Evaluation of Intraocular Pressure and Other Biomechanical Parameters to Distinguish between Subclinical Keratoconus and Healthy Corneas
Abstract
:1. Introduction
2. Materials and Methods
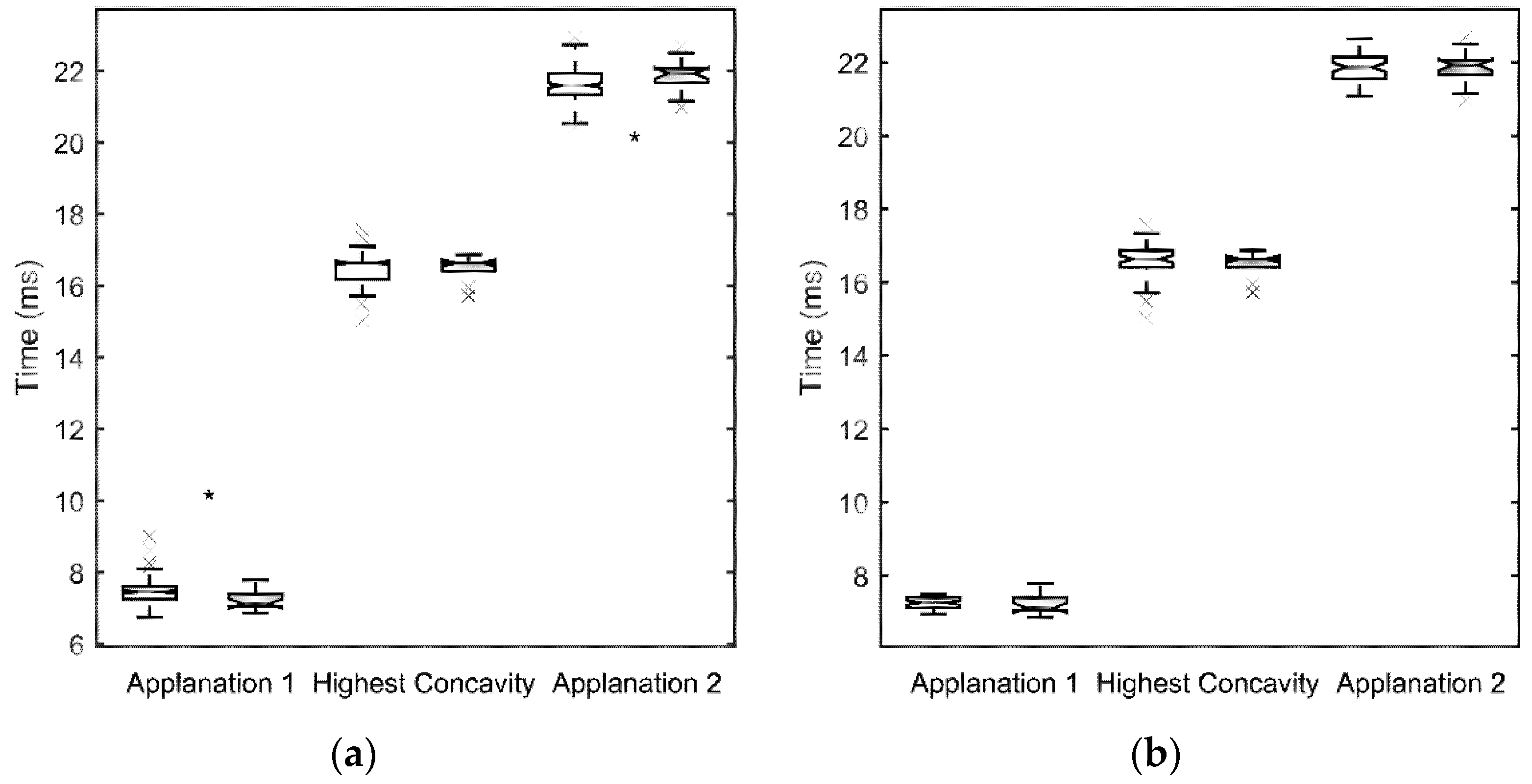
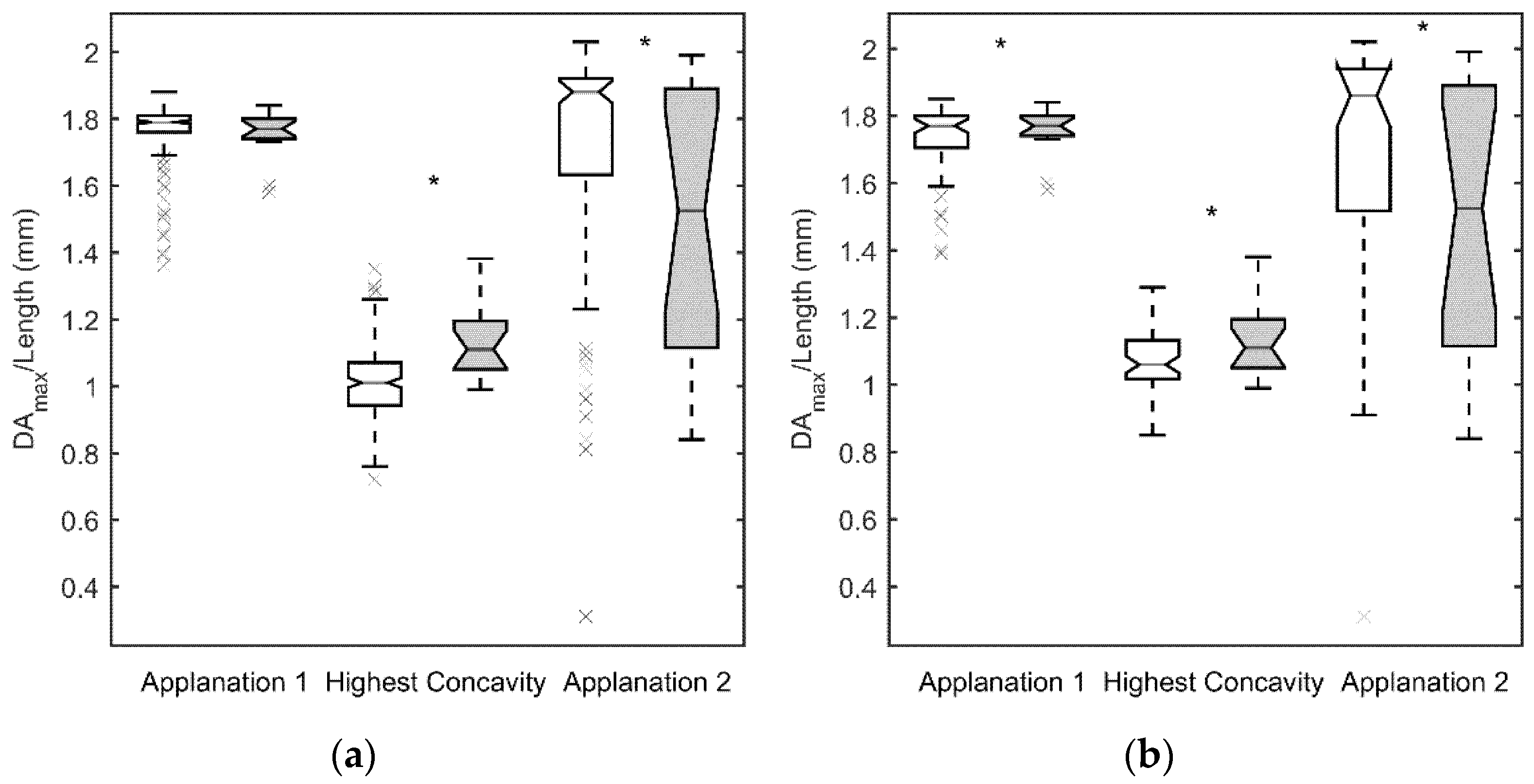
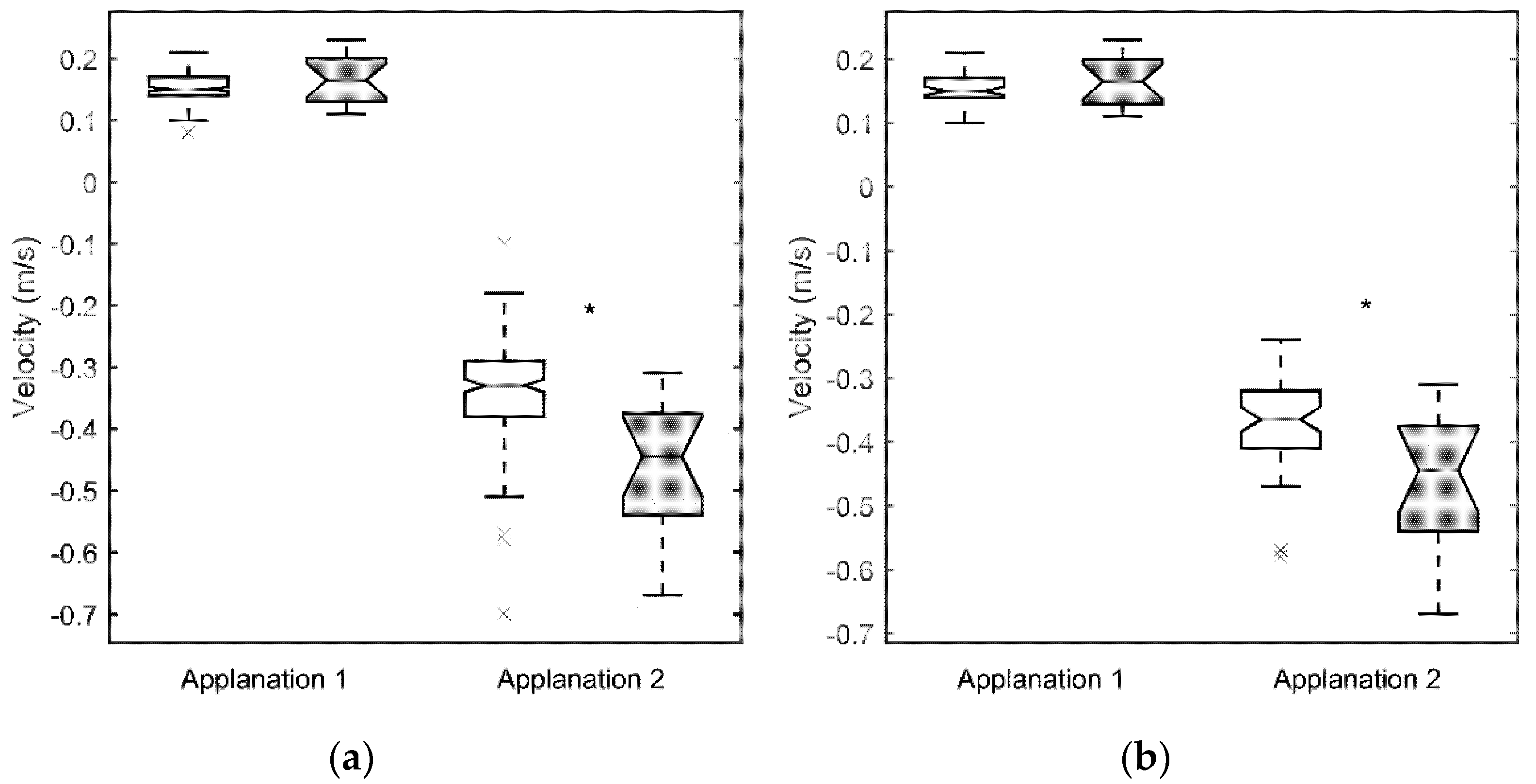
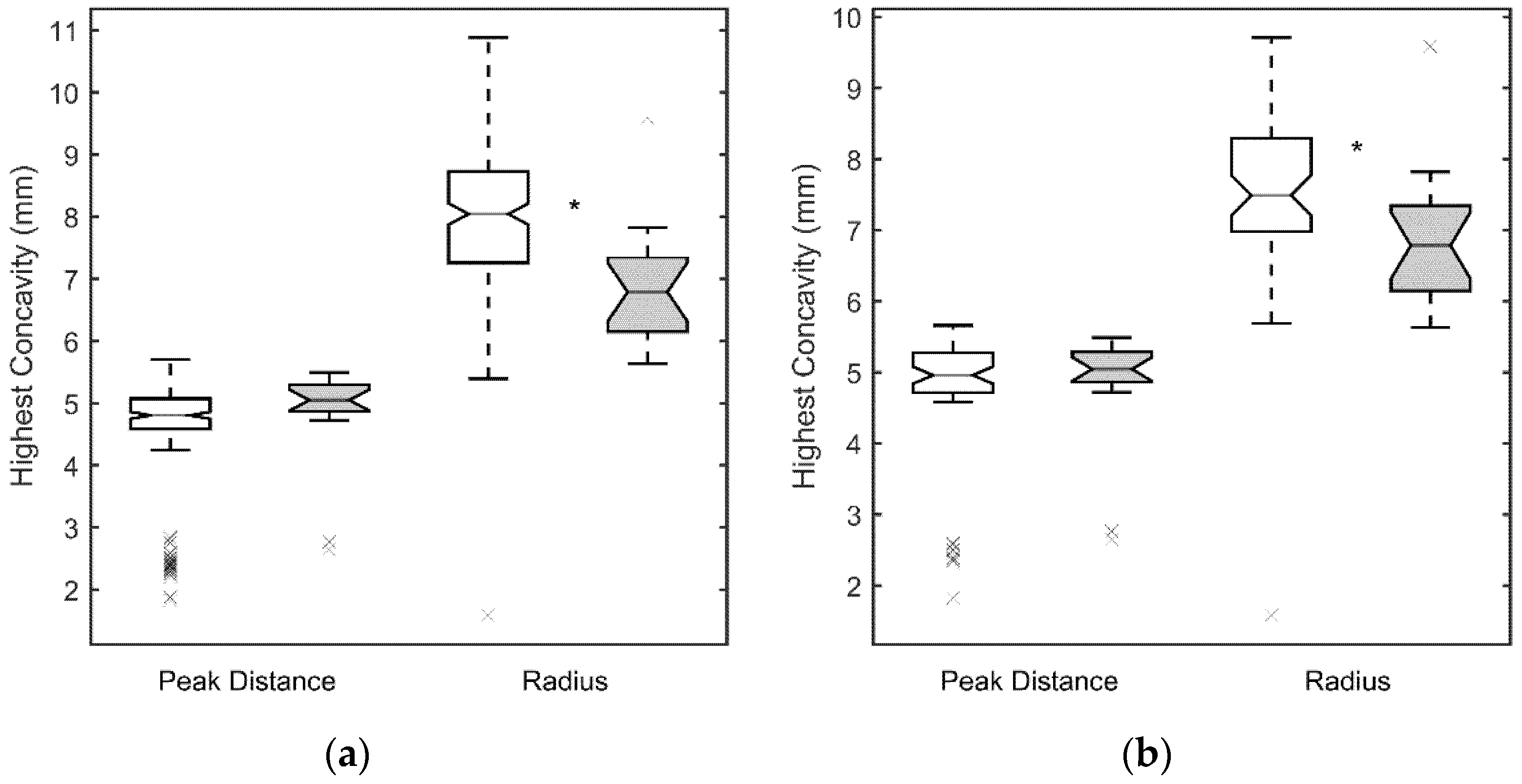
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brookes, N.; Loh, I.-P.; Clover, G.; Poole, C.; Sherwin, T. Involvement of corneal nerves in the progression of keratoconus. Exp. Eye Res. 2003, 77, 515–524. [Google Scholar] [CrossRef]
- Erie, J.C.; Patel, S.V.; McLaren, J.W.; Nau, C.B.; Hodge, D.O.; Bourne, W.M. Keratocyte density in keratoconus. A confocal microscopy study. Am. J. Ophthalmol. 2002, 134, 689–695. [Google Scholar] [CrossRef]
- Meek, K.M.; Tuft, S.J.; Huang, Y.; Gill, P.S.; Hayes, S.; Newton, R.H.; Bron, A.J. Changes in Collagen Orientation and Distribution in Keratoconus Corneas. Investig. Opthalmol. Vis. Sci. 2005, 46, 1948–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esporcatte, L.P.G.; Salomão, M.Q.; Lopes, B.T.; Vinciguerra, P.; Vinciguerra, R.; Roberts, C.; Elsheikh, A.; Dawson, D.G.; Ambrósio, R. Biomechanical Diagnostics of the Cornea. Int. Ophthalmol. Clin. Summer 2017, 57, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Vellara, H.R.; Patel, D.V. Biomechanical properties of the keratoconic cornea: A review. Clin. Exp. Optom. 2015, 98, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, D.R.; Fischer, R.W.; Winterhalter, K.H.; Witmer, R.; Vaughan, L. Comparative studies of collagens in normal and keratoconus corneas. Exp. Eye Res. 1988, 46, 431–442. [Google Scholar] [CrossRef]
