The Phenolic Antioxidant 3,5-dihydroxy-4-methoxybenzyl Alcohol (DHMBA) Prevents Enterocyte Cell Death under Oxygen-Dissolving Cold Conditions through Polyphyletic Antioxidant Actions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cold Preservation and Subsequent Rewarming
2.4. Experimental Protocol
2.4.1. Protocol 1
2.4.2. Protocol 2
2.5. Evaluations
2.5.1. Viability and Injury
2.5.2. Lactate Dehydrogenase (LDH) Assay
2.5.3. Mitochondrial Membrane Potential
2.5.4. Lipid Peroxidation Assay
2.5.5. Cell Survival and Death
2.5.6. Western Blotting
2.6. Statistics
3. Results
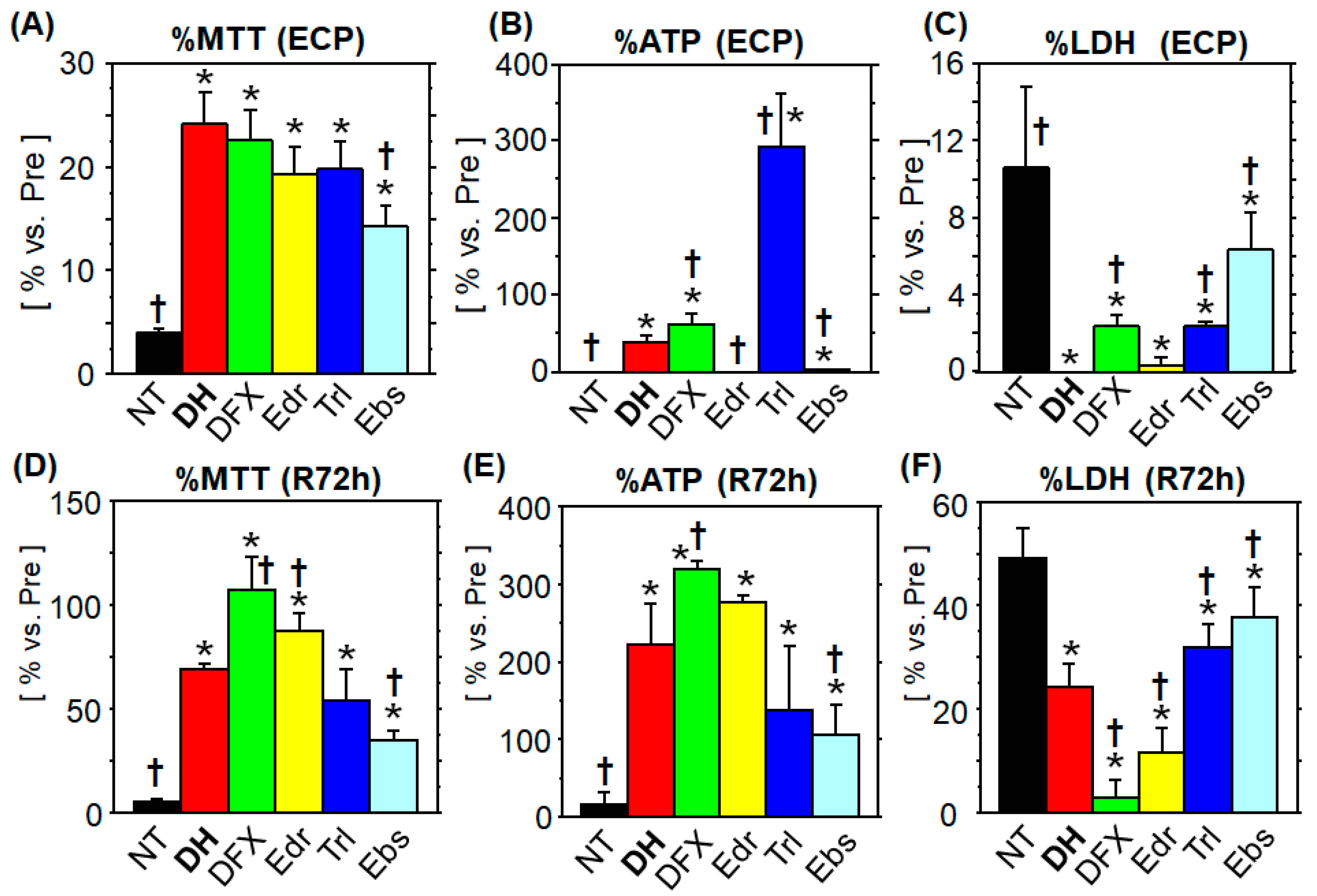
3.1. Comparison of the Cytoprotective Ability
3.1.1. MTT Assay
3.1.2. ATP Content
3.1.3. LDH Leakage
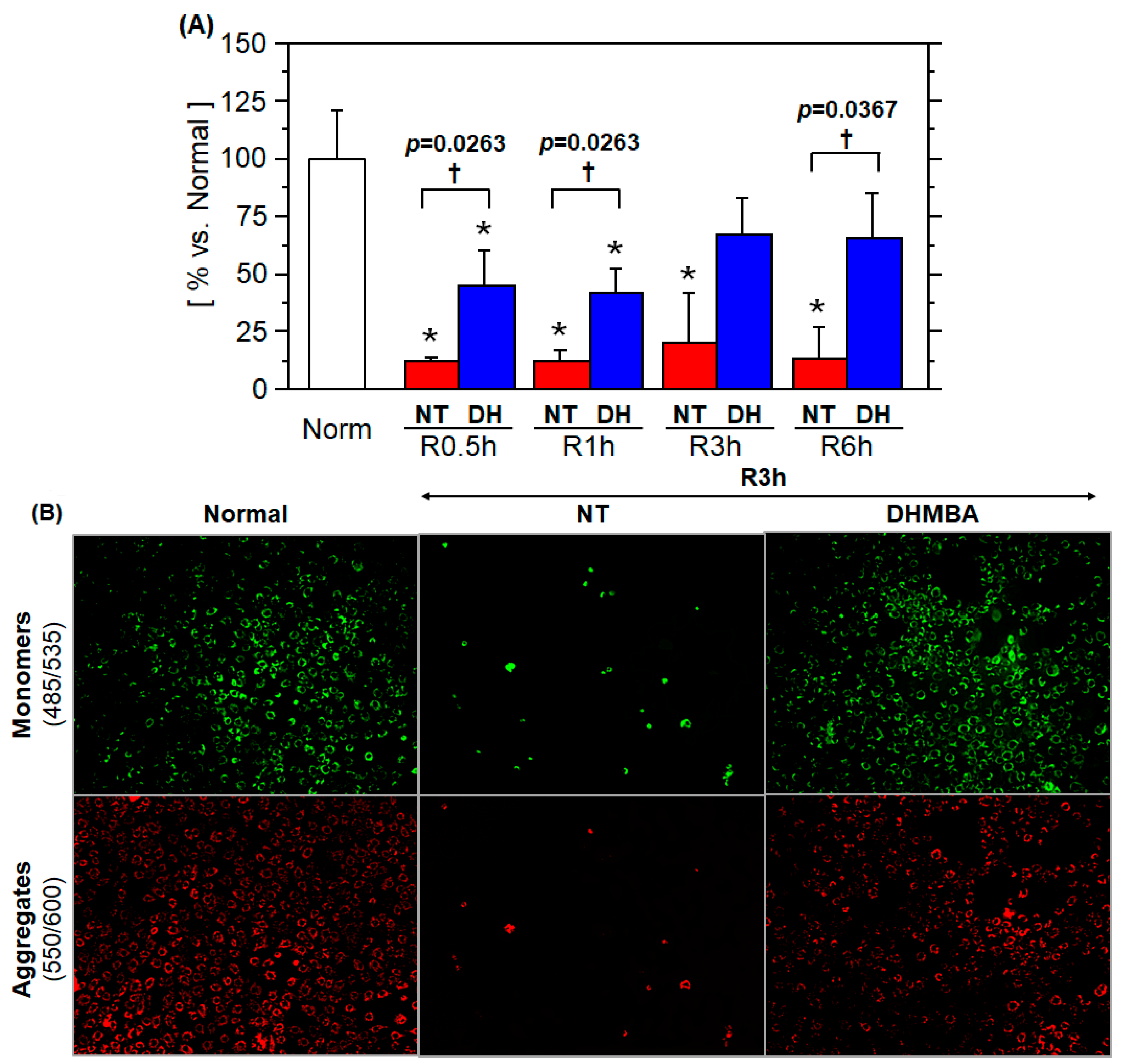
3.2. Mitochondrial Membrane Potential
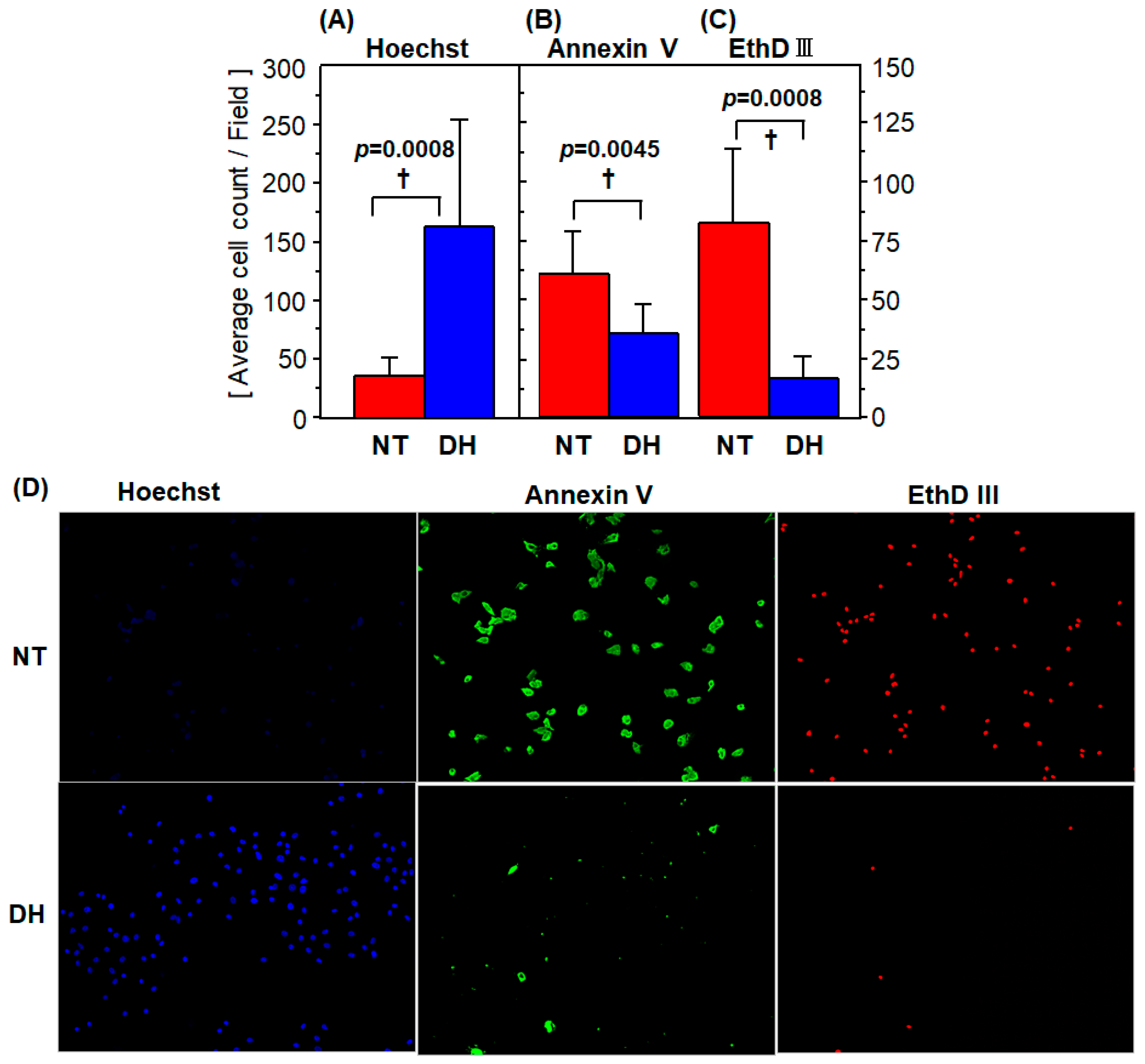
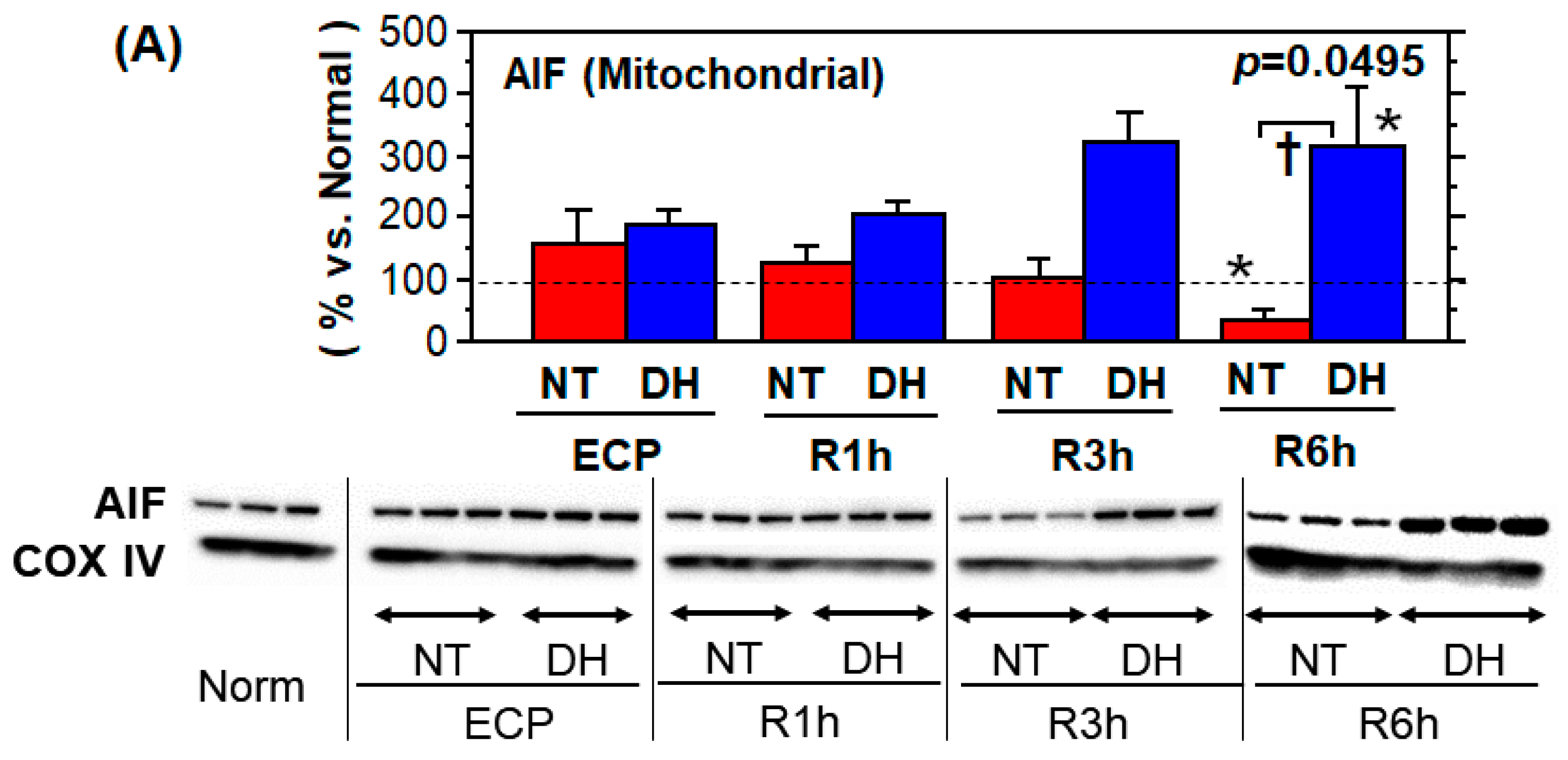
3.3. Cell Survival and Death
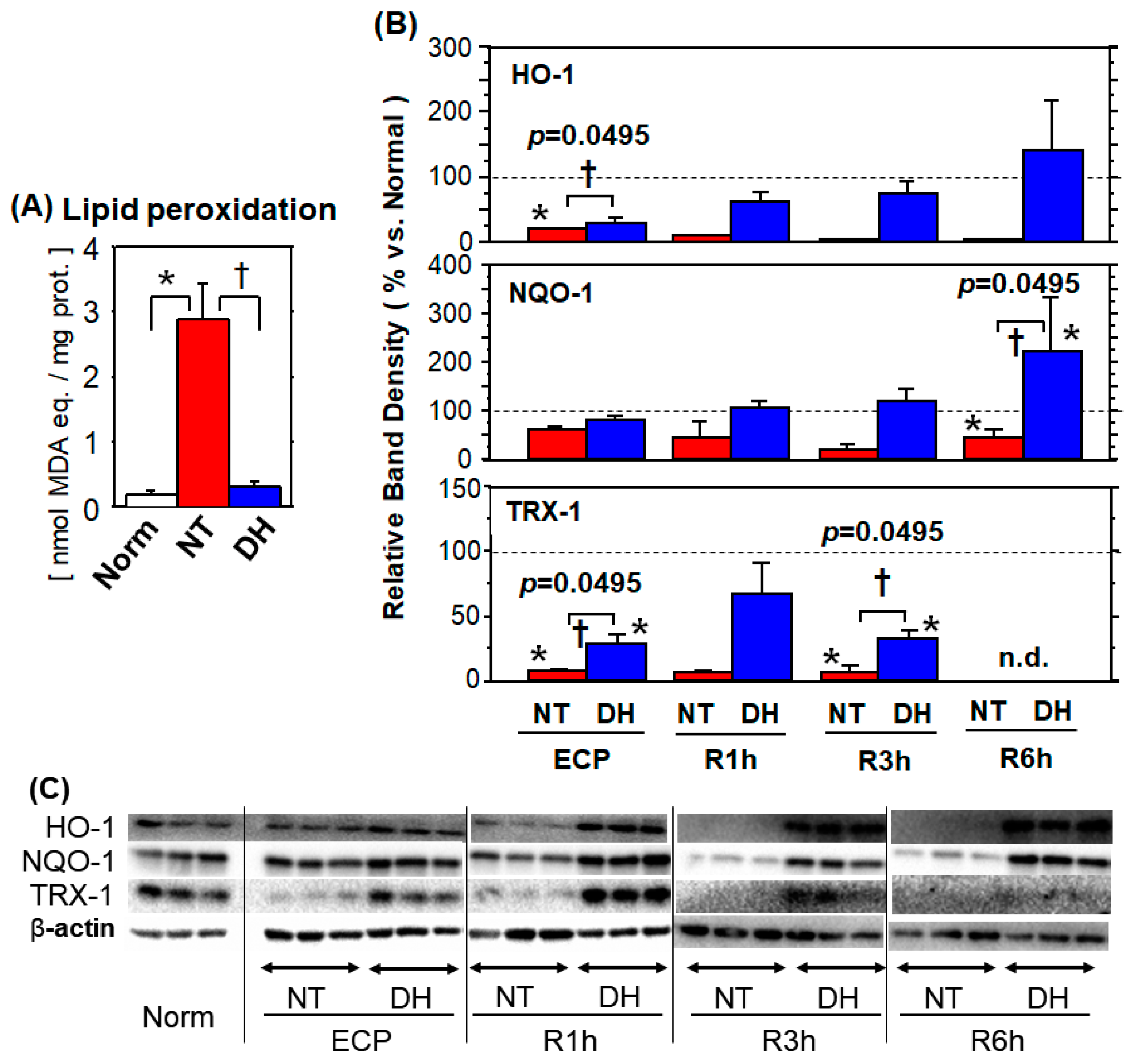
3.4. Oxidative Stress
3.4.1. Lipid Peroxidation
3.4.2. Antioxidant Enzymes
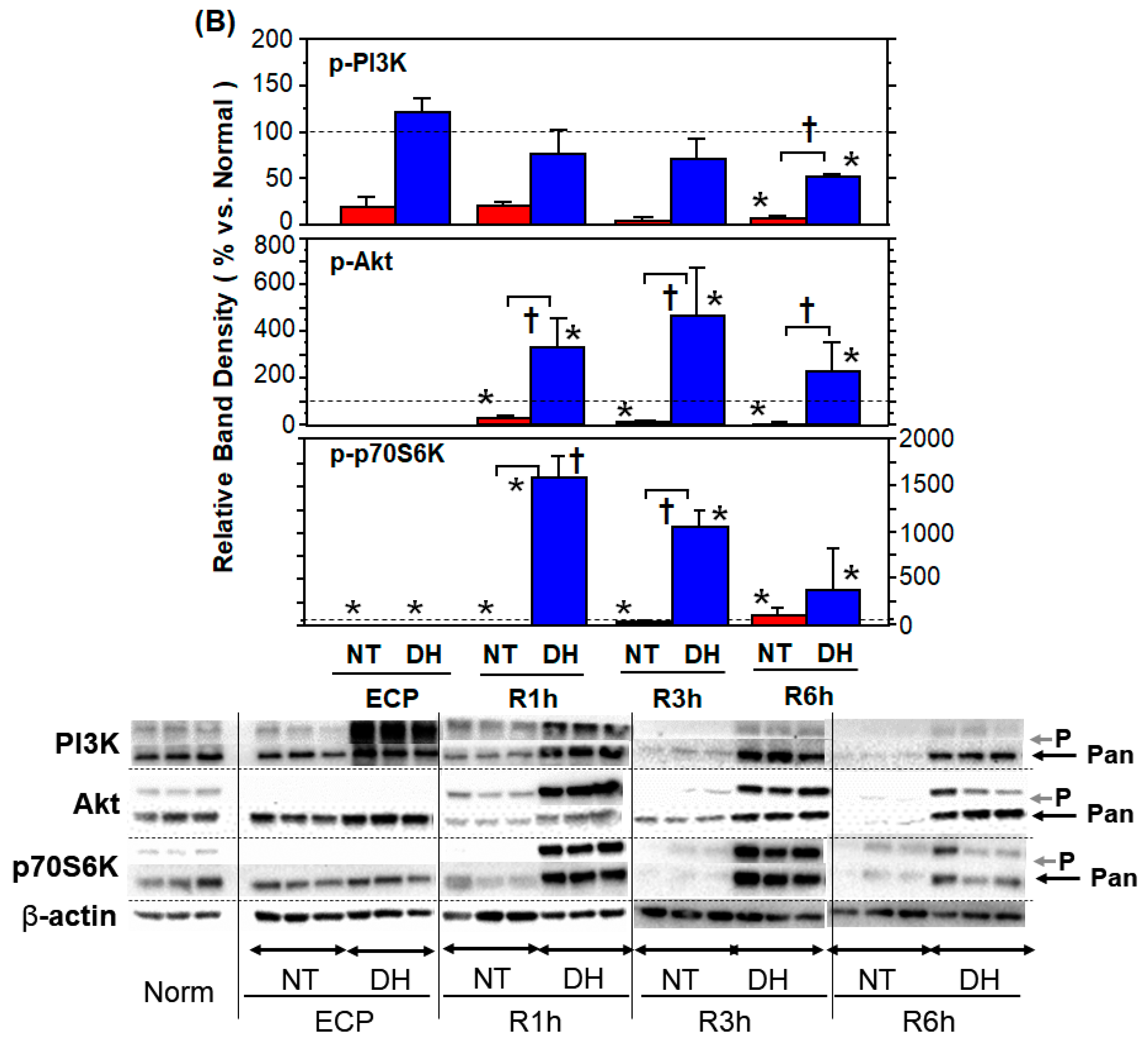
3.5. Stress Responses and Survival Signals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cicalese, L.; Sileri, P.; Green, M.; Abu-Elmagd, K.; Kocoshis, S.; Reyes, J. Bacterial translocation in clinical intestinal transplantation. Transplant. 2001, 71, 1414–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yandza, T.; Tauc, M.; Canioni, D.; Rogel-Gaillard, C.; Bernard, G.; Bernard, A.; Gugenheim, J. Effect of Polyethylene Glycol in Pig Intestinal Allotransplantation Without Immunosuppression. J. Surg. Res. 2012, 176, 621–628. [Google Scholar] [CrossRef]
