Sleep Well and Recover Faster with Less Pain—A Narrative Review on Sleep in the Perioperative Period
Abstract
1. Introduction
1.1. What Do We Know about Interactions between Pain and Sleep?
1.2. Aim of the Present Review
2. Perioperative Sleep Disturbances
2.1. Sleep Disturbances and Their Consequences
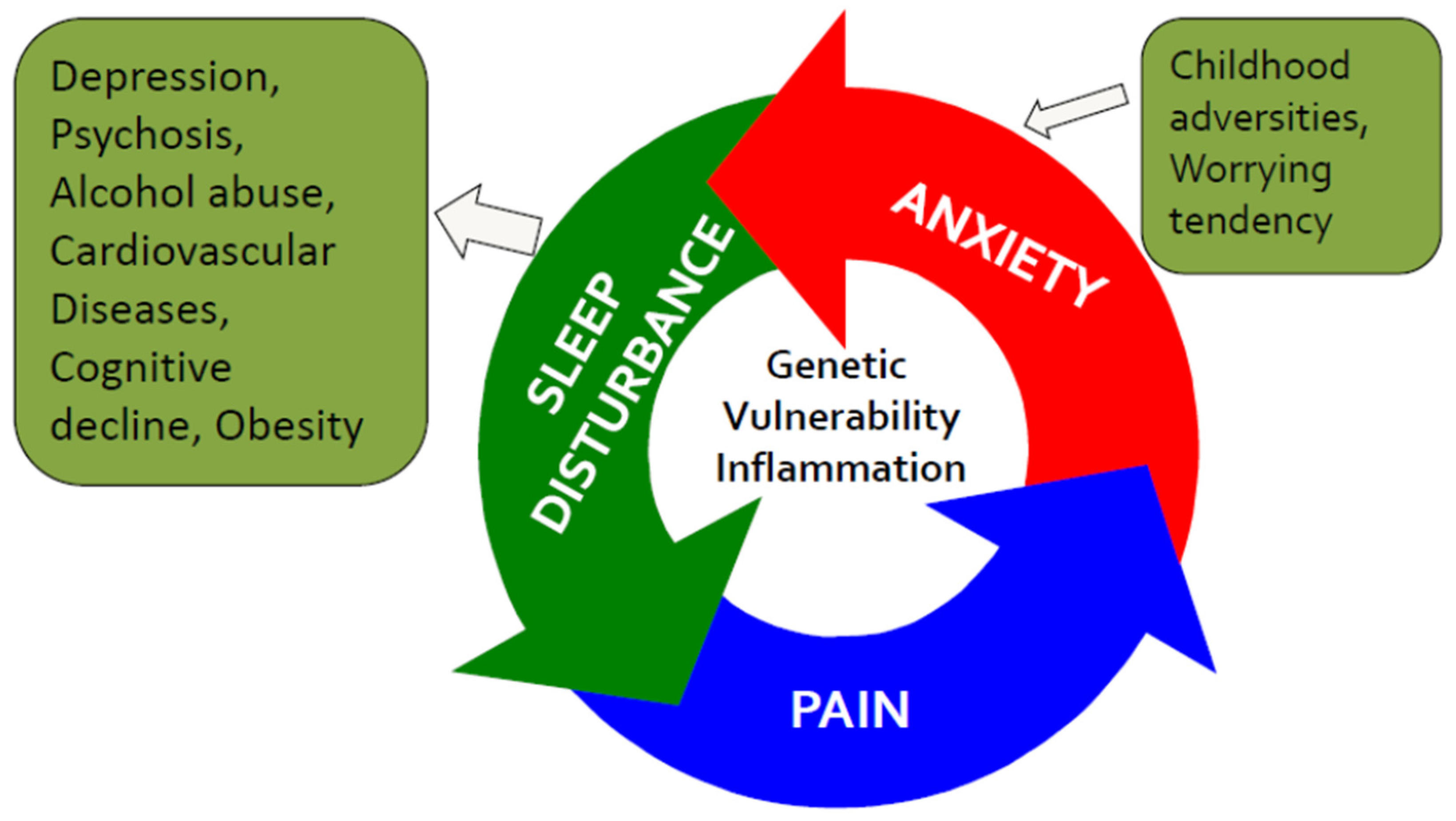
2.2. Sleep, Pain, and Anxiety—The Vicious Circle
2.3. Factors Associating with Postoperative Sleep Quality
2.4. Surgery-Related Causes of Sleep Disturbances
2.5. Anesthesia- and Analgesia-Related Associations with Sleep Disturbances
2.6. External Causes for Postsurgical Sleep Disturbances: Better Sleep Quality at Home
3. Measuring Sleep
3.1. Objective Sleep Quality
3.2. Subjective Measures of Sleep Quality: Questionnaires
4. Why Is It Important to Promote Good Sleep Quality after Surgery?
Quality of Sleep and Risk of Postoperative Acute Pain and Pain Persistence
5. How to Improve Sleep Pre- and Postoperatively
5.1. Non-Pharmacological Treatment
5.2. Pharmacological Treatment (Pre-, Peri-, and Postoperatively)
6. Summary and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leger, D.; Poursain, B.; Neubauer, D.; Uchiyama, M. An international survey of sleeping problems in the general population. Curr. Med. Res. Opin. 2008, 24, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; LeBlanc, M.; Daley, M.; Gregoire, J.P.; Merette, C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006, 7, 123–130. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Mantyselka, P.T.; Turunen, J.H.; Ahonen, R.S.; Kumpusalo, E.A. Chronic pain and poor self-rated health. JAMA 2003, 290, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Pogatzki-Zahn, E.; Aduckathil, S.; Peelen, L.M.; Kappen, T.H.; van Wijck, A.J.; Kalkman, C.J.; Meissner, W. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology 2014, 120, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, T.J.; Andersen, K.G.; Bruce, J.; Haasio, L.; Sipila, R.; Scott, N.W.; Ripatti, S.; Kehlet, H.; Kalso, E. Clinical Prediction Model and Tool for Assessing Risk of Persistent Pain After Breast Cancer Surgery. J. Clin. Oncol. 2017, 35, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J. Pathophysiology of postoperative pain. Pain 2011, 152, S33–S40. [Google Scholar] [CrossRef]
- Voscopoulos, C.; Lema, M. When does acute pain become chronic? Br. J. Anaesth. 2010, 105 (Suppl. S1), i69–i85. [Google Scholar] [CrossRef]
- Chouchou, F.; Khoury, S.; Chauny, J.M.; Denis, R.; Lavigne, G.J. Postoperative sleep disruptions: A potential catalyst of acute pain? Sleep Med. Rev. 2014, 18, 273–282. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The Association of Sleep and Pain: An Update and a Path Forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef]
- Harvey, A.G. A cognitive model of insomnia. Behav. Res. Ther. 2002, 40, 869–893. [Google Scholar] [CrossRef]
- Sipila, R.M.; Haasio, L.; Meretoja, T.J.; Ripatti, S.; Estlander, A.M.; Kalso, E.A. Does expecting more pain make it more intense? Factors associated with the first week pain trajectories after breast cancer surgery. Pain 2017. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, T.J.; Leidenius, M.H.; Tasmuth, T.; Sipila, R.; Kalso, E. Pain at 12 months after surgery for breast cancer. JAMA 2014, 311, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.A.; Olechowski, C.; Rashiq, S.; Verrier, M.J.; Kerr, B.; Witmans, M.; Baker, G.; Joyce, A.; Dick, B.D. A Critical Review of Neurobiological Factors Involved in the Interactions between Chronic Pain, Depression, and Sleep Disruption. Clin. J. Pain 2016, 32, 327–336. [Google Scholar] [CrossRef]