- Herber, R.; Ramm, L.; Spoerl, E.; Raiskup, F.; Pillunat, L.E.; Terai, N. Assessment of corneal biomechanical parameters in healthy and keratoconic eyes using dynamic bidirectional applanation device and dynamic Scheimpflug analyzer. J. Cataract. Refract. Surg. 2019, 45, 778–788. [Google Scholar] [CrossRef]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Shah, S.; Laiquzzaman, M.; Bhojwani, R.; Mantry, S.; Cunliffe, I. Assessment of the biomechanical properties of the cornea with the ocular re-sponse analyzer in normal and keratoconic eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3026–3031. [Google Scholar] [CrossRef] [Green Version]
- Galletti, J.G.; Pförtner, T.; Bonthoux, F.F. Improved keratoconus detection by ocular response analyzer testing after considera-tion of corneal thickness as a confounding factor. J. Refract. Surg. 2012, 28, 202–208. [Google Scholar] [CrossRef]
- Kara, N.; Altinkaynak, H.; Baz, O.; Goker, Y. Biomechanical Evaluation of Cornea in Topographically Normal Relatives of Patients with Keratoconus. Cornea 2013, 32, 262–266. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.L.; Yang, S.L.; Yan, X.M.; Li, H.L. Examination and discriminant analysis of corneal biomechanics with CorVis ST in keratoconus and subclinical keratoconus. Beijing Da Xue Xue Bao Yi Xue Ban 2019, 51, 881–886. [Google Scholar]
- Yang, K.; Xu, L.; Fan, Q.; Zhao, D.; Ren, S. Repeatability and comparison of new Corvis® ST parameters in normal and keratoconus eyes. Sci. Rep. 2019, 9, 15379. [Google Scholar] [CrossRef]
- Yang, K.; Xu, L.; Fan, Q.; Gu, Y.; Song, P.; Zhang, B.; Zhao, D.; Pang, C.; Ren, S. Evaluation of new Corvis® ST parameters in normal, Post-LASIK, Post-LASIK keratectasia and keratoconus eyes. Sci. Rep. 2020, 10, 5676. [Google Scholar] [CrossRef]