- Elsabbagh, A.M.; Hawksworth, J.; Khan, K.M.; Kaufman, S.S.; Yazigi, N.A.; Kroemer, A.; Smith, C.; Fishbein, T.M.; Matsumoto, C.S. Long-term survival in visceral transplant recipients in the new era: A single-center experience. Arab. Archaeol. Epigr. 2019, 19, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Fukai, M.; Shibata, K.; Sakamoto, S.; Wakayama, K.; Ishikawa, T.; Kawamura, N.; Fujiyoshi, M.; Shimamura, T.; Taketomi, A. Heavy Water (D2O) containing preservation solution reduces hepatic cold preservation and reperfusion injury in an Isolated Perfused Rat Liver (IPRL) Model. J. Clin. Med. 2019, 8, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, A.; Kimizuka, K.; Stolz, D.B.; Neto, J.S.; Kaizu, T.; Choi, A.M.K.; Uchiyama, T.; Zuckerbraun, B.S.; Nalesnik, M.A.; Otterbein, L.E.; et al. Carbon Monoxide Inhalation Protects Rat Intestinal Grafts from Ischemia/Reperfusion Injury. Am. J. Pathol. 2003, 163, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, T.; Salehi, P.; Walker, J.; Avila, J.; Madsen, K.; Lakey, J.; Kuroda, Y.; Churchill, T.A. Ameliorating Small Bowel Injury Using a Cavitary Two-Layer Preservation Method with Perfluorocarbon and a Nutrient-Rich Solution. Arab. Archaeol. Epigr. 