The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Anthropometric Data
2.3. Blood Pressure Measurements and Instrumental Examinations
2.4. Laboratory Analyses
2.5. Definitions
2.6. Statistical Analysis
3. Results
Correlations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Q.; Lin, W.; Liu, C.; Zhou, Y.; Chen, T.; Zhang, L.; Zhang, X.; Yu, S.; Wu, Q.; Jin, Z.; et al. Prevalence and epidemiological determinants of metabolically obese but normal-weight in Chinese population. BMC Public Health 2020, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The effect of menopause on metabolic syndrome: Cross-sectional results from the Canadian Longitudinal Study on Aging. Menopause 2020, 27, 999–1009. [Google Scholar] [CrossRef]
- Pasquali, R.; Casanueva, F.; Haluzik, M.; Van Hulsteijn, L.; LeDoux, S.; Monteiro, M.P.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guideline: Endocrine work-up in obesity. Eur. J. Endocrinol. 2020, 182, G1–G32. [Google Scholar] [PubMed] [Green Version]
- Kim, S.H.; Després, J.P.; Koh, K.K. Obesity and cardiovascular disease: Friend or foe? Eur. Heart J. 2016, 37, 3560–3568. [Google Scholar] [PubMed] [Green Version]
- Xu, H.; Jin, C.; Guan, Q. Causal Effects of Overall and Abdominal Obesity on Insulin Resistance and the Risk of Type 2 Diabetes Mellitus: A Two-Sample Mendelian Randomization Study. Front. Genet. 2020, 11, 603. [Google Scholar] [PubMed]
- Allison, D.; Zhu, S.; Plankey, M.; Faith, M.; Heo, M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int. J. Obes. 2002, 26, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.M.; Su, H.; Thomas, D.M.; Heo, M.; Golnabi, A.H.; Pietrobelli, A.; Heymsfield, S.B. Tri-Ponderal Mass Index vs Body Mass Index in Estimating Body Fat During Adolescence. JAMA Pediatr. 2017, 171, 629–636. [Google Scholar]
- Rankinen, T.; Kim, S.-Y.; Pérusse, L.; Després, J.-P.; Bouchard, C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int. J. Obes. 1999, 23, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. For the AlkaMeSy Study Group Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indexes of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Sartorio, A. Indexes of adiposity and body composition in the prediction of metabolic syndrome in obese children and adolescents: Which is the best? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Radetti, G.; Fanolla, A.; Lupi, F.; Sartorio, A.; Grugni, G. Accuracy of Different Indexes of Body Composition and Adiposity in Identifying Metabolic Syndrome in Adult Subjects with Prader-Willi Syndrome. J. Clin. Med. 2020, 9, 1646. [Google Scholar] [CrossRef]
- Deurenberg, P. International consensus conference on impedance. Age Nutr. 1994, 5, 142–145. [Google Scholar]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Bedogni, G.; Agosti, F.; De Col, A.; Marazzi, N.; Tagliaferri, A.; Sartorio, A. Comparison of dual-energy X-ray absorptiometry, air displacement plethysmography and bioelectrical impedance analysis for the assessment of body composition in morbidly obese women. Eur. J. Clin. Nutr. 2013, 67, 1129–1132. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. WHO Fact Sheet No. 311. 2014. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/# (accessed on 8 April 2017).
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Chen, C. Growth Charts of Body Mass Index (BMI) with Quantile Regression. 2005. Available online: https://www.researchgate.net/profile/Colin-Chen-4/publication/220979218_Growth_Charts_of_Body_Mass_Index_BMI_With_Quantile_Regression/links/02bfe50ef9479c0a2f000000/Growth-Charts-of-Body-Mass-Index-BMI-With-Quantile-Regression.pdf (accessed on 1 May 2021).
