Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
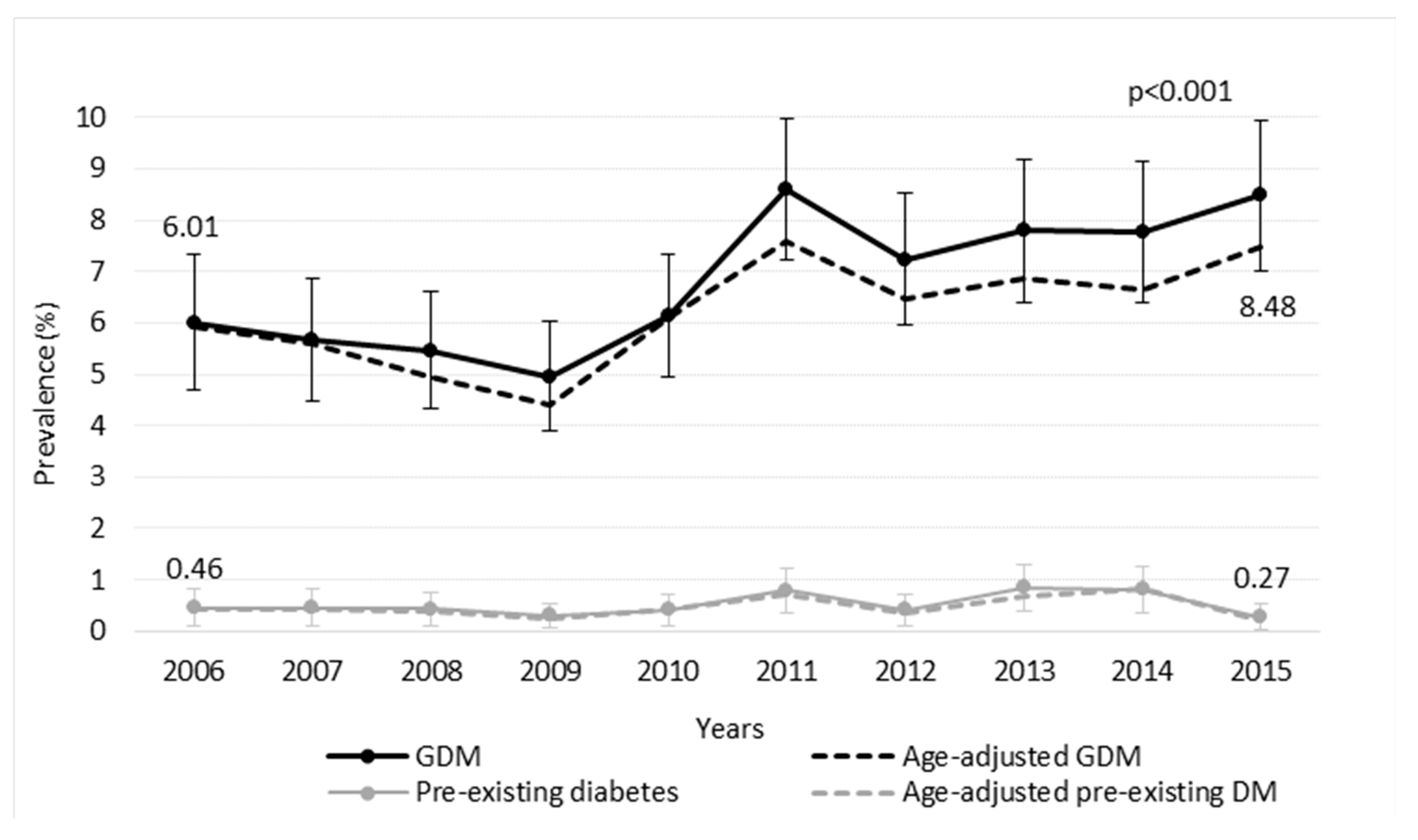
3. Results
3.1. Pregnancy Outcomes of Twin Pregnancies According to Glycaemic Status
3.2. Interaction between Twin Gestations and Glycaemic Status in Relation to Adverse Perinatal Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eades, C.E.; Cameron, D.M.; Evans, J.M.M. Prevalence of gestational diabetes mellitus in Europe: A meta-analysis. Diabetes Res. Clin. Pract. 2017, 129, 173–181. [Google Scholar] [CrossRef]
- Buckley, B.S.; Harreiter, J.; Damm, P.; Corcoy, R.; Chico, A.; Simmons, D.; Vellinga, A.; Dunne, F. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet. Med. 2012, 29, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Gortazar, L.; Flores-Le Roux, J.A.; Benaiges, D.; Sarsanedas, E.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Goday, A. Trends in prevalence of gestational diabetes and perinatal outcomes in Catalonia, Spain, 2006 to 2015: The Diagestcat Study. Diabetes Metab. Res. Rev. 2019, 35, e3151. [Google Scholar] [CrossRef]
- Gortazar, L.; Goday, A.; Flores-Le Roux, J.A.; Sarsanedas, E.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Benaiges, D. Trends in prevalence of pre-existing diabetes and perinatal outcomes: A large, population-based study in Catalonia, Spain, 2006–2015. BMJ Open Diabetes Res. Care 2020, 8, e001254. [Google Scholar] [CrossRef] [PubMed]
- Mackin, S.T.; Nelson, S.M.; Kerssens, J.J.; Wood, R.; Wild, S.; Colhoun, H.M.; Leese, G.P.; Philip, S.; Lindsay, R.S. Diabetes and pregnancy: National trends over a 15 Year Period. Diabetologia 2018, 61, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30 (Suppl. 2), S141–S146. [Google Scholar] [CrossRef]
- Getahun, D.; Nath, C.; Ananth, C.V.; Chavez, M.R.; Smulian, J.C. Gestational diabetes in the United States: Temporal trends 1989 through 2004. Am. J. Obstet. Gynecol. 2008, 198, 525.e1–525.e5. [Google Scholar] [CrossRef]
- Bell, R.; Bailey, K.; Cresswell, T.; Hawthorne, G.; Critchley, J.; Lewis-Barned, N. Trends in prevalence and outcomes of pregnancy in women with pre-existing type I and type II diabetes. BJOG 2008, 115, 445–452. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.M.; Damm, P. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Jovanovič, L.; Liang, Y.; Weng, W.; Hamilton, M.; Chen, L.; Wintfeld, N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes Metab. Res. Rev. 2015, 31, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Chauhan, S.P. Epidemiology of twinning in developed countries. Semin. Perinatol. 2012, 36, 156–161. [Google Scholar] [CrossRef]
