Continuous Glucose Monitoring in the Intensive Care Unit Following Total Pancreatectomy with Islet Autotransplantation in Children: Establishing Accuracy of the Dexcom G6 Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Procedures
2.2. Statistical Analysis
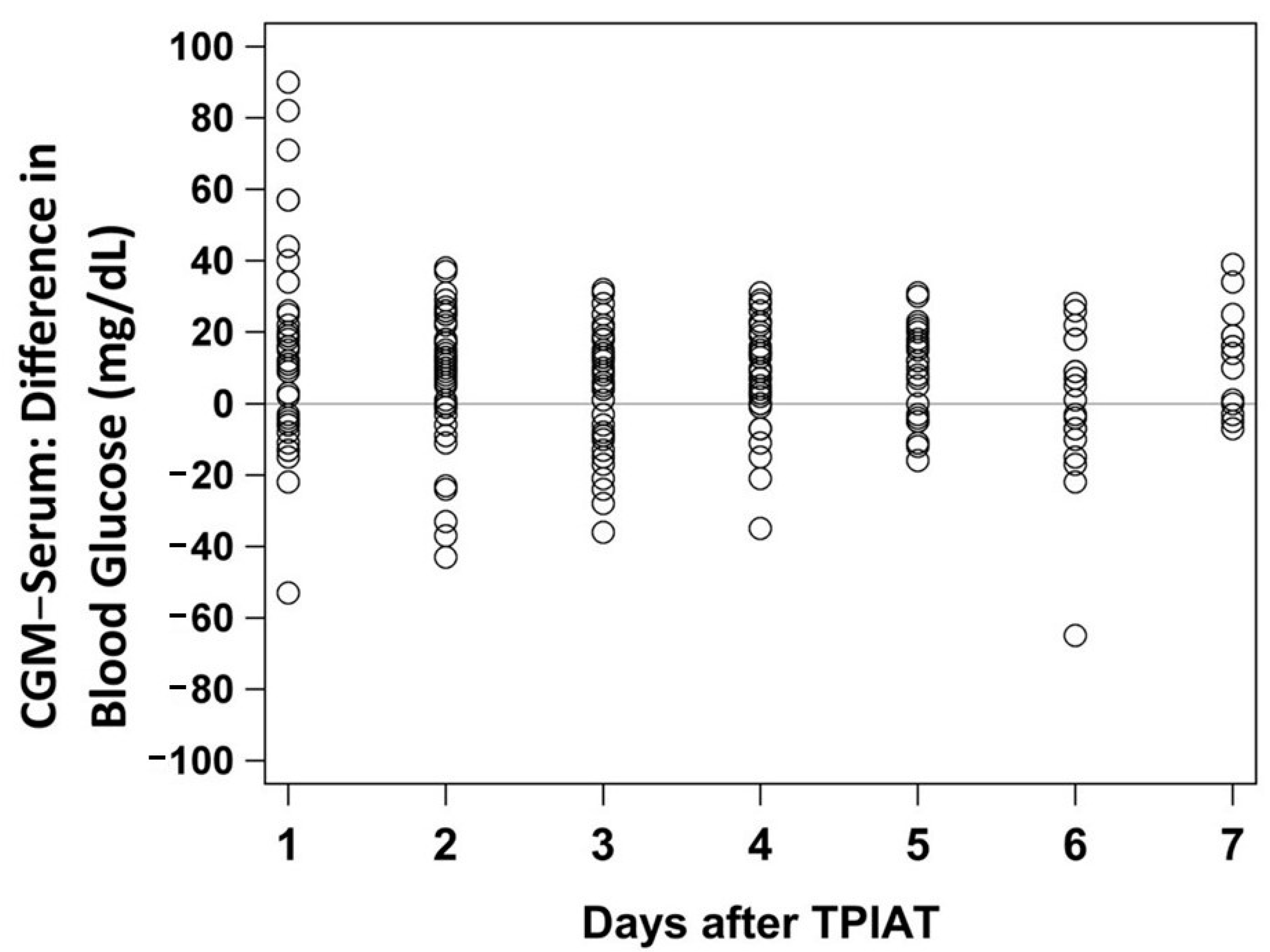
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Tamborlane, W.V.; Beck, R.W.; Bode, B.W.; Buckingham, B.; Chase, H.P.; Clemons, R.; Fiallo-Scharer, R.; Fox, L.A.; Gilliam, L.K.; et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N. Engl. J. Med. 2008, 359, 1464–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, B.E.; Wolfsdorf, J.I. Monitoring of Pediatric Type 1 Diabetes. Front. Endocrinol. 2020, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Discover Dexcom Continuous Glucose Monitoring (CGM) Technology. Available online: https://provider.dexcom.com/dexcom-cgm (accessed on 3 March 2021).
- Free Style Libre 2 System. Available online: https://provider.myfreestyle.com/freestyle-libre-2-accuracy.html (accessed on 21 March 2021).
