Neutrophil-to-Lymphocyte Ratio, a Novel Inflammatory Marker, as a Predictor of Bipolar Type in Depressed Patients: A Quest for Biological Markers
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschfeld, R. Differential diagnosis of bipolar disorder and major depressive disorder. J. Affect. Disord. 2014, 169, S12–S16. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Merikangas, K.; Akiskal, H.; Angst, J.; Greenberg, P.; Hirschfeld, R.; Petukhova, M.; Kessler, R. Lifetime And 12-Month Prev-alence Of Bipolar Spectrum Disorder in The National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2007, 64, 543. [Google Scholar] [CrossRef]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar Disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of role due to common physical and mental conditions: Results from the WHO World Mental Health surveys. Mol. Psychiatry 2010, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Prim. 2018, 4, 18008. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Kapczinski, F.; Andreazza, A.; Dean, O.; Giorlando, F.; Maes, M.; Yücel, M.; Gama, C.; Dodd, S.; Dean, B.; et al. Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011, 35, 804–817. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain Sci. 2017, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Ivković, M.; Pantović-Stefanović, M.; Dunjić-Kostić, B.; Jurišić, V.; Lačković, M.; Totić-Poznanović, S.; Jovanović, A.; Damjanović, A. Neutrophil-To-Lymphocyte Ratio Predicting Suicide Risk in Euthymic Patients with Bipolar Disorder: Moderatory Effect of Family History. Compr. Psychiatry 2016, 66, 87–95. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Horstman, L.L.; Jy, W.; Ahn, Y.S.; Zivadinov, R.; Maghzi, A.H.; Etemadifar, M.; Alexander, J.S.; Minagar, A. Role of platelets in neuroinflammation: A wide-angle perspective. J. Neuroinflammation 2010, 7, 10. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Wachowicz, B. Platelet haemostatic function in psychiatric disorders: Effects of antidepressants and antipsychotic drugs. World J. Biol. Psychiatry 2016, 18, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, C.; O’Loughlin, E.; Calcagno, N.; Madore, C.; Butovsky, O. Differential contribution of microglia and monocytes in neurodegenerative diseases. J. Neural Transm. 2017, 125, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Fornaguera-Trías, J.; Sheridan, J.F. Stress-Induced Microglia Activation and Monocyte Trafficking to the Brain Underlie the Development of Anxiety and Depression. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Dantzer, R., Capuron, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 155–172. ISBN 978-3-319-51151-1. [Google Scholar]