- Cole, T.J.; Freeman, J.V.; Preece, M.A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat. Med. 1998, 17, 407–429. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Srinivasan, S.R.; Chen, W.; Malina, R.M.; Bouchard, C.; Berenson, G.S. Body Mass Index, Waist Circumference, and Clustering of Cardiovascular Disease Risk Factors in a Biracial Sample of Children and Adolescents. Pediatrics 2004, 114, e198–e205. [Google Scholar] [CrossRef] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef]
- Werida, R.; Khairat, I.; Khedr, N.F. Effect of atorvastatin versus rosuvastatin on inflammatory biomarkers and LV function in type 2 diabetic patients with dyslipidemia. Biomed. Pharmacother. 2021, 135, 111179. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2011, 13, 275–286. [Google Scholar] [CrossRef]
- Park, H.J.; Hong, Y.H.; Cho, Y.J.; Lee, J.E.; Yun, J.M.; Kwon, H.; Kim, S.H. Trends and Cut-Point Changes in Obesity Parameters by Age Groups Considering Metabolic Syndrome. J. Korean Med. Sci. 2018, 33, e47. [Google Scholar] [CrossRef] [Green Version]
- Kerkadi, A.; Suleman, D.; Abu Salah, L.; Lotfy, C.; Attieh, G.; Bawadi, H.; Shi, Z. Adiposity Indicators as Cardio-Metabolic Risk Predictors in Adults from Country with High Burden of Obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, ume 13, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Hübers, M.; Pourhassan, M.; Braun, W.; Geisler, C.; Müller, M. Definition of new cut-offs of BMI and waist circumference based on body composition and insulin resistance: Differences between children, adolescents and adults. Obes. Sci. Pract. 2017, 3, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Grugni, G.; Crinò, A.; Bedogni, G.; Cappa, M.; Sartorio, A.; Corrias, A.; Di Candia, S.; Gargantini, L.; Iughetti, L.; Pagano, C.; et al. Metabolic syndrome in adult patients with Prader–Willi syndrome. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1134–1140. [Google Scholar] [CrossRef]
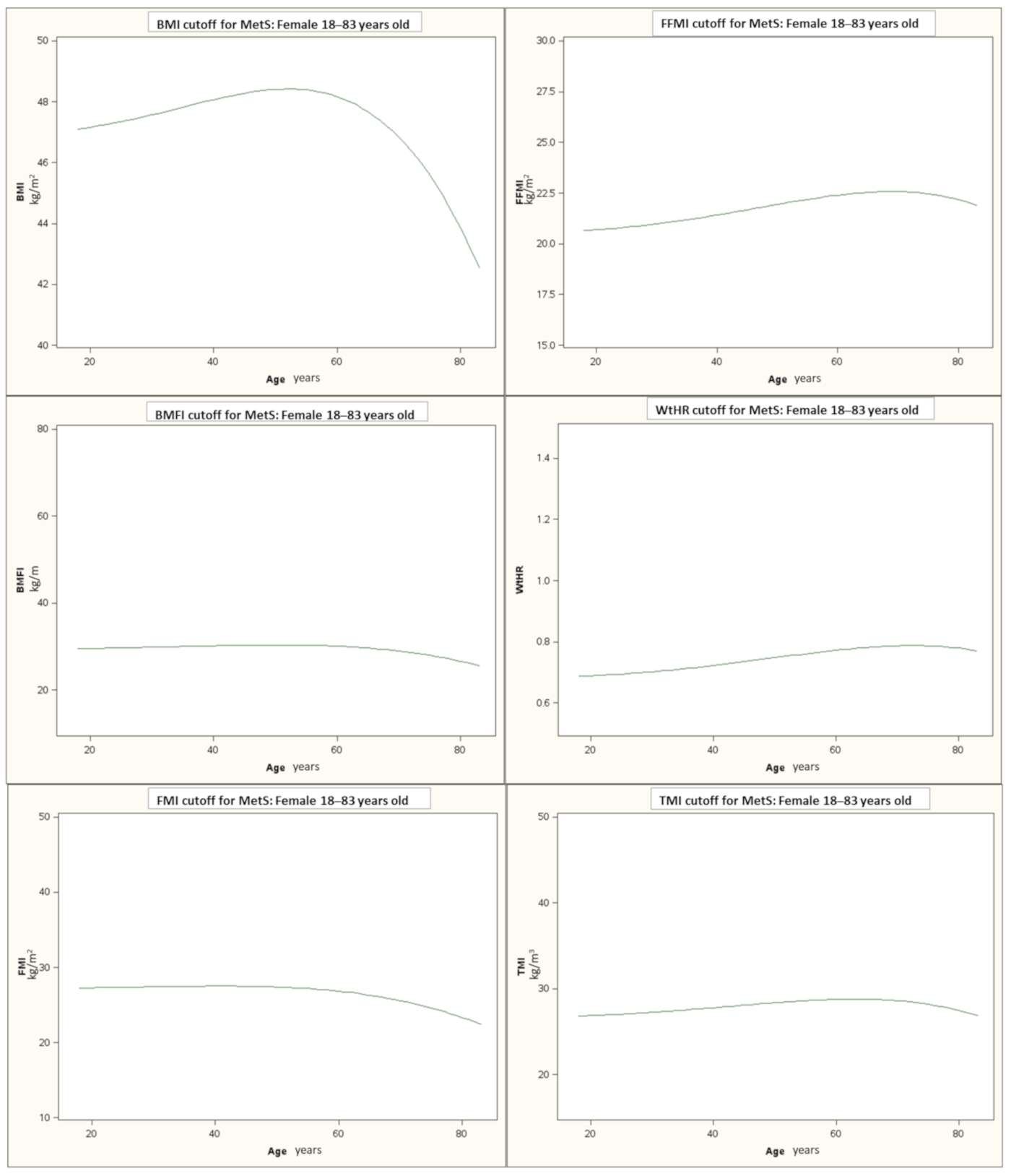

| All | MetS− | MetS+ | p | |
|---|---|---|---|---|