2004, 4, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.J.; Castillo, E.G.; Salehi, P.; Avila, J.; Lakey, J.R.T.; Churchill, T.A. A novel technique of hypothermic luminal perfusion for small bowel preservation. Transplantation 2003, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Kobayashi, N.; Ishikawa, T.; Wakayama, K.; Shimada, S.; Umemoto, K.; Ohtani, S.; Fujiyoshi, M.; Yamashita, K.; Shimamura, T.; et al. 14-3-3ζ-Mediated stimulation of oxidative phosphorylation exacerbates oxidative damage under hypothermic oxygenated conditions in Human Renal Tubular Cells (HK-2). Transplant. Proc. 2016, 48, 1288–1291. [Google Scholar] [CrossRef]
- Salehi, P.; Walker, J.; Madsen, K.; Churchill, T.A. Control of oxidative stress in small bowel: Relevance to organ preservation. Surgery 2006, 139, 317–323. [Google Scholar] [CrossRef]
- Mohr, A.; Brockmann, J.G.; Becker, F. HTK-N: Modified Histidine-Tryptophan-Ketoglutarate Solution—A Promising New Tool in Solid Organ Preservation. Int. J. Mol. Sci. 2020, 21, 6468. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.M.; Sun, S.; Schechner, R.S.; Tellis, V.A.; Alt, E.R.; Greenstein, S.M. Improved small intestinal preservation after lazaroid u74389g treatment and cold storage in university of wisconsin solution. Transplantation 1995, 59, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.-P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and Characterization of a Phenolic Antioxidant from the Pacific Oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Fuda, H.; Watanabe, M.; Hui, S.-P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Chiba, H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef]