- Minogue, A.M. Role of infiltrating monocytes/macrophages in acute and chronic neuroinflammation: Effects on cognition, learning and affective behaviour. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yu, Z.; Sakai, M.; Tomita, H. Linking Activation of Microglia and Peripheral Monocytic Cells To The Patho-physiology Of Psychiatric Disorders. Front Cell Neurosci. 2016, 10, 144. [Google Scholar] [CrossRef]
- Wang, J.-R.; Chen, Z.; Yang, K.; Yang, H.-J.; Tao, W.-Y.; Li, Y.-P.; Jiang, Z.-J.; Bai, C.-F.; Yin, Y.-C.; Duan, J.-M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil Lymphocyte Ratio As A Measure Of Systemic In-flammation In Prevalent Chronic Diseases In Asian Population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 483, 48–56. [Google Scholar] [CrossRef]
- Jung, J.; Lee, E.; Suh, C.; Kim, H. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are associated with disease activity in Polymyalgia Rheumatica. J. Clin. Lab. Anal. 2019, 33, e23000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zulfic, Z.; Weickert, C.; Weickert, T.; Liu, D.; Myles, N.; Galletly, C. Neutrophil–Lymphocyte Ratio—A Simple, Accessible Measure of Inflammation, Morbidity and Prognosis in Psychiatric Disorders? Australas. Psychiatry 2020, 28, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-Q.; Huang, L.-D.; Dai, R.-J.; Chen, N.-D.; Hu, W.-J.; Shan, Y.-F. Neutrophil-lymphocyte ratio: A controversial marker in predicting Crohn’s disease severity. Int. J. Clin. Exp. Pathol. 2015, 8, 14779–14785. [Google Scholar]
- Islas-Vazquez, L.; Aguilar-Cazares, D.; Galicia-Velasco, M.; Rumbo-Nava, U.; Meneses-Flores, M.; Luna-Rivero, C.; Lopez-Gonzalez, J.S. IL-6, NLR, and SII Markers and Their Relation with Alterations in CD8+ T-Lymphocyte Subpopulations in Patients Treated for Lung Adenocarcinoma. Biology 2020, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Kulaksizoglu, B.; Kulaksizoglu, S. Relationship Between Neutrophil/Lymphocyte Ratio with Oxidative Stress and Psycho-pathology in Patients with Schizophrenia. Neuropsychiatr. Dis. Treat. 2016, 12, 1999–2005. [Google Scholar] [CrossRef]
- Jacomb, I.; Stanton, C.; Vasudevan, R.; Powell, H.; O’Donnell, M.; Lenroot, R.; Bruggemann, J.; Balzan, R.; Galletly, C.; Liu, D.; et al. C-Reactive Protein: Higher During Acute Psychotic Episodes and Related to Cortical Thickness in Schizophrenia and Healthy Controls. Front. Immunol. 2018, 9, 2230. [Google Scholar] [CrossRef] [PubMed]
- Mert, D.G.; Terzi, H. Mean platelet volume in bipolar disorder: The search for an ideal biomarker. Neuropsychiatr. Dis. Treat. 2016, 12, 2057–2062. [Google Scholar] [CrossRef]
- Özdin, S.; Sarisoy, G.; Böke, Ö. A Comparison of The Neutrophil-Lymphocyte, Platelet-Lymphocyte and Monocyte-Lymphocyte Ratios in Schizophrenia And Bipolar Disorder Patients—A Retrospective File Review. Nord. J. Psychiatry 2017, 71, 509–512. [Google Scholar] [CrossRef]
- Özdin, S.; Usta, M.B. A comparison of inflammatory markers in manic and euthymic states of bipolar disorder. Nord. J. Psychiatry 2021, 75, 124–129. [Google Scholar] [CrossRef]