- Babiloni, A.; De Koninck, B.P.; Beetz, G.; De Beaumont, L.; Martel, M.O.; Lavigne, G.J. Sleep and pain: Recent insights, mechanisms, and future directions in the investigation of this relationship. J. Neural Transm. 2020, 127, 647–660. [Google Scholar] [CrossRef]
- Afolalu, E.F.; Ramlee, F.; Tang, N.K.Y. Effects of sleep changes on pain-related health outcomes in the general population: A systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med. Rev. 2018, 39, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Lallukka, T.; Petrie, K.J.; Steingrimsdottir, O.A.; Stubhaug, A.; Nielsen, C.S. Sleep and pain sensitivity in adults. Pain 2015, 156, 1433–1439. [Google Scholar] [CrossRef]
- Krenk, L.; Jennum, P.; Kehlet, H. Sleep disturbances after fast-track hip and knee arthroplasty. Br. J. Anaesth. 2012, 109, 769–775. [Google Scholar] [CrossRef]
- Kain, Z.N.; Caldwell-Andrews, A.A. Sleeping characteristics of adults undergoing outpatient elective surgery: A cohort study. J. Clin. Anesth. 2003, 15, 505–509. [Google Scholar] [CrossRef]
- Gogenur, I.; Wildschiotz, G.; Rosenberg, J. Circadian distribution of sleep phases after major abdominal surgery. Br. J. Anaesth. 2008, 100, 45–49. [Google Scholar] [CrossRef]
- Beydon, L.; Rauss, A.; Lofaso, F.; Liu, N.; Cherqui, D.; Goldenberg, F.; Bonnet, F. Survey of the quality of sleep during the perioperative period. Study of factors predisposing to insomnia. Ann. Fr. Anesth. Reanim. 1994, 13, 669–674. [Google Scholar] [CrossRef]
- Chen, A.F.; Orozco, F.R.; Austin, L.S.; Post, Z.D.; Deirmengian, C.A.; Ong, A.C. Prospective Evaluation of Sleep Disturbances After Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 330–332. [Google Scholar] [CrossRef]
- Sateia, M.J.; Doghramji, K.; Hauri, P.J.; Morin, C.M. Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 2000, 23, 243–308. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Frojmark, M.; Palagini, L.; Rucker, G.; Riemann, D.; et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Liao, D.; Bixler, E.O. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med. Rev. 2013, 17, 241–254. [Google Scholar] [CrossRef]
- Sexton, C.E.; Sykara, K.; Karageorgiou, E.; Zitser, J.; Rosa, T.; Yaffe, K.; Leng, Y. Connections Between Insomnia and Cognitive Aging. Neurosci. Bull. 2020, 36, 77–84. [Google Scholar] [CrossRef]
- Cedernaes, J.; Osorio, R.S.; Varga, A.W.; Kam, K.; Schioth, H.B.; Benedict, C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med. Rev. 2017, 31, 102–111. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Miksiewicz, T.; Kritikou, I.; Shaffer, M.L.; Liao, D.; Basta, M.; Bixler, E.O. Unveiling the longitudinal association between short sleep duration and the incidence of obesity: The Penn State Cohort. Int. J. Obes. 2014, 38, 825–832. [Google Scholar] [CrossRef]
- Sivertsen, B.; Pallesen, S.; Glozier, N.; Bjorvatn, B.; Salo, P.; Tell, G.S.; Ursin, R.; Overland, S. Midlife insomnia and subsequent mortality: The Hordaland health study. BMC Public Health 2014, 14, 720. [Google Scholar] [CrossRef]
- Lallukka, T.; Sivertsen, B.; Kronholm, E.; Bin, Y.S.; Overland, S.; Glozier, N. Association of sleep duration and sleep quality with the physical, social, and emotional functioning among Australian adults. Sleep Health 2018, 4, 194–200. [Google Scholar] [CrossRef]