- Elham, R.; Jafarzadehpur, E.; Hashemi, H.; Amanzadeh, K.; Shokrollahzadeh, F.; Yekta, A.A.; Khabazkhoob, M. Keratoconus diagnosis using Corvis® ST measured biomechanical parameters. J. Curr. Ophthalmol. 2017, 29, 175–181. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, Y.; Yan, Z.; Tian, M.; Zhao, J.; Zhou, X. Relationship Among Corneal Stiffness, Thickness, and Biomechanical Parameters Measured by Corvis® ST, Pentacam and ORA in Keratoconus. Front. Physiol. 2019, 10, 740. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Li, Y.; Song, Y.; Wang, Z. Early Diagnosis of Keratoconus in Chinese Myopic Eyes by Combining Corvis® ST with Pen-tacam. Curr. Eye Res. 2020, 45, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Koc, M.; Aydemir, E.; Tekin, K.; Inanc, M.; Kosekahya, P.; Kiziltoprak, H. Biomechanical Analysis of Subclinical Keratoconus With Normal Topographic, Topometric, and Tomographic Findings. J. Refract. Surg. 2019, 35, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Wang, Y.M.; Yu, M.; Jhanji, V. Comparison of Corneal Tomography and a New Combined Tomographic Biomechanical Index in Subclinical Keratoconus. J. Refract. Surg. 2018, 34, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.; Siebert, M.; Katz, T.; Frings, A.; Mehlan, J.; Druchkiv, V.; Bühren, J.; Linke, S.J. Tomographic and Biomechanical Scheimpflug Imaging for Keratoconus Characterization: A Validation of Current Indices. J. Refract. Surg. 2018, 34, 840–847. [Google Scholar] [CrossRef]
- Koh, S.; Ambrósio, R.; Inoue, R.; Maeda, N.; Miki, A.; Nishida, K. Detection of Subclinical Corneal Ectasia Using Corneal Tomographic and Biomechanical Assessments in a Japanese Population. J. Refract. Surg. 2019, 35, 383–390. [Google Scholar] [CrossRef]