- Mayda, H.; Ahsen, A.; Bagcioglu, E.; Ozturk, A.; Bahceci, B.; Soyucok, E.; Baspinar, E.; Ulu, M. Effect of Increased Neutro-phil-To-Lymphocyte Ratio (NLR) And Decreased Mean Platelet Volume (MPV) Values on Inflammation in Acute Mania. Noro Psikiyatr. Ars. 2016, 53, 317–320. [Google Scholar] [CrossRef]
- Kalelioglu, T.; Akkus, M.; Karamustafalioglu, N.; Genc, A.; Genc, E.S.; Cansiz, A.; Emul, M. Neutrophil-lymphocyte and platelet-lymphocyte ratios as inflammation markers for bipolar disorder. Psychiatry Res. 2015, 228, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Inanli, I.; Aydin, M.; Çaliskan, A.M.; Eren, I. Neutrophil/lymphocyte ratio, monocyte/lymphocyte ratio, and mean platelet volume as systemic inflammatory markers in different states of bipolar disorder. Nord. J. Psychiatry 2019, 73, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, M.G.; Cicek, I.E.; Inanli, I.; Caliskan, A.M.; Ercan, S.K.; Eren, I. Neutrophil/lymphocyte and platelet/lymphocyte ratios in all mood states of bipolar disorder. Psychiatry Clin. Psychopharmacol. 2017, 27, 278–282. [Google Scholar] [CrossRef]
- Mazza, M.G.; Lucchi, S.; Tringali, A.G.M.; Rossetti, A.; Botti, E.R.; Clerici, M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 229–236. [Google Scholar] [CrossRef]
- Atli, A.; Demir, S.; Bulut, M.; Okan İbiloğlu, A.; Güneş, M.; Kaya, M.; Demirpençe, Ö.; Sır, A. Neutrophil-Lymphocyte Ratio In Patients With Major Depressive Disorder Undergoing No Pharmacological Therapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2253–2258. [Google Scholar] [CrossRef]
- Kayhan, F.; Gündüz, Ş.; Ersoy, S.A.; Kandeğer, A.; Annagür, B.B. Relationships of neutrophil–lymphocyte and platelet–lymphocyte ratios with the severity of major depression. Psychiatry Res. 2017, 247, 332–335. [Google Scholar] [CrossRef]
- Sunbul, E.A.; Sunbul, M.; Yanartas, O.; Cengiz, F.; Bozbay, M.; Sari, I.; Güleç, H. Increased Neutrophil/Lymphocyte Ratio in Patients with Depression is Correlated with the Severity of Depression and Cardiovascular Risk Factors. Psychiatry Investig. 2016, 13, 121–126. [Google Scholar] [CrossRef]
- Arabska, J.; Łucka, A.; Magierski, R.; Sobów, T.; Wysokiński, A. Neutrophil-lymphocyte ratio is increased in elderly patients with first episode depression, but not in recurrent depression. Psychiatry Res. 2018, 263, 35–40. [Google Scholar] [CrossRef]
- Erdoğan, M.; Erdöl, M.; Öztürk, S.; Durmaz, T. Systemic Immune-Inflammation Index Is A Novel Marker to Predict Func-tionally Significant Coronary Artery Stenosis. Biomark. Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Ruta, V.; Man, A.; Alexescu, T.; Motoc, N.; Tarmure, S.; Ungur, R.; Todea, D.; Coste, S.; Valean, D.; Pop, M. Neutrophil-To-Lymphocyte Ratio and Systemic Immune-Inflammation Index—Biomarkers in Interstitial Lung Disease. Medicina 2020, 56, 381. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, D.; Dai, Z.; Li, X. Association between Systemic Immune-Inflammation Index and Diabetic Depression. Clin. Interv. Aging 2021, 16, 97–105. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, X.; Wang, W. Inflammation and Coronary Heart Disease Risk in Patients with Depression in China Mainland: A Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2020, 16, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Mazza, M.; Cavalli, G.; Ciceri, F.; Dagna, L.; Rovere-Querini, P. Can Cytokine Blocking Prevent Depression in COVID-19 Survivors? J. Neuroimmune Pharmacol. 2021, 16, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef]
- Osimo, E.; Pillinger, T.; Rodriguez, I.; Khandaker, G.; Pariante, C.; Howes, O. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5166 Patients And 5083 Controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, K.; Vinberg, M.; Kessing, L.V. Cytokines in bipolar disorder: A systematic review and meta-analysis. J. Affect. Disord. 2013, 144, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Guo, W.; Feng, Y.; Deng, H.; Li, G.; Nie, H.; Guo, G.; Yu, H.; Ma, Y.; Wang, J.; et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: A systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 21–32. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Lydholm, N.C.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M. Efficacy of Anti-Inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-Analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.; Kakar, R.; Berk, M.; Kessing, L.; Vinberg, M.; Baune, B.; Mansur, R.; Brietzke, E.; Goldstein, B.; McIntyre, R. Anti-Inflammatory Agents in The Treatment of Bipolar Depression: A Systematic Review and Meta-Analysis. Bipolar Disord. 2016, 18, 89–101. [Google Scholar] [CrossRef]
- Rosenblat, J.D. Targeting the immune system in the treatment of bipolar disorder. Psychopharmacology 2019, 236, 2909–2921. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Surace, T.; Vanella, A.; Meo, V.; Patania, F.; Furnari, R.; Signorelli, M.S.; Aguglia, E. The effect of adjunctive nutraceuticals in bipolar disorder: A systematic review of randomized placebo-controlled trials. J. Affect. Disord. 2019, 252, 334–349. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar Disorder and Inflammation. Psychiatr. Clin. North Am. 2016, 39, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Leboyer, M.; Soreca, I.; Scott, J.; Frye, M.; Henry, C.; Tamouza, R.; Kupfer, D. Can Bipolar Disorder Be Viewed as a Multi-System Inflammatory Disease? J. Affect. Disord. 2012, 141, 1–10. [Google Scholar] [CrossRef]