- Pak, V.M.; Onen, S.H.; Bliwise, D.L.; Kutner, N.G.; Russell, K.L.; Onen, F. Sleep Disturbances in MCI and AD: Neuroinflammation as a Possible Mediating Pathway. Front. Aging Neurosci. 2020, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Palagini, L.; Drake, C.L.; Gehrman, P.; Meerlo, P.; Riemann, D. Early-life origin of adult insomnia: Does prenatal-early-life stress play a role? Sleep Med. 2015, 16, 446–456. [Google Scholar] [CrossRef] [PubMed]
- van Dalfsen, J.H.; Markus, C.R. The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: A systematic review. Sleep Med. Rev. 2018, 39, 187–194. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Crombez, G.; Linton, S.J. The fear-avoidance model of pain. Pain 2016, 157, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Norell-Clarke, A.; Jansson-Frojmark, M.; Tillfors, M.; Harvey, A.G.; Linton, S.J. Cognitive processes and their association with persistence and remission of insomnia: Findings from a longitudinal study in the general population. Behav. Res. 2014, 54, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O.; Singareddy, R.; Shaffer, M.L.; Calhoun, S.L.; Liao, D.; Basta, M.; Chrousos, G.P. Persistent insomnia: The role of objective short sleep duration and mental health. Sleep 2012, 35, 61–68. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T.; Zitting, K.M.; Wigren, H.K. Sleep, its regulation and possible mechanisms of sleep disturbances. Acta Physiol. 2013, 208, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.C.; Landolt, H.P. Sleep-Wake Neurochemistry. Sleep Med. Clin. 2018, 13, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg-Adamsen, S.; Kehlet, H.; Dodds, C.; Rosenberg, J. Postoperative sleep disturbances: Mechanisms and clinical implications. Br. J. Anaesth. 1996, 76, 552–559. [Google Scholar] [CrossRef]
- Wang, J.P.; Lu, S.F.; Guo, L.N.; Ren, C.G.; Zhang, Z.W. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery: A prospective cohort study. Medicine 2019, 98, e17708. [Google Scholar] [CrossRef]
- Chung, F.; Liao, P.; Elsaid, H.; Shapiro, C.M.; Kang, W. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology 2014, 120, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Liao, P.; Yegneswaran, B.; Shapiro, C.M.; Kang, W. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology 2014, 120, 287–298. [Google Scholar] [CrossRef]
- Yilmaz, S.; Aksoy, E.; Dogan, T.; Diken, A.I.; Yalcinkaya, A.; Ozsen, K. Angina severity predicts worse sleep quality after coronary artery bypass grafting. Perfusion 2016, 31, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.A.; Trinder, J.; Curtis, N. Sick and tired: Does sleep have a vital role in the immune system? Nat. Rev. Immunol. 2004, 4, 457–467. [Google Scholar] [CrossRef]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef]
- de Zambotti, M.; Covassin, N.; Tona, G.; Sarlo, M.; Stegagno, L. Sleep onset and cardiovascular activity in primary insomnia. J. Sleep Res. 2011, 20, 318–325. [Google Scholar] [CrossRef]
- Rosenberg-Adamsen, S.; Skarbye, M.; Wildschiodtz, G.; Kehlet, H.; Rosenberg, J. Sleep after laparoscopic cholecystectomy. Br. J. Anaesth. 1996, 77, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Kjolhede, P.; Langstrom, P.; Nilsson, P.; Wodlin, N.B.; Nilsson, L. The impact of quality of sleep on recovery from fast-track abdominal hysterectomy. J. Clin. Sleep Med. 2012, 8, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Shaw, I.R.; Lavigne, G.; Mayer, P.; Choiniere, M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: A preliminary study. Sleep 2005, 28, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Dimsdale, J.E.; Norman, D.; DeJardin, D.; Wallace, M.S. The effect of opioids on sleep architecture. J. Clin. Sleep Med. 2007, 3, 33–36. [Google Scholar] [PubMed]