- Hiersch, L.; Berger, H.; Okby, R.; Ray, J.G.; Geary, M.; McDonald, S.D.; Murray-Davis, B.; Riddell, C.; Halperin, I.; Hasan, H.; et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am. J. Obstet. Gynecol. 2019, 220, 102.e1–102.e8. [Google Scholar] [CrossRef]
- Institut d’Estadistica de Catalunya. IDESCAT. Available online: http://www.idescat.net (accessed on 1 December 2020).
- GEDE (Grupo Español de Diabetes y Embarazo). Asistencia a la gestante con diabetes. Guía de práctica clínica actualizada En 2014. Av. Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- Departament de Salut. Corbes de Referencia de Pes, Perímetre Cranial i Longitud en Néixer de Nounats D’embarassos Únics, de Bessons i de Trigèmins a Catalunya; Departament de Salut: Barcelona, Spain, 2008. [Google Scholar]
- González-González, N.L.; Medina, V.; Ruano, A.; Perales, A.; Pérez-Mendaña, J.M.; Melchor, J.C. Base de Datos Perinatales Nacionales 2004 (National Perinatal Data Sets 2004). Prog. Obstet. Ginecol. 2005, 49, 1084–1089. [Google Scholar]
- Lai, F.Y.; Johnson, J.A.; Dover, D.; Kaul, P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: A population-based study in Alberta, Canada, 2005–2011. J. Diabetes 2016, 8, 45–55. [Google Scholar] [CrossRef]
- Okby, R.; Weintraub, A.Y.; Sergienko, R.; Eyal, S. Gestational diabetes mellitus in twin pregnancies is not associated with adverse perinatal outcomes. Arch. Gynecol. Obstet. 2014, 290, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, A.R.S.; Jernman, R.M.; Gissler, M.; Nupponen, I.; Nuutila, M.E. Maternal complications in twin pregnancies in Finland during 1987–2014: A retrospective study. BMC Pregnancy Childbirth 2019, 19, 337. [Google Scholar] [CrossRef]
- Lambalk, C.B.; Boomsma, D.I.; de Boer, L.; de Koning, C.H.; Schoute, E.; Popp-Snijders, C.; Schoemaker, J. Increased levels and pulsatility of follicle-stimulating hormone in mothers of hereditary dizygotic twins. JCEM 1998, 83, 481–486. [Google Scholar] [CrossRef]
- Comisión Nacional de Reproducción Humana Asistida. Ministerio de Sanidad. Registro Nacional de Actividad y Resultados de los Centros y Servicios de Reproducción Humana Asistida. Available online: https://cnrha.sanidad.gob.es/registros/actividades.htm (accessed on 5 December 2020).