- Catalán-López, S.; Cadarso-Suárez, L.; López-Ratón, M.; Cadarso-Suárez, C. Corneal Biomechanics in Unilateral Keratoconus and Fellow Eyes with a Scheimpflug-based Tonometer. Optom. Vis. Sci. 2018, 95, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yang, K.; Li, P.; Liu, Y.; Liang, D.; Ren, S.; Zeng, Q. Assessment of Corneal Pachymetry Distribution and Morphologic Changes in Subclinical Kerato-conus with Normal Biomechanics. Biomed. Res. Int. 2019, 2019, 1748579. [Google Scholar] [CrossRef] [Green Version]
- Valbon, B.F.; Ambrósio, R., Jr.; Fontes, B.M.; Alves, M.R. Effects of age on corneal deformation by non-contact tonometry integrated with an ultra-high-speed (UHS) Scheimpflug camera. Arq. Bras. Oftalmol. 2013, 76, 229–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, G.; Hassan, Z.; Csutak, A.; Szalai, E.; Berta, A.; Modis, J.L. Repeatability of Ocular Biomechanical Data Measurements with a Scheimpflug-Based Noncontact Device on Normal Corneas. J. Refract. Surg. 2013, 29, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Xu, J.; Wei, A.; Deng, S.X.; Cui, X.; Yu, X.; Sun, X. A New Tonometer—The Corvis® ST Tonometer: Clinical Comparison with Noncontact and Goldmann Applanation Tonometers. Investig. Opthalmol. Vis. Sci. 2013, 54, 659–665. [Google Scholar] [CrossRef]
- Hon, Y.; Lam, A.K. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom. Vis. Sci. 2013, 90, e1–e8. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, L.; Dou, R.; Wu, W.; Wu, D.; Jhanji, V. Corneal Stiffness and Its Relationship with Other Corneal Biomechanical and Nonbiomechan-ical Parameters in Myopic Eyes of Chinese Patients. Cornea 2018, 37, 881–885. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Ambrósio, R., Jr.; Roberts, C.J.; Azzolini, C.; Vinciguerra, P. Biomechanical Characterization of Subclinical Keratoconus Without Topo-graphic or Tomographic Abnormalities. J. Refract. Surg. 2017, 33, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Peña-García, P.; Peris-Martínez, C.; Abbouda, A.; Ruiz-Moreno, J.M. Detection of subclinical keratoconus through non-contacttonometry and the use of discriminant biomechanical functions. J. Biomech. 2016, 49, 353–363. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Safeen, S.; Shah, S.; Laiquzzaman, M. Changes of Corneal Biomechanics with Keratoconus. Cornea 2012, 31, 849–854. [Google Scholar] [CrossRef]
- Smadja, D.; Touboul, D.; Cohen, A.; Doveh, E.; Santhiago, M.R.; Mello, G.R.; Krueger, R.R.; Colin, J. Detection of subclinical kerato-conus using an automated decision tree classification. Am. J. Ophthalmol. 2013, 156, 237–246. [Google Scholar] [CrossRef]
- Klyce, S.D. Chasing the suspect: Keratoconus. Br. J. Ophthalmol. 2009, 93, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio, R., Jr.; Nogueira, L.P.; Caldas, D.L.; Fontes, B.M.; Luz, A.; Cazal, J.O.; Alves, M.R.; Belin, M.W. Evaluation of corneal shape and biomechanics before LASIK. Int. Ophthalmol. Clin. 2011, 51, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Ishii, R.; Shimizu, K.; Igarashi, A. Evaluation of corneal elevation, pachymetry and keratometry in keratoconic eyes with respect to the stage of Amsler-Krumeich classification. Br. J. Ophthalmol. 