- Murphy, P.J.; Badia, P.; Myers, B.L.; Boecker, M.R.; Wright, K.P., Jr. Nonsteroidal anti-inflammatory drugs affect normal sleep patterns in humans. Physiol. Behav. 1994, 55, 1063–1066. [Google Scholar] [CrossRef]
- Verret, M.; Lauzier, F.; Zarychanski, R.; Perron, C.; Savard, X.; Pinard, A.M.; Leblanc, G.; Cossi, M.J.; Neveu, X.; Turgeon, A.F.; et al. Perioperative Use of Gabapentinoids for the Management of Postoperative Acute Pain: A Systematic Review and Meta-analysis. Anesthesiology 2020, 133, 265–279. [Google Scholar] [CrossRef]
- Brinck, E.C.; Tiippana, E.; Heesen, M.; Bell, R.F.; Straube, S.; Moore, R.A.; Kontinen, V. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2018, 12, CD012033. [Google Scholar] [CrossRef] [PubMed]
- Brinck, E.C.V.; Maisniemi, K.; Kankare, J.; Tielinen, L.; Tarkkila, P.; Kontinen, V.K. Analgesic Effect of Intraoperative Intravenous S-Ketamine in Opioid-Naive Patients After Major Lumbar Fusion Surgery Is Temporary and Not Dose-Dependent: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Anesth. Analg. 2021, 132, 69–79. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Aldecoa, C.; Bettelli, G.; Bilotta, F.; Sanders, R.D.; Audisio, R.; Borozdina, A.; Cherubini, A.; Jones, C.; Kehlet, H.; MacLullich, A.; et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur. J. Anaesthesiol. 2017, 34, 192–214. [Google Scholar] [CrossRef] [PubMed]
- Wesselius, H.M.; van den Ende, E.S.; Alsma, J.; Ter Maaten, J.C.; Schuit, S.C.E.; Stassen, P.M.; de Vries, O.J.; Kaasjager, K.H.A.H.; Haak, H.R.; van Doormaal, F.F.; et al. “Onderzoeks Consortium Acute Geneeskunde” Acute Medicine Research Consortium Quality and Quantity of Sleep and Factors Associated With Sleep Disturbance in Hospitalized Patients. JAMA Intern. Med. 2018, 178, 1201–1208. [Google Scholar] [CrossRef]
- de Zambotti, M.; Rosas, L.; Colrain, I.M.; Baker, F.C. The Sleep of the Ring: Comparison of the OURA Sleep Tracker Against Polysomnography. Behav. Sleep Med. 2019, 17, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef]
- Chee, N.I.Y.N.; Ghorbani, S.; Golkashani, H.A.; Leong, R.L.F.; Ong, J.L.; Chee, M.W.L. Multi-Night Validation of a Sleep Tracking Ring in Adolescents Compared with a Research Actigraph and Polysomnography. Nat. Sci. Sleep 2021, 13, 177–190. [Google Scholar] [CrossRef]
- Asgari Mehrabadi, M.; Azimi, I.; Sarhaddi, F.; Axelin, A.; Niela-Vilen, H.; Myllyntausta, S.; Stenholm, S.; Dutt, N.; Liljeberg, P.; Rahmani, A.M. Sleep Tracking of a Commercially Available Smart Ring and Smartwatch Against Medical-Grade Actigraphy in Everyday Settings: Instrument Validation Study. JMIR Mhealth Uhealth 2020, 8, e20465. [Google Scholar] [CrossRef]
- Allen, R.W.; Burney, C.P.; Davis, A.; Henkin, J.; Kelly, J.; Judd, B.G.; Ivatury, S.J. Deep Sleep and Beeps: Sleep Quality Improvement Project in General Surgery Patients. J. Am. Coll. Surg. 2021. [Google Scholar] [CrossRef]
- Natale, V.; Leger, D.; Bayon, V.; Erbacci, A.; Tonetti, L.; Fabbri, M.; Martoni, M. The consensus sleep diary: Quantitative criteria for primary insomnia diagnosis. Psychosom. Med. 2015, 77, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Fabbri, M.; Beracci, A.; Martoni, M.; Meneo, D.; Tonetti, L.; Natale, V. Measuring Subjective Sleep Quality: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, M.; Dockray, S.; Hendrickx, H.; Steptoe, A. Psychosocial factors and sleep efficiency: Discrepancies between subjective and objective evaluations of sleep. Psychosom. Med. 2011, 73, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiyama, K.; Nagayama, H.; Kudo, K.; Kojima, K.; Yamada, K. Discrepancy between subjective and objective sleep in patients with depression. Psychiatry Clin. Neurosci. 