- Dionisie, V.; Filip, G.A.; Manea, M.C.; Manea, M.; Riga, S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: A review of human and rodent research studies. Inflammopharmacology 2021, 29, 75–90. [Google Scholar] [CrossRef]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019, 6, 164–173. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Obuchowiczwa, E.; Goehler, L.; Brzeszcz, J. Depression’s Multiple Comorbidities Explained By (Neuro) Inflammatory and Oxidative & Nitrosative Stress Pathways. Neuroendocr. Lett. 2011, 32, 7–24. [Google Scholar]
- Schaefer, M.; Engelbrechta, M.A.; Gut, O.; Fiebich, B.L.; Bauer, J.; Schmidt, F.; Grunze, H.; Lieb, K. Interferon alpha (IFNα) and psychiatric syndromes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 731–746. [Google Scholar] [CrossRef]
- Mazza, M.; Tringali, A.; Rossetti, A.; Botti, R.; Clerici, M. Cross-Sectional Study of Neutrophil-Lymphocyte, Platelet-Lymphocyte and Monocyte-Lymphocyte Ratios in Mood Disorders. Gen Hosp. Psychiatry 2019, 58, 7–12. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Natale, A.; Amerio, A.; Cimpoesu, P.; Filioli, P.G.; Aguglia, E.; Amore, M.; Serafini, G.; Aguglia, A. Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte and Monocyte-to-Lymphocyte Ratio in Bipolar Disorder. Brain Sci. 2021, 11, 58. [Google Scholar] [CrossRef]
- Fernandes, B.; Steiner, J.; Molendijk, M.; Dodd, S.; Nardin, P.; Gonçalves, C.; Jacka, F.; Köhler, C.; Karmakar, C.; Carvalho, A.; et al. C-Reactive Protein Concentrations Across the Mood Spectrum in Bipolar Disorder: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2016, 3, 1147–1156. [Google Scholar] [CrossRef]
- Rowland, T.; Perry, B.; Upthegrove, R.; Barnes, N.; Chatterjee, J.; Gallacher, D.; Marwaha, S. Neurotrophins, Cytokines, Ox-idative Stress Mediators and Mood State in Bipolar Disorder: Systematic Review and Meta-Analyses. Br. J. Psychiatry 2018, 213, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-M.; Su, T.-P.; Li, C.-T.; Tsai, S.-J.; Chen, M.-H.; Tu, P.-C.; Chiou, W.-F. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disord. 2014, 17, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-M.; Chen, M.-H.; Hsu, J.-W.; Huang, K.-L.; Tu, P.-C.; Chang, W.-C.; Su, T.-P.; Li, C.T.; Lin, W.-C.; Tsai, S.-J. A comparison study of metabolic profiles, immunity, and brain gray matter volumes between patients with bipolar disorder and depressive disorder. J. Neuroinflammation 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Chang, W.-C.; Hsu, J.-W.; Huang, K.-L.; Tu, P.-C.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Bai, Y.-M. Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. J. Affect. Disord. 2019, 245, 8–15. [Google Scholar] [CrossRef]
- Bowden, C.L. A different depression: Clinical distinctions between bipolar and unipolar depression. J. Affect. Disord. 2005, 84, 117–125. [Google Scholar] [CrossRef]