| Number of subjects | 1528 | 611 | 917 | |
| Age (years) | 50.78 ± 14.04 | 46.05 ± 15.12 | 53.93 ± 12.31 | <0.0001 |
| SBP (mm/Hg) | 128.12 ± 13.52 | 124.88 ± 13.1 | 130.28 ± 13.37 | <0.0001 |
| DBP (mm/Hg) | 76.84 ± 7.57 | 75.91 ± 7.63 | 77.45 ± 7.47 | <0.0001 |
| TG (mg/dL) | 132.07 ± 60.38 | 98.32 ± 31.17 | 154.55 ± 64.53 | <0.0001 |
| HDL-C (mg/dL) | 50.86 ± 12.7 | 56.92 ± 12.17 | 46.81 ± 11.36 | <0.0001 |
| glycemia (mmol/L) | 98.06 ± 31.72 | 83.59 ± 12.01 | 107.71 ± 36.72 | <0.0001 |
| BMI (kg/m2) | 43.35 ± 5.93 | 42.55 ± 5.1 | 43.88 ± 6.37 | <0.0001 |
| WtHR | 0.7 ± 60.08 | 0.74 ± 0.07 | 0.78 ± 0.08 | <0.0001 |
| FMI (kg/m2) | 22.27 ± 5.16 | 21.82 ± 4.7 | 22.56 ± 5.43 | <0.005 |
| FFMI (kg/m2) | 21.08 ± 1.82 | 20.73 ± 1.64 | 21.32 ± 1.9 | <0.0001 |
| TMI (kg/m3) | 27.58 ± 4.08 | 26.98 ± 3.47 | 27.99 ± 4.4 | <0.0001 |
| BMFI (kg/m) | 27.15 ± 8.77 | 25.66 ± 7.53 | 28.14 ± 9. 38 | <0.0001 |
| WC (cm) | 119.94 ± 12.26 | 116.05 ± 11.34 | 122.54 ± 12.16 | <0.0001 |
| FM% | 50.82 ± 5.35 | 50.8 ± 5.43 | 50.84 ± 5.29 | 0.88 |
| FFM% | 49.18 ± 5.35 | 49.2 ± 5.43 | 49.16 ± 5.29 | 0.88 |
| SBP (mm/Hg) | DBP (mm/Hg) | HDL-C (mg/dL) | Glycemia (mg/dL) | TG (mg/dL) | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 0.19 (0.14–0.23); 0.03 | 0.19 (0.14–0.23); 0.03 | −0.08 (−0.13/−0.03); 0.01 | 0.09 (0.04–0.14); 0.01 | −0.04 (−0.09–0.01); 0.00 |
| WtHR | 0.19 (0.14–0.24); 0.04 | 0.18 (0.13–0.23); 0.03 | −0.09 (−0.14/−0.04); 0.01 | 0.1 (0.05–0.15); 0.01 | −0.01 (−0.06–0.04); 0.00 |
| FMI (kg/m2) | 0.19 (0.14–0.24); 0.04 | 0.19 (0.14–0.24); 0.05 | −0.05 (−0.1–0.0); 0.01 | 0.07 (0.02–0.12); 0.01 | −0.06 (−0.11/−0.01); 0.01 |
| FFMI (kg/m2) | 0.08 (0.03–0.13); 0.08 | 0.07 (0.02–0.12); 0.07 | −0.13 (−0.18/−0.08); 0.09 | 0.11 (0.06–0.16); 0.08 | 0.03 (−0.02–0.08); 0.07 |
| TMI (kg/m3) | 0.16 (0.11–0.21); 0.12 | 0.13 (0.08–0.18); 0.11 | −0.15 (−0.2/−0.1); 0.12 | 0.16 (0.11–0.21); 0.12 | 0.07 (0.02–0.12); 0.1 |
| BMFI (kg/m) | 0.17 (0.12–0.22); 0.04 | 0.17 (0.13–0.22); 0.04 | −0.07 (−0.12/−0.02); 0.01 | 0.1 (0.05–0.15); 0.02 | −0.05 (−0.1–0.0); 0.01 |