- Forlenza, G.P.; Nathan, B.M.; Moran, A.; Dunn, T.B.; Beilman, G.J.; Pruett, T.L.; Kovatchev, B.P.; Bellin, M.D. Accuracy of Continuous Glucose Monitoring in Patients After Total Pancreatectomy with Islet Autotransplantation. Diabetes Technol. Ther. 2016, 18, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, D.A.; Jiminez-Vega, J.M.; Hornung, L.N.; Chima, R.S.; Abu-El-Haija, M.; Lin, T.K.; Palermo, J.J.; Nathan, J.D. Continuous glucose monitoring following pancreatectomy with islet autotransplantation in children. Pediatr. Transplant. 2017, 21, e12998. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; Laffel, L.M.; Wadwa, R.P.; Garg, S.K. Performance of a Factory-Calibrated Real-Time Continuous Glucose Monitoring System Utilizing an Automated Sensor Applicator. Diabetes Technol. Ther. 2018, 20, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadwa, R.P.; Laffel, L.M.; Shah, V.N.; Garg, S.K. Accuracy of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System During 10 Days of Use in Youth and Adults with Diabetes. Diabetes Technol. Ther. 2018, 20, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.B.; Zhang, X.; Puhr, S.A.; Johnson, T.K.; Walker, T.C.; Balo, A.K.; Price, D. Performance of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System in Pediatric Participants With Type 1 Diabetes. J. Diabetes Sci. Technol. 2019, 13, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Castorino, K.; Polsky, S.; O’Malley, G.; Levister, C.; Nelson, K.; Farfan, C.; Brackett, S.; Puhr, S.; Levy, C.J. Performance of the Dexcom G6 Continuous Glucose Monitoring System in Pregnant Women with Diabetes. Diabetes Technol. Ther. 2020, 22, 943–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottino, R.; Bertera, S.; Grupillo, M.; Melvin, P.R.; Humar, A.; Mazariegos, G.; Moser, A.J.; Walsh, R.M.; Fung, J.; Gelrud, A.; et al. Isolation of human islets for autologous islet transplantation in children and adolescents with chronic pancreatitis. J. Transplant. 2012, 2012, 642787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ooi, C.Y.; Werlin, S.; Abu-El-Haija, M.; Barth, B.; Bellin, M.D.; Durie, P.R.; Fishman, D.S.; Freedman, S.D.; Gariepy, C.; et al. Risk Factors Associated With Pediatric Acute Recurrent and Chronic Pancreatitis: Lessons From INSPPIRE. JAMA Pediatr. 2016, 170, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Bellin, M.; Husain, S.Z.; Ahuja, M.; Barth, B.; Davis, H.; Durie, P.R.; Fishman, D.S.; Freedman, S.D.; Gariepy, C.E.; et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J. Pediatr. 2015, 166, 890–896.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellin, M.D.; Forlenza, G.P.; Majumder, K.; Berger, M.; Freeman, M.L.; Beilman, G.J.; Dunn, T.B.; Pruett, T.L.; Murati, M.; Wilhelm, J.J.; et al. Total Pancreatectomy With Islet Autotransplantation Resolves Pain in Young Children With Severe Chronic Pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, R.M.; Saavedra, J.R.; Lentz, G.; Guerron, A.D.; Scheman, J.; Stevens, T.; Trucco, M.; Bottino, R.; Hatipoglu, B. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J. Gastrointest. Surg. 2012, 16, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Schwarzenberg, S.J.; Cook, M.; Sutherland, D.E.; Chinnakotla, S. Pediatric Autologous Islet Transplantation. Curr. Diabetes Rep. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnakotla, S.; Beilman, G.J.; Dunn, T.B.; Bellin, M.D.; Freeman, M.L.; Radosevich, D.M.; Arain, M.; Amateau, S.K.; Mallery, J.S.; Schwarzenberg, S.J.; et al. Factors Predicting Outcomes After a Total Pancreatectomy and Islet Autotransplantation Lessons Learned From Over 500 Cases. Ann. Surg. 2015, 262, 610–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnakotla, S.; Bellin, M.D.; Schwarzenberg, S.J.; Radosevich, D.M.; Cook, M.; Dunn, T.B.; Beilman, G.J.; Freeman, M.L.; Balamurugan, A.N.; Wilhelm, J.; et al. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: Indication, surgical techniques, postoperative management, and long-term outcomes. Ann. Surg. 2014, 260, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.C.; Lin, Y.K.; Walsh, R.M.; Bottino, R.; Stevens, T.K.; Trucco, M.; Bena, J.; Faiman, C.; Hatipoglu, B.A. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J. Clin. Endocrinol. Metab. 2015, 100, 1765–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Total Pancreatectomy with Islet Autotransplantation (TPIAT). Available online: https://www.cincinnatichildrens.org/service/p/pancreas-care/treatments/tpiat (accessed on 25 October 2020).