- Gitlin, M.J. Antidepressants in bipolar depression: An enduring controversy. Int. J. Bipolar Disord. 2018, 6, 25. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Gama, C.S.; Kauer-Sant’Anna, M.; Lobato, M.I.; Belmonte-De-Abreu, P.; Kapczinski, F. Serum brain-derived neurotrophic factor in bipolar and unipolar depression: A potential adjunctive tool for differential diagnosis. J. Psychiatr. Res. 2009, 43, 1200–1204. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Supasitthumrong, T.; Teixeira, A.L.; Vieira, E.L.; Gattaz, W.F.; Benseñor, I.M.; Lotufo, P.A.; Lafer, B.; Berk, M.; Carvalho, A.F.; et al. Differences in the immune-inflammatory profiles of unipolar and bipolar depression. J. Affect. Disord. 2020, 262, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, T.; Lee, I.; Lee, S.; Chen, K.; Huang, S.; Yang, Y.; Lu, R.; Chen, P. C-Reactive Protein: A Differential Biomarker For Major Depressive Disorder and Bipolar II Disorder. World J. Biol. Psychiatry 2016, 18, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Zhang, C.; Chen, J.; Zhao, G.; Zhou, R.; Wang, F.; Xu, J.; Yang, T.; Su, Y.; Huang, J.; et al. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J. Affect. Disord. 2018, 237, 65–72. [Google Scholar] [CrossRef]
- Wollenhaupt-Aguiar, B.; Librenza-Garcia, D.; Bristot, G.; Przybylski, L.; Stertz, L.; Burque, R.K.; Ceresér, K.M.; Spanemberg, L.; Caldieraro, M.A.; Frey, B.N.; et al. Differential biomarker signatures in unipolar and bipolar depression: A machine learning approach. Aust. New Zealand J. Psychiatry 2019, 54, 393–401. [Google Scholar] [CrossRef] [PubMed]
| Total Sample (N = 182) | BD Manic Episode (N = 65) | BD Depressive Episode (N = 34) | MDD (N = 83) | p | |
|---|---|---|---|---|---|
| Gender, N (%) | 0.925 1 | ||||
| Males | 84 (46.2) | 31 (47.7) | 16 (47.1) | 37 (44.6) | |
| Females | 98 (53.8) | 34 (52.3) | 18 (52.9) | 46 (56.4) | |
| Age (years), mean ± SD | 44.41 ± 12.29 | 41.25 ± 11.6 | 47.59 ± 12.02 | 45.58 ± 12.45 | 0.0152 |
| Level of education, N (%) | 0.0242 | ||||
| ≤8 years | 38 (20.9) | 7 (10.8) | 7 (20.6) | 24 (28.9) | |
| 9–12 years | 93 (51.1) | 35 (53.8) | 17 (50.0) | 41 (49.4) | |
| >12 years | 51 (28) | 23 (35.4) | 10 (29.4) | 18 (21.7) | |
| Marital status, N (%) | 0.062 2 | ||||
| Single | 68 (37.4) | 35 (53.8) | 10 (29.4) | 23 (27.7) | |
| Married | 62 (34.1) | 15 (23.1) | 12 (35.3) | 35 (42.2) | |
| Divorced | 26 (14.3) | 6 (9.2) | 8 (23.5) | 12 (14.5) | |
| Widowed | 6 (3.3) | 0 | 0 | 6 (7.2) | |
| Domestic Partnership | 20 (11.0) | 9 (13.8) | 4 (11.8) | 7 (8.4) | |
| Smoking status, N (%) | 0.011 1 | ||||
| Yes | 105 (57.7) | 47 (72.3) | 18 (52.9) | 40 (48.2) | |
| No | 77 (42.3) | 18 (27.7) | 16 (47.1) | 43 (51.8) | |
| Psychotic symptoms, N (%) | <0.0011 | ||||
| Yes | 64 (35.2) | 43 (66.2) | 28 (82.4) | 15 (18.1) | |
| No | 118 (64.8) | 22 (33.8) | 6 (17.6) | 68 (81.9) | |
| BMI (kg/m2), mean ± SD | 24.92 ± 2.86 | 25.17 ± 2.56 | 25.47 ± 2.25 | 24.51 ± 3.25 | 0.449 2 |
| Total Sample | BD Manic Episode | BD Depressive Episode | MDD | p | |
|---|---|---|---|---|---|