2003, 57, 259–264. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Calhoun, S.L.; Bixler, E.O.; Karataraki, M.; Liao, D.; Vela-Bueno, A.; Jose Ramos-Platon, M.; Sauder, K.A.; Basta, M.; Vgontzas, A.N. Sleep misperception and chronic insomnia in the general population: Role of objective sleep duration and psychological profiles. Psychosom. Med. 2011, 73, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.M.; Nield, L.E.; Popel, N.; Cantor, W.J.; Plante, S.; Goldman, L.; Prabhakar, M.; Manlhiot, C.; McCrindle, B.W.; Miner, S.E. Symptoms of disturbed sleep predict major adverse cardiac events after percutaneous coronary intervention. Can. J. Cardiol. 2014, 30, 118–124. [Google Scholar] [CrossRef]
- Su, X.; Wang, D. Improve postoperative sleep: What can we do? Curr. Opin. Anaesthesiol. 2018, 31, 83–88. [Google Scholar] [CrossRef]
- Flink, B.J.; Rivelli, S.K.; Cox, E.A.; White, W.D.; Falcone, G.; Vail, T.P.; Young, C.C.; Bolognesi, M.P.; Krystal, A.D.; Trzepacz, P.T.; et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012, 116, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Todd, O.M.; Gelrich, L.; MacLullich, A.M.; Driessen, M.; Thomas, C.; Kreisel, S.H. Sleep Disruption at Home As an Independent Risk Factor for Postoperative Delirium. J. Am. Geriatr. Soc. 2017, 65, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Cremeans-Smith, J.K.; Millington, K.; Sledjeski, E.; Greene, K.; Delahanty, D.L. Sleep disruptions mediate the relationship between early postoperative pain and later functioning following total knee replacement surgery. J. Behav. Med. 2006, 29, 215–222. [Google Scholar] [CrossRef]
- Montes, A.; Roca, G.; Sabate, S.; Lao, J.I.; Navarro, A.; Cantillo, J.; Canet, J.; GENDOLCAT Study Group. Genetic and Clinical Factors Associated with Chronic Postsurgical Pain after Hernia Repair, Hysterectomy, and Thoracotomy: A Two-year Multicenter Cohort Study. Anesthesiology 2015, 122, 1123–1141. [Google Scholar] [CrossRef]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Andersen, K.G.; Duriaud, H.M.; Jensen, H.E.; Kroman, N.; Kehlet, H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain 2015, 156, 2413–2422. [Google Scholar] [CrossRef]
- Katz, J.; Seltzer, Z. Transition from acute to chronic postsurgical pain: Risk factors and protective factors. Expert Rev. Neurother 2009, 9, 723–744. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.; Stamer, U.M.; Pogatzki-Zahn, E.; Zaslansky, R.; Tanase, N.V.; Perruchoud, C.; Kranke, P.; Komann, M.; Lehman, T.; Meissner, W.; et al. Chronic postsurgical pain in Europe: An observational study. Eur. J. Anaesthesiol. 2015, 32, 725–734. [Google Scholar] [CrossRef]
- Mustonen, L.; Aho, T.; Harno, H.; Sipila, R.; Meretoja, T.; Kalso, E. What Makes Surgical Nerve Injury Painful? A 4-9 Year Follow-Up of Patients with Intercostobrachial Nerve Resection in Women Treated for Breast Cancer. Pain 2018, 160. [Google Scholar] [CrossRef]
- Schnabel, A.; Yahiaoui-Doktor, M.; Meissner, W.; Zahn, P.K.; Pogatzki-Zahn, E.M. Predicting poor postoperative acute pain outcome in adults: An international, multicentre database analysis of risk factors in 50,005 patients. Pain Rep. 2020, 5, e831. [Google Scholar] [CrossRef] [PubMed]