- Joko, S.; Watanabe, M.; Fuda, H.; Takeda, S.; Furukawa, T.; Hui, S.-P.; Shrestha, R.; Chiba, H. Comparison of chemical structures and cytoprotection abilities between direct and indirect antioxidants. J. Funct. Foods 2017, 35, 245–255. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Okabe, H.; Hui, S.-P.; Fuda, H.; Furukawa, T.; Takeda, S.; Shrestha, R.; Miura, Y.; Watanabe, M.; Chiba, H. Mass Spectrometric Quantification of Amphipathic, Polyphenolic Antioxidant of the Pacific Oyster (Crassostrea Gigas). Anal. Sci. 2015, 31, 1341–1344. [Google Scholar] [CrossRef] [Green Version]
- Dengu, F.; Abbas, S.H.; Ebeling, G.; Nasralla, D. Normothermic Machine Perfusion (NMP) of the Liver as a Platform for Therapeutic Interventions during Ex-Vivo Liver Preservation: A Review. J. Clin. Med. 2020, 9, 1046. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Sugimura, N.; Fujisawa, A.; Yamamoto, Y. Stabilizers of edaravone aqueous solution and their action mechanisms. 1. Sodium bisulfite. J. Clin. Biochem. Nutr. 2017, 61, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Slaughter, A.; Kotter, C.V.; Moore, E.E.; Hauser, C.J.; Itagaki, K.; Wohlauer, M.; Frank, D.N.; Silliman, C.; Banerjee, A.; et al. A “clean case” of systemic injury. Shock 2015, 44, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Brinkkoetter, P.