| White blood cells (103 cells/mm3) | 7.5 ± 1.87 | 8.13 ± 2 | 7.38 ± 1.78 | 7.05 ± 1.66 | 0.0021 |
| Neutrophils (103 cells/mm3) | 4.34 ± 1.61 | 5.13 ± 1.63 | 4.40 ± 1.68 | 3.68 ± 1.24 | 0.0002 |
| Lymphocytes (103 cells/mm3) | 2.38 ± 0.73 | 2.25 ± 0.67 | 2.24 ± 0.63 | 2.54 ± 0.77 | 0.0222 |
| Monocytes (103 cells/mm3) | 0.61 ± 0.17 | 0.64 ± 0.18 | 0.57 ± 0.13 | 0.61 ± 0.18 | 0.072 2 |
| Platelets (103 cells/mm3) | 264.76 ± 59.05 | 264.65 ± 52.26 | 255.35 ± 52.93 | 268.7 ± 65.63 | 0.683 2 |
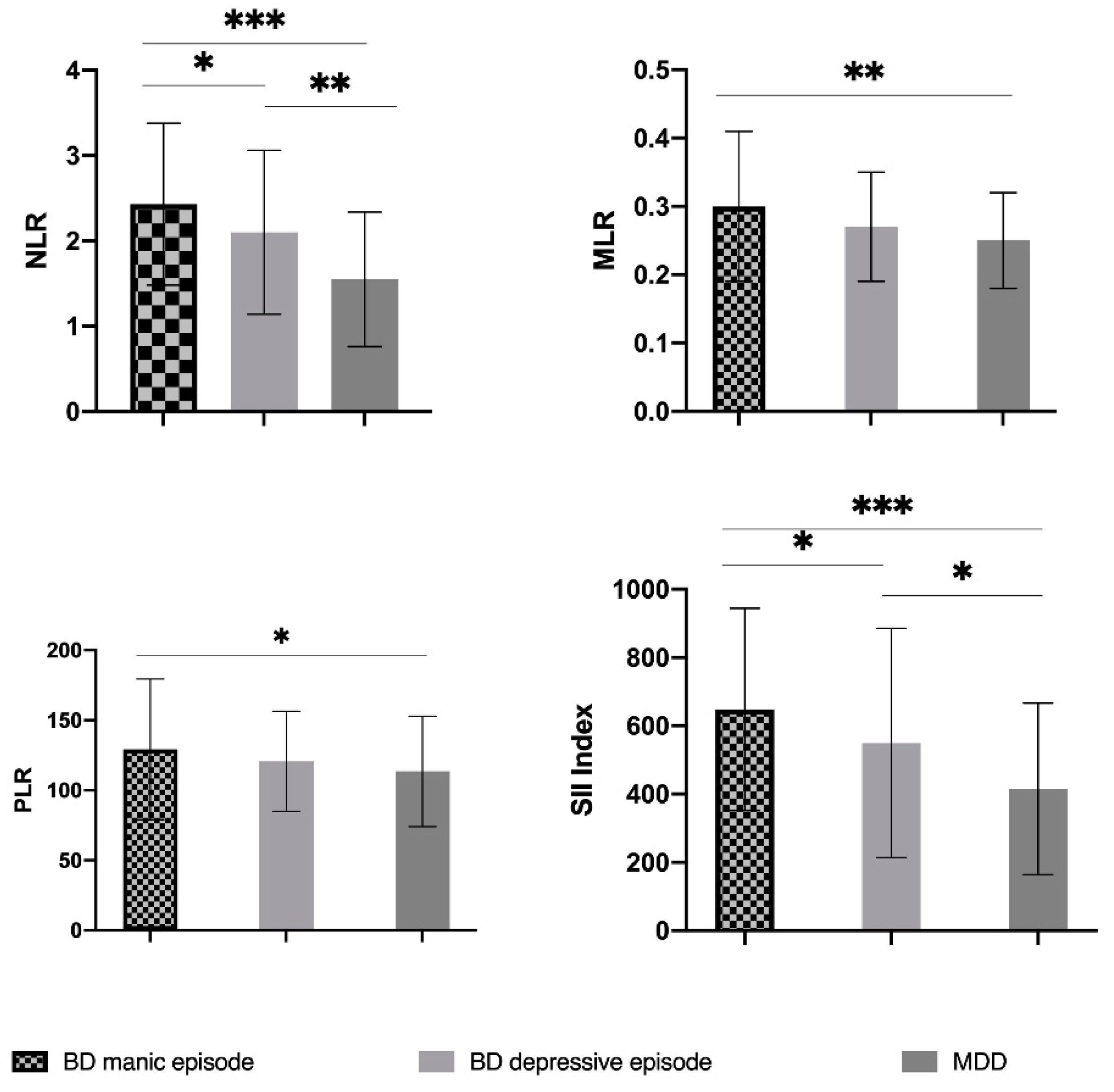
| NLR | 1.97 ± 0.97 | 2.43 ± 0.95 | 2.10 ± 0.96 | 1.55 ± 0.79 | 0.0002 |
| PLR | 120.47 ± 43.56 | 129.21 ± 50.19 | 120.78 ± 35.66 | 113.5 ± 39.47 | 0.068 2 |
| MLR | 0.27 ± 0.09 | 0.30 ± 0.11 | 0.27 ± 0.08 | 0.25 ± 0.07 | 0.0032 |
| SII Index | 523.53 ± 303.58 | 648.21 ± 295.79 | 549.40 ± 335.78 | 415.13 ± 251.45 | 0.0002 |
| Glycemia (mg/dL) | 92.03 ± 11.78 | 91.58 ± 12.16 | 94.5 ± 12.32 | 91.06 ± 11.41 | 0.158 1 |
| Total Cholesterol (mg/dL) | 189.42 ± 45.80 | 177.18 ± 47.49 | 193.32 ± 49.30 | 197.40 ± 41.26 | 0.0072 |
| Triglycerides (mg/dL) | 131.0 ± 79.90 | 138.40 ± 101.95 | 135.68 ± 78.41 | 123.29 ± 58.25 | 0.919 2 |
| BD Mania vs. BD Depression | BD Mania vs. MDD | BD Depression vs. MDD | |
|---|---|---|---|
| White blood cells (103 cells/mm3) 1 | 0.072 | <0.001 | 0.344 |
| Neutrophils (103 cells/mm3) 2 | 0.009 | <0.001 | 0.038 |
| Lymphocytes (103 cells/mm3) 2 | 0.754 | 0.030 | 0.016 |
| Monocytes (103 cells/mm3) 2 | 0.023 | 0.126 | 0.368 |
| Platelets (103 cells/mm3) 2 | 0.470 | 0.951 | 0.391 |
| NLR 2 | 0.047 | <0.001 | 0.001 |
| PLR 2 | 0.635 | 0.034 | 0.113 |
| MLR 2 | 0.095 | 0.001 | 0.368 |
| SII index 2 | 0.033 | <0.001 | 0.015 |
| Binary logistic regression: NLR Univariate analysis, R2 Nagelkerke = 0.108, p = 0.002 | ||||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for Exp(B) | ||
| Lower | Upper | |||||||
| 0.778 | 0.301 | 6.692 | 1 | 0.010 | 2.178 | 1.208 | 3.927 | |
| Binary logistic regression: SII Index Univariate analysis, R2 Nagelkerke = 0.059, p = 0.026 | ||||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for Exp(B) | ||
| Lower | Upper | |||||||
| SII Index | 0.002 | 0.001 | 3.950 | 1 | 0.047 | 1.002 | 1.000 | 1.003 |
| Binary logistic regression: Multivariate analysis, Model 2, R2 Nagelkerke = 0.235, p = 0.021, Method: Enter | ||||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for Exp(B) | ||
| Lower | Upper | |||||||
| NLR | 0.914 | 0.328 | 7.780 | 1 | 0.005 | 2.495 | 1.312 | 4.744 |
| Age | 0.032 | 0.022 | 2.204 | 1 | 0.138 | 1.033 | 0.990 | 1.078 |
| Gender | 0.077 | 0.478 | 0.026 | 1 | 0.872 | 1.080 | 0.423 | 2.753 |
| BMI | 0.172 | 0.092 | 3.520 | 1 | 0.061 | 1.188 | 0.992 | 1.421 |
| Smoking status | −0.350 | 0.482 | 0.527 | 1 | 0.468 | 0.705 | 0.274 | 1.812 |
| Glycemia | 0.019 | 0.020 | 0.926 | 1 | 0.336 | 1.020 | 0.980 | 1.061 |
| Total Cholesterol | −0.014 | 0.006 | 4.552 | 1 | 0.033 | 0.987 | 0.974 | .999 |