- Biarnés, M.; Montolio, M.; Nacher, V.; Raurell, M.; Soler, J.; Montanya, E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002, 51, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Interfering Substances and Risks. Available online: https://www.dexcom.com/interference (accessed on 25 October 2020).
- Tellez, S.E.; Hornung, L.N.; Courter, J.D.; Abu-El-Haija, M.; Nathan, J.D.; Lawson, S.A.; Elder, D.A. Inaccurate Glucose Sensor Values After Hydroxyurea Administration. Diabetes Technol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dexcom Continuous Glucose Monitoring. Available online: https://www.dexcom.com/g6-cgm-system (accessed on 25 October 2020).
- Reiterer, F.; Polterauer, P.; Schoemaker, M.; Schmelzeisen-Redecker, G.; Freckmann, G.; Heinemann, L.; Del Re, L. Significance and Reliability of MARD for the Accuracy of CGM Systems. J. Diabetes Sci. Technol. 2017, 11, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| TPIAT Patients | |
|---|---|
| n = 25 | |
| TPIAT age (years) | 11.2 (9.2–14.0) |
| Sex (male) | 16 (64%) |
| Race (White/Caucasian) | 24 (96%) |
| Ethnicity (Non-Hispanic) | 23/23 (100%) |
| Weight percentile | 73.8 (60.7–90.9) |
| Height percentile | 60.5 (32.0–71.3) |
| BMI percentile | 81.9 (63.2–93.5) |
| Total islet equivalents/kg (IEQ/kg) | 4518 (3154–7028) |
| Genetic testing positive | 18 (72%) |
| PRSS1 | 10/22 (45%) |
| SPINK1 | 6/24 (25%) |
| CFTR | 7/22 (32%) |
| CTRC | 4/21 (19%) |
| More than 1 gene affected | 7 (28%) |
| Exocrine insufficiency | 8/24 (33%) |
| MARD | |
|---|---|
| Overall | 14.6% |
| Day 1 | 19.8% |
| Day 2 | 15.2% |
| Day 3 | 14.0% |
| Day 4 | 12.1% |
| Day 5 | 11.4% |
| Day 6 | 13.2% |
| Day 7 | 14.1% |
| CGM vs. Serum | n = 183 |
|---|---|
| Mean absolute difference (MAD) ± SD | 14.7 ± 10.3 |
| Mean absolute relative difference (MARD) | 13.4% |
| Mean difference ± Standard Deviation | 6.7 ± 16.7 |
| Median difference (Interquartile Range) | 9.0 (−4.0, 18.0) |
| Within ± 20 mg/dL | 134 (73%) |
| Within ± 15 mg/dL | 107 (58%) |
| Within ± 10 mg/dL | 75 (41%) |
| %20/20 | 143 (78%) |
| %15/15 | 117 (64%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segev, N.; Hornung, L.N.; Tellez, S.E.; Courter, J.D.; Lawson, S.A.; Nathan, J.D.; Abu-El-Haija, M.; Elder, D.A. Continuous Glucose Monitoring in the Intensive Care Unit Following Total Pancreatectomy with Islet Autotransplantation in Children: Establishing Accuracy of the Dexcom G6 Model. J. Clin. Med. 2021, 10, 1893. https://doi.org/10.3390/jcm10091893
Segev N, Hornung LN, Tellez SE, Courter JD, Lawson SA, Nathan JD, Abu-El-Haija M, Elder DA. Continuous Glucose Monitoring in the Intensive Care Unit Following Total Pancreatectomy with Islet Autotransplantation in Children: Establishing Accuracy of the Dexcom G6 Model. Journal of Clinical Medicine. 2021; 10(9):1893. https://doi.org/10.3390/jcm10091893
Chicago/Turabian StyleSegev, Natalie, Lindsey N. Hornung, Siobhan E. Tellez, Joshua D. Courter, Sarah A. Lawson, Jaimie D. Nathan, Maisam Abu-El-Haija, and Deborah A. Elder. 2021. "Continuous Glucose Monitoring in the Intensive Care Unit Following Total Pancreatectomy with Islet Autotransplantation in Children: Establishing Accuracy of the Dexcom G6 Model" Journal of Clinical Medicine 10, no. 9: 1893. https://doi.org/10.3390/jcm10091893
APA StyleSegev, N., Hornung, L. N., Tellez, S. E., Courter, J. D., Lawson, S. A., Nathan, J. D., Abu-El-Haija, M., & Elder, D. A. (2021). Continuous Glucose Monitoring in the Intensive Care Unit Following Total Pancreatectomy with Islet Autotransplantation in Children: Establishing Accuracy of the Dexcom G6 Model. Journal of Clinical Medicine, 10(9), 1893. https://doi.org/10.3390/jcm10091893