-T.; Song, H.; Lösel, R.; Schnetzke, U.; Gottmann, U.; Feng, Y.; Hanusch, C.; Beck, G.C.; Schnuelle, P.; Wehling, M.; et al. Hypothermic Injury: The Mitochondrial Calcium, ATP and ROS Love-Hate Triangle out of Balance. Cell. Physiol. Biochem. 2008, 22, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Joshi, M.; Herlenius, G.; Olausson, M. Improved intestinal preservation using an intraluminal macromolecular solution: Evidence from a rat model. Transplantation 2010, 89, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Hayashi, T.; Yokota, R.; Shimamura, T.; Suzuki, T.; Taniguchi, M.; Matsushita, M.; Furukawa, H.; Todo, S. Lipid peroxidation during ischemia depends on ischemia time in warm ischemia and reperfusion of rat liver. Free. Radic. Biol. Med. 2005, 38, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Shiraishi, M.; Naito, Y.; Torii, Y.; Nakamura, Y.; Osawa, T. Activation of Stress Signaling Pathways by the End Product of Lipid Peroxidation. J. Biol. Chem. 1999, 274, 2234–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villuendas-Rey, Y.; Alvarez-Idaboy, J.R.; Galano, A. Assessing the Protective Activity of a Recently Discovered Phenolic Compound against Oxidative Stress Using Computational Chemistry. J. Chem. Inf. Model. 2015, 55, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Tancharoen, S.; Takeshige, N.; Yoshitomi, M.; Morioka, M.; Murai, Y.; Tanaka, E. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 13909–13930. [Google Scholar] [CrossRef] [Green Version]