- Fox, N.S.; Gerber, R.S.; Saltzman, D.H.; Gupta, S.; Fishman, A.Y.; Klauser, C.K.; Rebarber, A. Glycemic control in twin pregnancies with gestational diabetes: Are we improving or worsening outcomes? J. Matern. Fetal Neonatal Med. 2016, 29, 1041–1045. [Google Scholar] [CrossRef]
- Dinham, G.K.; Henry, A.; Lowe, S.A.; Nassar, N.; Lui, K.; Spear, V.; Shand, A.W. Twin pregnancies complicated by gestational diabetes mellitus: A single centre cohort study. Diabet. Med. 2016, 33, 1659–1667. [Google Scholar] [CrossRef]
- McGrath, R.T.; Hocking, S.L.; Scott, E.S.; Seeho, S.K.; Fulcher, G.R.; Glastras, S.J. Outcomes of twin pregnancies complicated by gestational diabetes: A meta-analysis of observational studies. J. Perinatol. 2017, 37, 360–368. [Google Scholar] [CrossRef]
- Simoes, T.; Queiros, A.; Correia, L.; Rocha, T.; Dias, E.; Blickstein, I. Gestational diabetes mellitus complicating twin pregnancies. J. Perinat. Med. 2011, 39, 437–440. [Google Scholar] [CrossRef] [PubMed]
- González, N.L.G.; Goya, M.; Bellart, J.; Lopez, J.; Sancho, M.A.; Mozas, J.; Medina, V.; Padrón, E.; Megia, A.; Pintado, P.; et al. Obstetric and perinatal outcome in women with twin pregnancy and gestational diabetes. J. Matern. Fetal Neonatal Med. 2012, 25, 1084–1089. [Google Scholar] [CrossRef]
- Schwartz, D.B.; Daoud, Y.; Zazula, P.; Goyert, G.; Bronsteen, R.; Wright, D.; Copes, J. Gestational diabetes mellitus: Metabolic and blood glucose parameters in singleton versus twin pregnancies. Am. J. Obstet. Gynecol. 1999, 181, 912–914. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shah, B.R. Impact of twin gestation and fetal sex on maternal risk of diabetes during and after pregnancy. Diabetes Care 2016, 39, e110–e111. [Google Scholar] [CrossRef]
- Luo, Z.C.; Simonet, F.; Wei, S.Q.; Xu, H.; Rey, E.; Fraser, W.D. Diabetes in pregnancy may differentially affect neonatal outcomes for twins and singletons. Diabet. Med. 2011, 28, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Corrado, F.; Caputo, F.; Facciola, G.; Mancuso, A. Gestational glucose intolerance in multiple pregnancy. Diabetes Care 2003, 26, 1646. [Google Scholar] [CrossRef][Green Version]
- Buhling, K.J.; Henrich, W.; Starr, E.; Lubke, M.; Bertram, S.; Siebert, G.; Dudenhausen, J.W. Risk for gestational diabetes and hypertension for women with twin pregnancy compared to singleton pregnancy. Arch. Gynecol. Obstet. 2003, 269, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Rauh-Hain, J.A.; Rana, S.; Tamez, H.; Wang, A.; Cohen, B.; Cohen, A.; Brown, F.; Ecker, J.L.; Karumanchi, S.A.; Thadhani, R. Risk for developing gestational diabetes in women with twin pregnancies. J. Matern. Fetal Neonatal Med. 2009, 22, 293–299. [Google Scholar] [CrossRef]
- Yamashita, H.; Shao, J.; Friedman, J.E. Physiologic and molecular alterations in carbohydrate metabolism during pregnancy and gestational diabetes mellitus. Clin. Obstet. Gynecol. 2000, 43, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sivan, E.; Maman, E.; Homko, C.J.; Lipitz, S.; Cohen, S.; Schiff, E. Impact of fetal reduction on the incidence of gestational diabetes. Obstet. Gynecol. 2002, 99, 91–94. [Google Scholar] [CrossRef] [PubMed]