| Sensitivity | Specificity | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | 22.4% | 88.2% | 74.0% | 43.1% | 1.90 | 0.88 |
| BMFI (kg/m) | 33.2% | 77.6% | 68.9% | 43.6% | 1.48 | 0.86 |
| FMI (kg/m2) | 18.3% | 89.0% | 71.5% | 42.1% | 1.67 | 0.92 |
| FFMI (kg/m2) | 35.1% | 80.0% | 72.5% | 45.1% | 1.76 | 0.81 |
| WtHR | 63.0% | 60.4% | 70.5% | 52.1% | 1.59 | 0.61 |
| TMI (kg/m3) | 41.5% | 68.9% | 66.7% | 44.0% | 1.34 | 0.85 |
| All Subjects | Age 18–44 Years | Age 45–54 Years | Age 55+ Years | |
|---|---|---|---|---|
| BMI (kg/m2) | 0.55 (0.52–0.58) ° | 0.51 (0.45–0.56) | 0.59 (0.53–0.65) ° | 0.55 (0.51–0.6) |
| BMFI (kg/m) | 0.57 (0.54–0.6) & | 0.54 (0.48–0.59) & | 0.62 (0.56–0.67) & | 0.57 (0.53–0.62) & |
| FMI (kg/m2) | 0.52 (0.5–0.55) | 0.5 (0.45–0.56) | 0.59 (0.53–0.64) | 0.54 (0.5–0.59) |
| FFMI (kg/m2) | 0.59 (0.56–0.62) + | 0.54 (0.48–0.59) | 0.57 (0.51–0.63) | 0.56 (0.51–0.61) |
| WtHR | 0.66 (0.63–0.68) * | 0.61 (0.55–0.66) * | 0.64 (0.58–0.7) * | 0.62 (0.58–0.67) * |
| TMI (kg/m3) | 0.56 (0.53–0.59) ^ | 0.51 (0.45–0.56) | 0.57 (0.51–0.63) | 0.55 (0.5–0.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Tamini, S.; Cicolini, S.; Sartorio, A. The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity. J. Clin. Med. 2021, 10, 1975. https://doi.org/10.3390/jcm10091975
Radetti G, Fanolla A, Grugni G, Lupi F, Tamini S, Cicolini S, Sartorio A. The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity. Journal of Clinical Medicine. 2021; 10(9):1975. https://doi.org/10.3390/jcm10091975
Chicago/Turabian StyleRadetti, Giorgio, Antonio Fanolla, Graziano Grugni, Fiorenzo Lupi, Sofia Tamini, Sabrina Cicolini, and Alessandro Sartorio. 2021. "The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity" Journal of Clinical Medicine 10, no. 9: 1975. https://doi.org/10.3390/jcm10091975
APA StyleRadetti, G., Fanolla, A., Grugni, G., Lupi, F., Tamini, S., Cicolini, S., & Sartorio, A. (2021). The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity. Journal of Clinical Medicine, 10(9), 1975. https://doi.org/10.3390/jcm10091975