- Guven, A.; Tunc, T.; Topal, T.; Kul, M.; Korkmaz, A.; Gundogdu, G.; Onguru, O.; Öztürk, H. α-Lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg. Today 2008, 38, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, N.; Wang, R.; Zhang, Y.; Chen, J.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. N-Acetyl Serotonin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Antioxidants 2020, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Soriano, F.X.; Baxter, P.; Murray, L.M.; Sporn, M.B.; Gillingwater, T.H.; Hardingham, G.E. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol. Cells 2009, 27, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxid. Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef] [Green Version]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear Heme Oxygenase-1 (HO-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-oxidant Defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [Green Version]
- Jez, M.; Ciesla, M.; Stepniewski, J.; Langrzyk, A.; Muchova, L.; Vitek, L.; Jozkowicz, A.; Dulak, J. Valproic acid downregulates heme oxygenase-1 independently of Nrf2 by increasing ubiquitination and proteasomal degradation. Biochem. Biophys. Res. Commun. 2017, 485, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Nelin, V.E.; Locy, M.L.; Jin, Y.; Tipple, T.E. Thioredoxin-1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L389–L395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-M.; Kim, J.-Y.; Lee, S.; Park, J.-H. Adrenomedullin protects against hypoxia/reoxygenation-induced cell death by suppression of reactive oxygen species via thiol redox systems. FEBS Lett. 2009, 584, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedr, R.M.; Ahmed, A.A.; Kamel, R.; Raafat, E.M. Sitagliptin attenuates intestinal ischemia/reperfusion injury via cAMP/PKA, PI3K/Akt pathway in a glucagon-like peptide 1 receptor-dependent manner. Life Sci. 2018, 211, 31–39. [Google Scholar] [CrossRef]
- Ban, K.; Kozar, R.A. Protective Role of p70S6K in Intestinal Ischemia/Reperfusion Injury in Mice. PLoS ONE 2012, 7, e41584. [Google Scholar] [CrossRef]
- Shimada, S.; Fukai, M.; Wakayama, K.; Ishikawa, T.; Kobayashi, N.; Kimura, T.; Yamashita, K.; Kamiyama, T.; Shimamura, T.; Taketomi, A.; et al. Hydrogen sulfide augments survival signals in warm ischemia and reperfusion of the mouse liver. Surg. Today 2014, 45, 892–903. [Google Scholar] [CrossRef]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prévost, M.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000, 14, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, Y.; Zhao, Y.; Zhang, J.; Liu, B.; Jiao, S.; Zhang, X. Astragaloside IV reduces neuronal apoptosis and parthanatos in ischemic injury by preserving mitochondrial hexokinase-II. Free Radic. Biol. Med. 2019, 131, 251–263. [Google Scholar] [CrossRef]