- González González, N.L.; González Dávila, E.; Goya, M.; Vega, B.; Hernández Suarez, M.; Bartha, J.L. Twin pregnancy among women with pregestational type 1 or type 2 diabetes mellitus. Int. J. Gynecol. Obstet. 2014, 126, 83–87. [Google Scholar] [CrossRef]
- Mourad, M.; Too, G.; Gyamfi-Bannerman, C.; Zork, N. Hypertensive disorders of pregnancy in twin gestations complicated by gestational diabetes. J. Matern. Fetal Neonatal Med. 2019, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.A.; Herranz, L.; Barquiel, B.; Hillman, N.; Burgos, M.A.; Pallardo, L.F. Influence of gestational diabetes mellitus on neonatal weight outcome in twin pregnancies. Diabet. Med. 2014, 31, 1651–1656. [Google Scholar] [CrossRef]
- Darke, J.; Glinianaia, S.V.; Marsden, P.; Bell, R. Pregestational diabetes is associated with adverse outcomes in twin pregnancies: A regional register-based study. Acta Obstet. Gynecol. Scand. 2016, 95, 339–346. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef] [PubMed]

| Normoglycaemic n= 14,785 | GDM n = 1088 | OR, GDM vs. Non-DM | Pre-Existing DM n = 83 | OR, Pre-Existing DM vs. Non-DM | ANOVA p Value | |
|---|---|---|---|---|---|---|
| Age (years) | 33.6 ± 5.1 | 35.3± 5.0 | 35.1 ± 6.1 | p < 0.001 | ||
| Chronic hypertension, n (%) | 127 (0.86) | 18 (1.65) | 1.81 (1.10–2.99) p = 0.020 | 3 (3.61) | 4.09 (1.27–13.14) p = 0.018 | |
| Dyslipidaemia, n (%) | 26 (0.18) | 2 (0.18) | 1.05 (0.25–4.44) p = 0.949 | 1 (1.20) | 7.06 (0.94–52.78) p = 0.057 | |
| Smoking, n (%) | 583 (3.94) | 54 (4.96) | 1.40 (1.05–1.87) p = 0.002 | 3 (3.61) | 0.98 (0.31–3.11) p = 0.969 | |
| Pre-eclampsia, n (%) | 1116 (7.55) | 124 (11.39) | 1.43 (1.17–1.74) p < 0.001 | 13 (15.66) | 2.04 (1.12–3.72) p = 0.020 | |
| Preterm birth, n (%) | 7373 (51.84) | 566 (53.04) | 1.06 (0.94–1.21) p = 0.327 | 55 (69.62) | 2.15 (1.33–3.48) p = 0.002 | |
| Caesarean section, n (%) | 10,072 (68.12) | 714 (65.63) | 0.82 (0.71–0.93) p = 0.002 | 61 (73.49) | 1.20 (0.73–1.97) p = 0.468 | |
| Stillbirth, n (%) | 473 (3.20) | 26 (2.39) | 0.76 (0.51–1.14) p = 0.189 | 2 (2.41) | 0.77 (0.18–3.31) p = 0.712 | |
| Macrosomia, n (%) | 22 (0.08) | 1 (0.05) | 0.595 (0.08–4.43) p = 0.612 | 0 (0) | 0 p = 0.966 | |
| LGA (>90th), n (%) | 3247 (11.63) | 273 (12.95) | 1.12 (0.98–1.28) p = 0.09 | 33 (20.89) | 1.98 (1.35–2.91) p = 0.001 | |
| SGA (<10th), n (%) | 2339 (8.37) | 202 (9.58) | 1.15 (0.99–1.34) p = 0.078 | 8 (5.06) | 0.58 (0.28–1.19) p = 0.134 | |
| Mean birth weight (g) | 2333 ± 503 | 2336 ± 486 | 2295 ± 616 | p = 0.304 |
| Twins vs. Singleton | Gestational Diabetes | Pre-Existing Diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B Coefficient Normoglycaemic Pregnancies p Value | OR Twins vs. Singleton p Value | B Coefficient Singleton Pregnancies p Value | OR GDM vs. Non-DM (Singleton) p Value | OR GDM vs. Non-DM (Twins) p Value | Interaction GDM and Twins B Coefficient p Value | Beta Coefficient Singleton Pregnancies p Value | OR Pre-Existing DM vs. Non-DM (Singleton) p Value | OR Pre-Existing DM vs. Non-DM (Twins) p Value | Interaction Pre-Existing DM and Twins B Coefficient p Value | |
