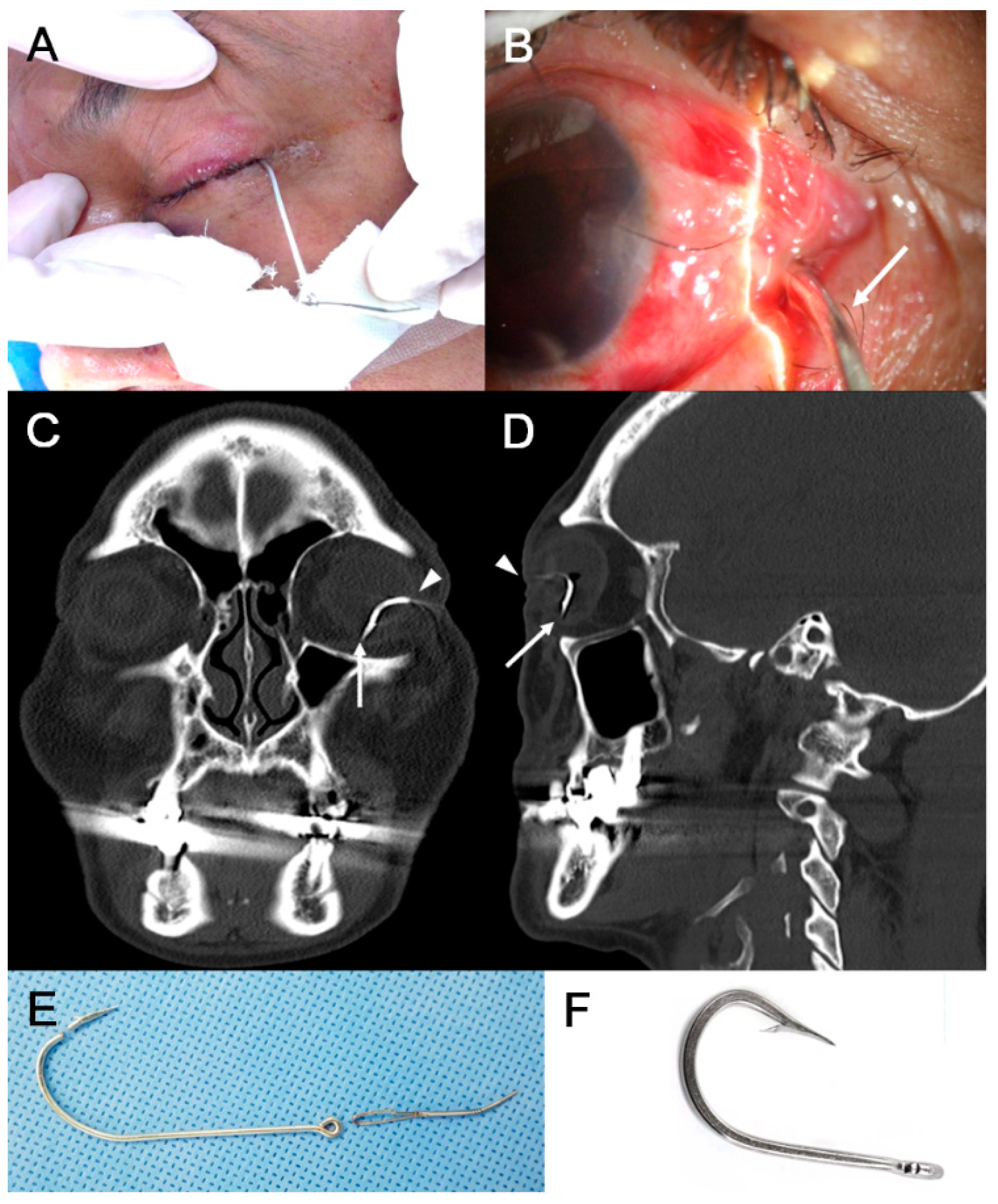

| Variable | Eyes with IOFB |
|---|---|
| Number of eyes | 52 |
| Age (yrs) | 46.7 ± 15.8 |
| Men/women | 50/2 |
| Right eye/left eye | 22/30 |
| Mechanisms of injury | |
| Hammering | 17 (32.7%) |
| Electric grass trimmer | 13 (25.0%) |
| Drilling/grinding | 7 (13.5%) |
| Cutting | 3 (5.8%) |
| Fishhook | 2 (3.8%) |
| Welding | 2 (3.8%) |
| Stabbed by the pencil | 2 (3.8%) |
| Car accident | 1 (1.9%) |
| Miscellaneous/unknown | 5 (9.6%) |
| Initial VA | |
| ≥20/40 | 9 (17.3%) |
| 20/50–20/200 | 9 (17.3%) |
| 20/300–FC | 13 (25.0%) |
| HM–LP | 20 (38.5%) |
| NLP | 1 (1.9%) |
| Initial VA (logMAR) | 1.60 ± 0.93 |
| Variable | Eyes with IOFB |
|---|---|
| Location of IOFB | |
| Cornea, full thickness | 4 (7.7%) |
| Iris | 6 (11.5%) |
| Anterior chamber | 1 (1.9%) |
| Lens | 3 (5.8%) |
| Vitreous | 8 (15.4%) |
| Retina/choroid | 30 (57.7%) |
| Penetrating site | |
| Cornea | 31 (59.6%) |
| Sclera | 9 (17.3%) |
| Corneosclera | 12 (23.1%) |
| Material of IOFB | |
| Metal | 40 (76.9%) |
| Stone | 5 (9.6%) |
| Glass | 3 (5.8%) |
| Pencil lead | 2 (3.8%) |
| Other | 2 (3.8%) |
| Size of IOFB (mm) | 5.16 ± 5.50 |
| Variable | Eyes with IOFB |
|---|---|
| Corneal injury | 43 (82.7%) |
| Hyphema | 12 (23.1%) |
| Iris injury | 25 (48.1%) |
| Traumatic cataract/lens injury | 32 (61.5%) |
| Vitreous hemorrhage | 25 (48.1%) |
| Retinal hemorrhage | 25 (48.1%) |
| Retinal tear | 32 (61.5%) |
| Retinal detachment | 21 (40.4%) |
| Choroidal detachment | 8 (15.4%) |
| Eyelid injury | 4 (7.7%) |
| Endophthalmitis | 4 (7.7%) |
| Variable | Eyes with IOFB |
|---|---|
| Trauma to primary repair (day) | 1.88 ± 4.44 |
| IOFB initially retrieved | 45 (86.5%) |
| Primary surgical procedures performed | |
| Anterior chamber washout | 6 (11.5%) |
| Phacoemulsification of cataract | 9 (17.3%) |
| Pars plana lensectomy | 18 (34.6%) |
| Pars plana vitrectomy | 33 (63.5%) |
| Additional surgical procedures performed | |
| Anterior chamber washout | 2 (3.8%) |
| Phacoemulsification of cataract | 4 (7.7%) |
| Pars plana lensectomy | 3 (5.8%) |
| Pars plana vitrectomy | 18 (34.6%) |
| Penetrating keratoplasty | 3 (5.8%) |
| Scleral buckling | 5 (9.6%) |
| Glaucoma surgery | 1 (1.9%) |
| Enucleation | 0 (0%) |
| Number of surgical procedures performed | 1.72 ± 0.83 |
| Final VA | |
| ≥20/40 | 17 (32.7%) |
| 20/50–20/200 | 10 (19.2%) |
| 20/300–FC | 8 (15.4%) |
| HM–LP | 11 (21.2%) |