2014, 98, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Ortak, H.; Yeter, V.; Alim, S.; Sayn, O.; Tas, U.; Sönmez, B. Mapping corneal thickness using dual-scheimpflug imaging at different stages of kerato-conus. Cornea 2013, 32, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Miháltz, K.; Kovács, I.; Takács, Á.; Nagy, Z.Z. Evaluation of keratometric, pachymetric, and elevation parameters of keratoconus cor-neas with pentacam. Cornea 2009, 28, 976–980. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, U.; Loiacono, C.; Richiardi, L.; Turco, D.; Mutani, B.; Grignolo, F.M. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology 2008, 115, 1534–1539. [Google Scholar] [CrossRef]
- Ventura, B.V.; Machado, A.P.; Ambrósio, R., Jr.; Ribeiro, G.; Araújo, L.N.; Luz, A.; Lyra, J.M. Analysis of waveform-derived ORA parameters in early forms of kerato-conus and normal corneas. J. Refract. Surg. 2013, 29, 637–643. [Google Scholar] [CrossRef]
- Tian, L.; Huang, Y.-F.; Wang, L.-Q.; Bai, H.; Wang, Q.; Jiang, J.-J.; Wu, Y.; Gao, M. Corneal Biomechanical Assessment Using Corneal Visualization Scheimpflug Technology in Keratoconic and Normal Eyes. J. Ophthalmol. 2014, 2014, 147516. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.; Jones, R.; Nguyen, T.; Grazier, J. Stress-controlled viscoelastic tensile response of bovine cornea. J. Biomech. 2007, 40, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.; Fontes, B.M.; Lopes, B.; Ramos, I.; Schor, P.; Ambrósio, R., Jr. ORA waveform-derived biomechanical parameters to distinguish normal from ker-atoconic eyes. Arq. Bras. Oftalmol. 2013, 76, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Data | Sex | Control Group | SCKC Group |
|---|---|---|---|
| Age (years) | Men | 31 ± 7 | 26 ± 13 |
| Women | 33 ± 8 | 31 ± 19 | |
| p-value | 0.67 | 0.50 | |
| Number of eyes | Men | 83 | 8 |