| Triglycerides | 0.005 | 0.004 | 1.597 | 1 | 0.206 | 1.005 | 0.997 | 1.012 |
| Level of education | 0.601 | 0.341 | 3.104 | 1 | 0.078 | 1.824 | 0.935 | 3.559 |
| Marital status | 0.141 | 0.199 | 0.503 | 1 | 0.478 | 1.151 | 0.780 | 1.699 |
| Constant | −9.503 | 3.461 | 7.540 | 1 | 0.006 | 0.000 | ||
| Binary logistic regression: Multivariate analysis, Model 3, R2 Nagelkerke = 0.248, p = 0.022, Method: Enter | ||||||||
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for Exp(B) | ||
| Lower | Upper | |||||||
| NLR | 1.670 | 0.768 | 4.730 | 1 | 0.030 | 5.311 | 1.179 | 23.919 |
| SII Index | −0.003 | 0.002 | 1.221 | 1 | 0.269 | 0.997 | 0.993 | 1.002 |
| Age | 0.035 | 0.022 | 2.455 | 1 | 0.117 | 1.035 | 0.991 | 1.081 |
| Gender | 0.112 | 0.482 | 0.054 | 1 | 0.816 | 1.119 | 0.435 | 2.877 |
| Smoking status | −0.202 | 0.502 | 0.162 | 1 | 0.687 | 0.817 | 0.306 | 2.184 |
| BMI | 0.171 | 0.093 | 3.423 | 1 | 0.064 | 1.187 | 0.990 | 1.423 |
| Glycemia | 0.024 | 0.021 | 1.342 | 1 | 0.247 | 1.025 | 0.983 | 1.068 |
| Total Cholesterol | −0.012 | 0.006 | 3.630 | 1 | 0.057 | 0.988 | 0.975 | 1.000 |
| Triglycerides | 0.004 | 0.004 | 1.094 | 1 | 0.295 | 1.004 | 0.996 | 1.012 |
| Level of Education | 0.544 | 0.345 | 2.493 | 1 | 0.114 | 1.723 | 0.877 | 3.385 |
| Marital status | 0.162 | 0.202 | 0.648 | 1 | 0.421 | 1.176 | 0.792 | 1.747 |
| Constant | −10.33 | 3.607 | 8.206 | 1 | 0.004 | 0.000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionisie, V.; Filip, G.A.; Manea, M.C.; Movileanu, R.C.; Moisa, E.; Manea, M.; Riga, S.; Ciobanu, A.M. Neutrophil-to-Lymphocyte Ratio, a Novel Inflammatory Marker, as a Predictor of Bipolar Type in Depressed Patients: A Quest for Biological Markers. J. Clin. Med. 2021, 10, 1924. https://doi.org/10.3390/jcm10091924
Dionisie V, Filip GA, Manea MC, Movileanu RC, Moisa E, Manea M, Riga S, Ciobanu AM. Neutrophil-to-Lymphocyte Ratio, a Novel Inflammatory Marker, as a Predictor of Bipolar Type in Depressed Patients: A Quest for Biological Markers. Journal of Clinical Medicine. 2021; 10(9):1924. https://doi.org/10.3390/jcm10091924
Chicago/Turabian StyleDionisie, Vlad, Gabriela Adriana Filip, Mihnea Costin Manea, Robert Constantin Movileanu, Emanuel Moisa, Mirela Manea, Sorin Riga, and Adela Magdalena Ciobanu. 2021. "Neutrophil-to-Lymphocyte Ratio, a Novel Inflammatory Marker, as a Predictor of Bipolar Type in Depressed Patients: A Quest for Biological Markers" Journal of Clinical Medicine 10, no. 9: 1924. https://doi.org/10.3390/jcm10091924
APA StyleDionisie, V., Filip, G. A., Manea, M. C., Movileanu, R. C., Moisa, E., Manea, M., Riga, S., & Ciobanu, A. M. (2021). Neutrophil-to-Lymphocyte Ratio, a Novel Inflammatory Marker, as a Predictor of Bipolar Type in Depressed Patients: A Quest for Biological Markers. Journal of Clinical Medicine, 10(9), 1924. https://doi.org/10.3390/jcm10091924