| NLP | 6 (11.5%) |
| Final VA (logMAR) | 1.28 ± 1.13 |
| Globe loss/survival | 4/48 |
| Follow-up period (months) | 62.56 ± 71.29 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (yrs) | 1.002 (0.967–1.038) | 0.922 | ||||
| Male sex (vs. female) | 1.000 (0) | 0.922 | ||||
| Initial VA (logMAR) | 2.913 (1.477–6.560) | 0.004 | 2.096 (0.735–6.524) | 0.174 | ||
| Characteristics of IOFB | ||||||
| Posterior segment IOFB | 25.000 (4.272–480.065) | 0.003 | 6.066 (0.601–145.906) | 0.164 | 11.556 (1.695–234.465) | 0.033 |
| Material of IOFB | 4.059 (1.035–20.430) | 0.058 | 0.448 (0.111–1.128) | 0.190 | ||
| Size of IOFB (mm) | 1.035 (0.935–1.163) | 0.519 | ||||
| Clinical presentations | ||||||
| Vitreous hemorrhage | 4.250 (1.374–14.230) | 0.015 | 0.640 (0.058–5.509) | 0.692 | ||
| Retinal hemorrhage | 6.107 (1.915–21.606) | 0.003 | 1.991 (0.206–20.573) | 0.546 | ||
| Retinal detachment | 12.375 (3.470–54.025) | <0.001 | 5.569 (0.966–44.478) | 0.070 | 4.781 (1.186–22.428) | 0.034 |
| Endophthalmitis | 1.000 (0.112–8.894) | 1.000 | ||||
| Management factors | ||||||
| Trauma to IOFB removal time (day) | 0.885 (0.605–1.044) | 0.339 | ||||
| IOFB extraction with primary repair | 0.350 (0.047–1.812) | 0.237 | ||||
| Number of surgical procedures performed | 1.704 (0.895–3.477) | 0.118 | 0.550 (0.179–1.507) | 0.261 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.C.; Lee, S.Y.; Yoon, C.K.; Park, U.C.; Heo, J.W.; Lee, E.K. Intraocular Foreign Body: Diagnostic Protocols and Treatment Strategies in Ocular Trauma Patients. J. Clin. Med. 2021, 10, 1861. https://doi.org/10.3390/jcm10091861
Jung HC, Lee SY, Yoon CK, Park UC, Heo JW, Lee EK. Intraocular Foreign Body: Diagnostic Protocols and Treatment Strategies in Ocular Trauma Patients. Journal of Clinical Medicine. 2021; 10(9):1861. https://doi.org/10.3390/jcm10091861
Chicago/Turabian StyleJung, Hyun Chul, Sang Yoon Lee, Chang Ki Yoon, Un Chul Park, Jang Won Heo, and Eun Kyoung Lee. 2021. "Intraocular Foreign Body: Diagnostic Protocols and Treatment Strategies in Ocular Trauma Patients" Journal of Clinical Medicine 10, no. 9: 1861. https://doi.org/10.3390/jcm10091861
APA StyleJung, H. C., Lee, S. Y., Yoon, C. K., Park, U. C., Heo, J. W., & Lee, E. K. (2021). Intraocular Foreign Body: Diagnostic Protocols and Treatment Strategies in Ocular Trauma Patients. Journal of Clinical Medicine, 10(9), 1861. https://doi.org/10.3390/jcm10091861