| Women | 100 | 5 |
| Total Normal Sample | IOP and CCP Matched Normal Sample | |||||
|---|---|---|---|---|---|---|
| Variable | AUC | Lower ICL | Upper ICL | AUC | Lower ICL | Upper ICL |
| 1st PCS | 0.8695 * | 0.7452 | 0.9269 | 0.7800 * | 0.6073 | 0.8906 |
| A2V | 0.8343 * | 0.6948 | 0.9176 | 0.7524 * | 0.5815 | 0.8814 |
| Radius | 0.8203 * | 0.6572 | 0.9142 | 0.7230 * | 0.5662 | 0.8506 |
| DAmax | 0.8047 * | 0.7052 | 0.8869 | 0.6629 | 0.4710 | 0.7929 |
| A2length | 0.6987 * | 0.5244 | 0.8496 | 0.6767 | 0.4970 | 0.8266 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peris-Martínez, C.; Díez-Ajenjo, M.A.; García-Domene, M.C.; Pinazo-Durán, M.D.; Luque-Cobija, M.J.; del Buey-Sayas, M.Á.; Ortí-Navarro, S. Evaluation of Intraocular Pressure and Other Biomechanical Parameters to Distinguish between Subclinical Keratoconus and Healthy Corneas. J. Clin. Med. 2021, 10, 1905. https://doi.org/10.3390/jcm10091905
Peris-Martínez C, Díez-Ajenjo MA, García-Domene MC, Pinazo-Durán MD, Luque-Cobija MJ, del Buey-Sayas MÁ, Ortí-Navarro S. Evaluation of Intraocular Pressure and Other Biomechanical Parameters to Distinguish between Subclinical Keratoconus and Healthy Corneas. Journal of Clinical Medicine. 2021; 10(9):1905. https://doi.org/10.3390/jcm10091905
Chicago/Turabian StylePeris-Martínez, Cristina, María Amparo Díez-Ajenjo, María Carmen García-Domene, María Dolores Pinazo-Durán, María José Luque-Cobija, María Ángeles del Buey-Sayas, and Susana Ortí-Navarro. 2021. "Evaluation of Intraocular Pressure and Other Biomechanical Parameters to Distinguish between Subclinical Keratoconus and Healthy Corneas" Journal of Clinical Medicine 10, no. 9: 1905. https://doi.org/10.3390/jcm10091905