- Bankaitis, E.D.; Ha, A.; Kuo, C.J.; Magness, S.T. Reserve Stem Cells in Intestinal Homeostasis and Injury. Gastroenterology 2018, 155, 1348–1361. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukai, M.; Nakayabu, T.; Ohtani, S.; Shibata, K.; Shimada, S.; Sakamoto, S.; Fuda, H.; Furukawa, T.; Watanabe, M.; Hui, S.-P.; et al. The Phenolic Antioxidant 3,5-dihydroxy-4-methoxybenzyl Alcohol (DHMBA) Prevents Enterocyte Cell Death under Oxygen-Dissolving Cold Conditions through Polyphyletic Antioxidant Actions. J. Clin. Med. 2021, 10, 1972. https://doi.org/10.3390/jcm10091972
Fukai M, Nakayabu T, Ohtani S, Shibata K, Shimada S, Sakamoto S, Fuda H, Furukawa T, Watanabe M, Hui S-P, et al. The Phenolic Antioxidant 3,5-dihydroxy-4-methoxybenzyl Alcohol (DHMBA) Prevents Enterocyte Cell Death under Oxygen-Dissolving Cold Conditions through Polyphyletic Antioxidant Actions. Journal of Clinical Medicine. 2021; 10(9):1972. https://doi.org/10.3390/jcm10091972
Chicago/Turabian StyleFukai, Moto, Takuya Nakayabu, Shintaro Ohtani, Kengo Shibata, Shingo Shimada, Soudai Sakamoto, Hirotoshi Fuda, Takayuki Furukawa, Mitsugu Watanabe, Shu-Ping Hui, and et al. 2021. "The Phenolic Antioxidant 3,5-dihydroxy-4-methoxybenzyl Alcohol (DHMBA) Prevents Enterocyte Cell Death under Oxygen-Dissolving Cold Conditions through Polyphyletic Antioxidant Actions" Journal of Clinical Medicine 10, no. 9: 1972. https://doi.org/10.3390/jcm10091972