- Giusti, E.M.; Lacerenza, M.; Manzoni, G.M.; Castelnuovo, G. Psychological and psychosocial predictors of chronic postsurgical pain: A systematic review and meta-analysis. Pain 2021, 162, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.H.; Hartley, R.L.; Leung, A.A.; Ronksley, P.E.; Jette, N.; Casha, S.; Riva-Cambrin, J. Preoperative predictors of poor acute postoperative pain control: A systematic review and meta-analysis. BMJ Open 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.E.; Bovbjerg, D.H.; Montgomery, G.H.; Weltz, C.; Goldfarb, A.; Pace, B.; Silverstein, J.H. Disrupted sleep the night before breast surgery is associated with increased postoperative pain. J. Pain Symptom Manag. 2009, 37, 352–362. [Google Scholar] [CrossRef]
- Sipila, R.; Kemp, H.; Harno, H.; Rice, A.S.C.; Kalso, E. Health-related quality of life and pain interference in two patient cohorts with neuropathic pain: Breast cancer survivors and HIV patients. Scand. J. Pain 2021. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.L.; Zinboonyahgoon, N.; Flowers, K.M.; Hruschak, V.; Fields, K.G.; Patton, M.E.; Schwartz, E.; Azizoddin, D.; Soens, M.; King, T.; et al. Prediction of Persistent Pain Severity and Impact 12 Months After Breast Surgery Using Comprehensive Preoperative Assessment of Biopsychosocial Pain Modulators. Ann. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Belanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.; Reynolds, C.; Monk, T.; Berman, S.; Kupfer, D. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. May 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Zoomer, J.; Peder, R.; Rubin, A.; Lavie, P. Mini Sleep Questionnaire for screening large populations for EDS complaints. In Sleep’84; Koella, W.P., Ruther, E., Schulz, H., Eds.; Gustav Fischer: Stuttgart, Germany, 1985; pp. 467–470. [Google Scholar]
- Natale, V.; Fabbri, M.; Tonetti, L.; Martoni, M. Psychometric goodness of the Mini Sleep Questionnaire. Psychiatry Clin. Neurosci. 2014, 68, 568–573. [Google Scholar] [CrossRef]
- Espie, C.A.; Kyle, S.D.; Hames, P.; Gardani, M.; Fleming, L.; Cape, J. The Sleep Condition Indicator: A clinical screening tool to evaluate insomnia disorder. BMJ Open 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Richards, K.C.; O’Sullivan, P.S.; Phillips, R.L. Measurement of sleep in critically ill patients. J. Nurs. Meas. 2000, 8, 131–144. [Google Scholar] [CrossRef]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med. 2021, 17, 263–298. [Google Scholar] [CrossRef]
- Kuula, L.; Halonen, R.; Kajanto, K.; Lipsanen, J.; Makkonen, T.; Peltonen, M.; Pesonen, A.K. The Effects of Presleep Slow Breathing and Music Listening on Polysomnographic Sleep Measures—A pilot trial. Sci. Rep. 2020, 10, 7427–7429. [Google Scholar] [CrossRef] [PubMed]
- Sarkamo, T.; Tervaniemi, M.; Laitinen, S.; Forsblom, A.; Soinila, S.; Mikkonen, M.; Autti, T.; Silvennoinen, H.M.; Erkkila, J.; Laine, M.; et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 2008, 131, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U. The effect of music intervention in stress response to cardiac surgery in a randomized clinical trial. Heart Lung 2009, 38, 201–207. [Google Scholar] [CrossRef]
- Selvanathan, J.; Pham, C.; Nagappa, M.; Peng, P.W.H.; Englesakis, M.; Espie, C.A.; Morin, C.M.; Chung, F. Cognitive behavioral therapy for insomnia in patients with chronic pain—A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2021, 60, 101460. [Google Scholar] [CrossRef]
- van Straten, A.; van der Zweerde, T.; Kleiboer, A.; Cuijpers, P.; Morin, C.M.; Lancee, J. Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Med. Rev. 2018, 38, 3–16. [Google Scholar] [CrossRef]
