Which Parameter Influences Local Disease-Free Survival after Radiation Therapy Due to Osteolytic Metastasis? A Retrospective Study with Pre- and Post-Radiation Therapy MRI including Diffusion-Weighted Images
Abstract
:1. Introduction
2. Materials and Methods
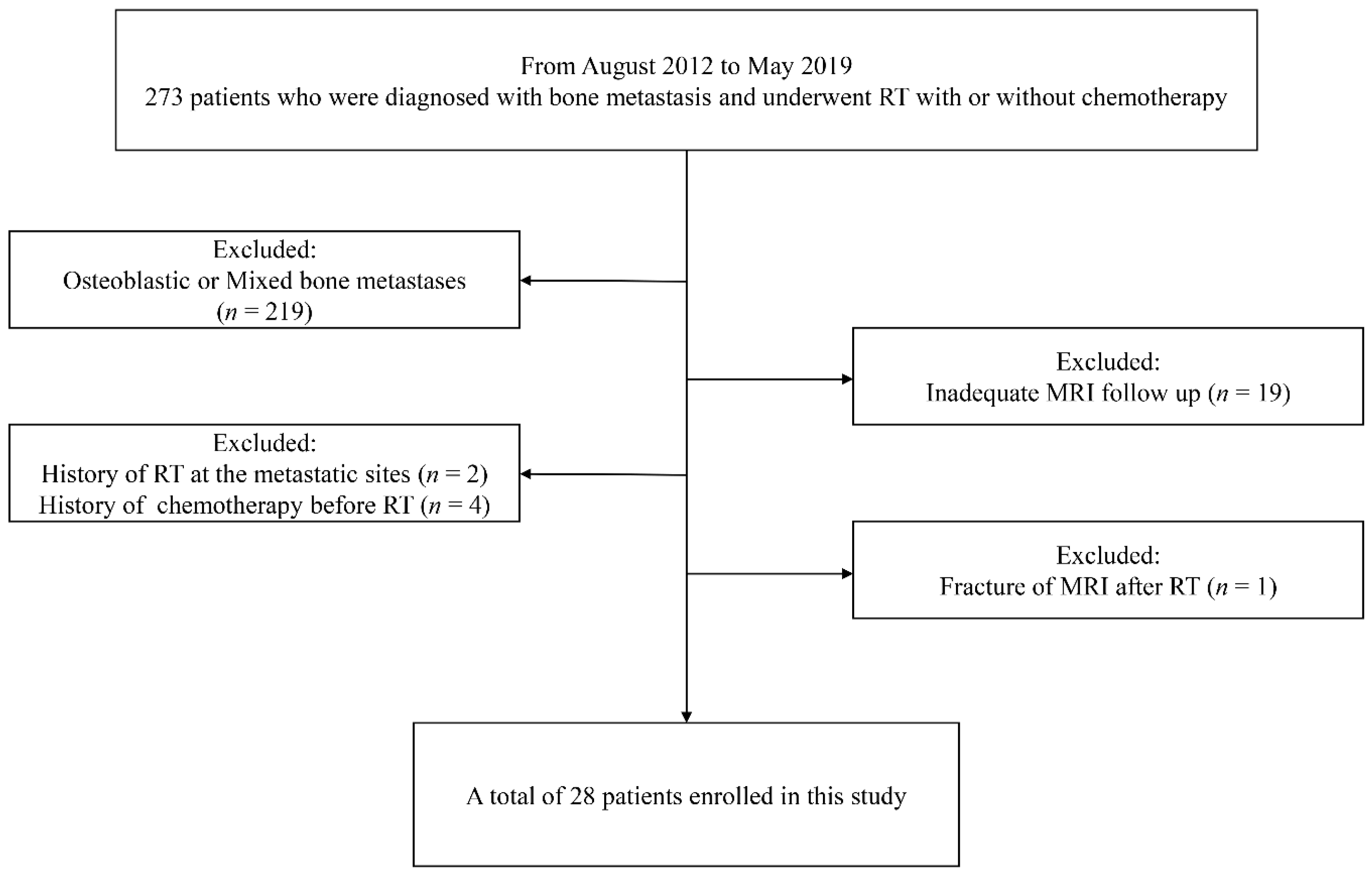
2.1. Patients
2.2. MRI Protocols
2.3. Image Analysis
2.4. Treatment Response Evaluation
2.5. Statistical Analysis
3. Results
3.1. Comparison of MRI-Derived Parameters between Pre- and Post-RT
3.2. Associations between Range-Ratio of MRI Parameters and Local Disease Progression-Free Survival (LDPFS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Gogbashian, A. Bony metastases: Assessing response to therapy with whole-body diffusion MRI. Cancer Imaging 2011, 11, S129–S145. [Google Scholar] [CrossRef]
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8, 25691–25699. [Google Scholar] [CrossRef] [Green Version]
- Byun, W.M.; Shin, S.O.; Chang, Y.; Lee, S.J.; Finsterbusch, J.; Frahm, J. Diffusion-weighted MR imaging of metastatic disease of the spine: Assessment of response to therapy. AJNR Am. J. Neuroradiol. 2002, 23, 906–912. [Google Scholar]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J.; Group, E.G.W. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25 (Suppl. 3), iii124–iii137. [Google Scholar] [CrossRef]
- Yu, T.; Choi, C.W.; Kim, K.S. Treatment outcomes of stereotactic ablative radiation therapy for non-spinal bone metastases: Focus on response assessment and treatment indication. Br. J. Radiol. 2019, 92, 20181048. [Google Scholar] [CrossRef]
- McDonald, R.; Probyn, L.; Poon, I.; Erler, D.; Brotherston, D.; Soliman, H.; Cheung, P.; Chung, H.; Chu, W.; Loblaw, A.; et al. Tumor Response After Stereotactic Body Radiation Therapy to Nonspine Bone Metastases: An Evaluation of Response Criteria. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 879–881. [Google Scholar] [CrossRef]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef]
- Charles-Edwards, E.M.; deSouza, N.M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging 2006, 6, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Biffar, A.; Dietrich, O.; Sourbron, S.; Duerr, H.R.; Reiser, M.F.; Baur-Melnyk, A. Diffusion and perfusion imaging of bone marrow. Eur. J. Radiol. 2010, 76, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Koh, D.M. Diffusion MR imaging for monitoring of treatment response. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, S.; Capasso, R.; Urraro, F. Assessing response to radiation therapy treatment of bone metastases: Short-term followup of radiation therapy treatment of bone metastases with diffusion-weighted magnetic resonance imaging. J. Radiother 2014, 698127. [Google Scholar] [CrossRef]