| Pre-eclampsia | 1.68 p < 0.001 | 5.38 (5.04–5.74) p < 0.001 | 0.532 p < 0.001 | 1.70 (1.59–1.82) p < 0.001 | 1.43 (1.17–1.74) p < 0.001 | −0.12 p = 0.275 | 1.37 p < 0.001 | 3.95 (3.44–4.51) p < 0.001 | 2.04 (1.12–3.72) p = 0.020 | −0.63 p = 0.045 |
| Preterm birth | 2.82 p < 0.001 | 16.8 (16.26–17.43) p < 0.001 | 0.18 p < 0.001 | 1.20 (1.15–1.25) p < 0.001 | 1.06 (0.94–1.21) p = 0.327 | −0.15 p = 0.028 | 1.15 p < 0.001 | 3.17 (2.91-–3.44) p < 0.001 | 2.15 (1.33–3.48) p = 0.002 | −0.429 p = 0.085 |
| Caesarean section | 1.73 p < 0.001 | 5.64 (5.44–5.84) p < 0.001 | 0.086 p < 0.001 | 1.09 (1.06–1.12) p < 0.001 | 0.82 (0.71–0.93) p = 0.002 | −0.30 p < 0.001 | 0.791 p < 0.001 | 2.20 (2.07–2.35) p < 0.001 | 1.20 (0.73–1.97) p = 0.468 | −0.621 p = 0.015 |
| Stillbirth | 1.92 p < 0.001 | 6.85 (6.21–7.57) p < 0.001 | −0.305 p = 0.001 | 0.74 (0.62–0.88) p < 0.001 | 0.76 (0.51–1.14) p = 0.189 | −0.025 p = 0.912 | 0.73 p < 0.001 | 2.08 (1.52–2.83) p < 0.001 | 0.77 (0.18–3.31) p = 0.712 | −1.072 p = 0.145 |
| Macrosomia | −4.406 p = 0.012 | 0.012 (0.008–0.019) p < 0.001 | 0.414 p < 0.001 | 1.51 (1.45–1.57) p < 0.001 | 0.595 (0.08–4.43) p = 0.612 | −0.905 p = 0.376 | 0.99 p < 0.001 | 2.68 (2.45–2.94) p < 0.001 | 0 p = 0.966 | −15.037 p = 0.996 |
| LGA | −0.14 p < 0.001 | 0.87 (0.84–0.90) p < 0.001 | 0.43 p < 0.001 | 1.53 (1.49–1.58) p < 0.001 | 1.12 (0.98–1.28) p = 0.09 | −0.302 p < 0.001 | 1.31 p < 0.001 | 3.69 (3.46–3.95) p < 0.001 | 1.98 (1.35–2.91) p = 0.001 | −0.610 p = 0.002 |
| SGA | −0.07 p = 0.001 | 0.93 (0.89–0.97) p < 0.001 | −0.137 p < 0.001 | 0.87 (0.83–0.91) p < 0.001 | 1.15 (0.99–1.34) p = 0.078 | 0.27 p = 0.001 | −0.55 p < 0.001 | 0.58 (0.50–0.60) p < 0.001 | 0.58 (0.28–1.19) p = 0.134 | −0.01 p = 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gortazar, L.; Flores-Le Roux, J.A.; Benaiges, D.; Sarsanedas, E.; Navarro, H.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Goday, A. Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study. J. Clin. Med. 2021, 10, 1937. https://doi.org/10.3390/jcm10091937
Gortazar L, Flores-Le Roux JA, Benaiges D, Sarsanedas E, Navarro H, Payà A, Mañé L, Pedro-Botet J, Goday A. Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study. Journal of Clinical Medicine. 2021; 10(9):1937. https://doi.org/10.3390/jcm10091937
Chicago/Turabian StyleGortazar, Lucia, Juana Antonia Flores-Le Roux, David Benaiges, Eugènia Sarsanedas, Humberto Navarro, Antonio Payà, Laura Mañé, Juan Pedro-Botet, and Albert Goday. 2021. "Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study" Journal of Clinical Medicine 10, no. 9: 1937. https://doi.org/10.3390/jcm10091937
APA StyleGortazar, L., Flores-Le Roux, J. A., Benaiges, D., Sarsanedas, E., Navarro, H., Payà, A., Mañé, L., Pedro-Botet, J., & Goday, A. (2021). Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study. Journal of Clinical Medicine, 10(9), 1937. https://doi.org/10.3390/jcm10091937