- Smith, M.T.; Finan, P.H.; Buenaver, L.F.; Robinson, M.; Haque, U.; Quain, A.; McInrue, E.; Han, D.; Leoutsakis, J.; Haythornthwaite, J.A. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: A randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015, 67, 1221–1233. [Google Scholar] [CrossRef]
- Lami, M.J.; Martínez, M.P.; Miró, E.; Sánchez, A.I.; Prados, G.; Cáliz, R.; Vlaeyen, J.W.S. Efficacy of combined cognitive-behavioral therapy for insomnia and pain in patients with fibromyalgia: A randomized controlled trial. Cogn. Ther. Res. 2018, 42, 63–79. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carrillo, C.; Sadeghi, N.; Breen, E.C.; Witarama, T.; Yokomizo, M.; Lavretsky, H.; Carroll, J.E.; Motivala, S.J.; et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep 2014, 37, 1543–1552. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Breen, E.C.; Witarama, T.; Carrillo, C.; Sadeghi, N.; Arevalo, J.M.; Ma, J.; Nicassio, P.; Bootzin, R.; et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: A randomized controlled trial. Biol. Psychiatry 2015, 78, 721–729. [Google Scholar] [CrossRef]
- Daly-Eichenhardt, A.; Scott, W.; Howard-Jones, M.; Nicolaou, T.; McCracken, L.M. Changes in Sleep Problems and Psychological Flexibility following Interdisciplinary Acceptance and Commitment Therapy for Chronic Pain: An Observational Cohort Study. Front. Psychol. 2016, 7, 1326. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Mera, A.; Hutton, B.; Burry, L.; Rosenberg, E.; MacDonald, E.; Luks, V. Pharmacological interventions to improve sleep in hospitalised adults: A systematic review. BMJ Open 2016, 6, e012108. [Google Scholar] [CrossRef]
- Krenk, L.; Jennum, P.; Kehlet, H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J. Clin. Sleep Med. 2014, 10, 321–326. [Google Scholar] [CrossRef]
- Furey, S.A.; Hull, S.G.; Leibowitz, M.T.; Jayawardena, S.; Roth, T. A randomized, double-blind, placebo-controlled, multicenter, 28-day, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J. Clin. Sleep Med. 2014, 10, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Andersen, L.P.; Werner, M.U.; Rosenberg, J.; Gogenur, I. A systematic review of peri-operative melatonin. Anaesthesia 2014, 69, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.T.; Hansen, M.V.; Andersen, L.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gogenur, I. Effect of Melatonin on Sleep in the Perioperative Period after Breast Cancer Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Sleep Med. 2016, 12, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, M.; Gao, Z.; Zhao, S.; Feng, X.; Chen, J.; Zhang, Y.; Maze, M. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: A double-blind, randomised clinical trial. Eur. J. Anaesthesiol. 2021, 38, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Cui, F.; Zhang, C.; Meng, Z.T.; Wang, D.X.; Ma, J.; Wang, G.F.; Zhu, S.N.; Ma, D. Low-dose Dexmedetomidine Improves Sleep Quality Pattern in Elderly Patients after Noncardiac Surgery in the Intensive Care Unit: A Pilot Randomized Controlled Trial. Anesthesiology 2016, 125, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Heerdt, P.M.; Fontes, M.; Rothman, D.L.; Volkow, N.D. Glymphatic System Function in Relation to Anesthesia and Sleep States. Anesth. Analg. 2019, 128, 747–758. [Google Scholar] [CrossRef]
- Eide, P.K.; Vinje, V.; Pripp, A.H.; Mardal, K.-A.; Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 2021, 144, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Purdon, P.L.; Sampson, A.; Pavone, K.J.; Brow, E.N. Clinical electroencephalography for anesthesiologists: Part i: Background and basic signatures. Anesthesiology 2015, 123, 937–960. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Lee, H.; Ding, F.; Sun, Q.; Al-Bizri, E.; Makaryus, R.; Probst, S.; Nedergaard, M.; Stein, E.A.; Lu, H. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway’y in rat brain when compared with high-dose isoflurane. Anesthesiology 2017, 127, 976–988. [Google Scholar] [CrossRef]

| Poor Subjective Sleep Quality Preoperatively |
| Symptoms of anxiety |
| Symptoms of depression |
| Surgical worry |
| Preoperative pain (surgical area or other chronic pain) |
| Severity of surgical trauma |
| Type of anesthesia (general anesthesia) |
| Type of postoperative analgesics (high dose of opioids) |
| External factors (e.g., light and noise in the ward) |
| Obstructive sleep apnea |
| Greater age |
| Coronary artery disease |
| Questionnaires | Number of Items | |
|---|---|---|
| Sleep diary [66,67] | Consensus Sleep Diary (CSD) suggest to include 9 themes. | Assessed themes: Time of getting into bed, time at which the individual attempts to fall asleep, sleep onset latency, number of awakenings, duration of awakenings, time of final awakening, final rise time, perceived sleep quality, additional space for open-ended comments. |
| Insomnia Severity Index (ISI) [89] | 7 Items | Assesses the severity of sleep onset, sleep maintenance difficulties (both nocturnal and early morning awakening), satisfaction with current sleep pattern, interference with daily functioning, impairment attributed to the sleep problem, and concern caused by the sleep problem. |
| Pittsburgh Sleep Quality Index (PSQI) [90] | 24 Items | Assesses sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medications, and daytime disturbance. |
| Mini-Sleep Questionnaire (MSQ) [91,92] | 10 Items | Assesses both symptoms of insomnia and excessive daytime sleepiness |
| Sleep Condition Indicator (SCI) [93] | 8 Items | Assesses concerns about sleep quality, getting to sleep, remaining asleep, daytime functioning, daytime performance, duration of sleep problem, nights per week having a sleep problem, and extent troubled by poor sleep. |
| Epworth Sleepiness Scale (ESS) [94] | 8 Items | Assesses the severity of daytime sleepiness, which is an important manifestation of sleep disorders. |
| The Richards-Campbell Sleep Questionnaire (RCSQ) [95] | 6 Items | Assesses in-hospital sleep quality: sleep depth, sleep latency, awakenings, returning to sleep, sleep quality, and noise disturbance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipilä, R.M.; Kalso, E.A. Sleep Well and Recover Faster with Less Pain—A Narrative Review on Sleep in the Perioperative Period. J. Clin. Med. 2021, 10, 2000. https://doi.org/10.3390/jcm10092000
Sipilä RM, Kalso EA. Sleep Well and Recover Faster with Less Pain—A Narrative Review on Sleep in the Perioperative Period. Journal of Clinical Medicine. 2021; 10(9):2000. https://doi.org/10.3390/jcm10092000
Chicago/Turabian StyleSipilä, Reetta M., and Eija A. Kalso. 2021. "Sleep Well and Recover Faster with Less Pain—A Narrative Review on Sleep in the Perioperative Period" Journal of Clinical Medicine 10, no. 9: 2000. https://doi.org/10.3390/jcm10092000
APA StyleSipilä, R. M., & Kalso, E. A. (2021). Sleep Well and Recover Faster with Less Pain—A Narrative Review on Sleep in the Perioperative Period. Journal of Clinical Medicine, 10(9), 2000. https://doi.org/10.3390/jcm10092000