- Gaeta, M.; Benedetto, C.; Minutoli, F.; D’Angelo, T.; Amato, E.; Mazziotti, S.; Racchiusa, S.; Mormina, E.; Blandino, A.; Pergolizzi, S. Use of diffusion-weighted, intravoxel incoherent motion, and dynamic contrast-enhanced MR imaging in the assessment of response to radiotherapy of lytic bone metastases from breast cancer. Acad. Radiol. 2014, 21, 1286–1293. [Google Scholar] [CrossRef]
- Just, N. Improving tumour heterogeneity MRI assessment with histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef] [Green Version]
- Schob, S.; Meyer, H.J.; Dieckow, J.; Pervinder, B.; Pazaitis, N.; Hohn, A.K.; Garnov, N.; Horvath-Rizea, D.; Hoffmann, K.T.; Surov, A. Histogram Analysis of Diffusion Weighted Imaging at 3T is Useful for Prediction of Lymphatic Metastatic Spread, Proliferative Activity, and Cellularity in Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 821. [Google Scholar] [CrossRef] [Green Version]
- Reischauer, C.; Froehlich, J.M.; Koh, D.M.; Graf, N.; Padevit, C.; John, H.; Binkert, C.A.; Boesiger, P.; Gutzeit, A. Bone metastases from prostate cancer: Assessing treatment response by using diffusion-weighted imaging and functional diffusion maps--initial observations. Radiology 2010, 257, 523–531. [Google Scholar] [CrossRef]
- Blackledge, M.D.; Collins, D.J.; Tunariu, N.; Orton, M.R.; Padhani, A.R.; Leach, M.O.; Koh, D.M. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: A feasibility study. PLoS ONE 2014, 9, e91779. [Google Scholar] [CrossRef]
- Messiou, C.; Collins, D.J.; Giles, S.; de Bono, J.S.; Bianchini, D.; de Souza, N.M. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur. Radiol. 2011, 21, 2169–2177. [Google Scholar] [CrossRef]
- Messiou, C.; Collins, D.J.; Morgan, V.A.; Desouza, N.M. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur. Radiol. 2011, 21, 1713–1718. [Google Scholar] [CrossRef]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J. Clin. Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kuroda, M.; Matsuya, R.; Kato, H.; Shibuya, K.; Oita, M.; Kawabe, A.; Matsuzaki, H.; Asaumi, J.; Murakami, J.; et al. In vitro experimental study of the relationship between the apparent diffusion coefficient and changes in cellularity and cell morphology. Oncol. Rep. 2009, 22, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, Y.; Hirai, T.; Yakushiji, T.; Katahira, K.; Shimomura, O.; Imuta, M.; Nakaura, T.; Utsunomiya, D.; Awai, K.; Yamashita, Y. Evaluation of diffusion-weighted imaging for the differential diagnosis of poorly contrast-enhanced and T2-prolonged bone masses: Initial experience. J. Magn. Reson. Imaging 2006, 23, 377–382. [Google Scholar] [CrossRef]
- Giles, S.L.; Messiou, C.; Collins, D.J.; Morgan, V.A.; Simpkin, C.J.; West, S.; Davies, F.E.; Morgan, G.J.; deSouza, N.M. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 2014, 271, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Dalili, D.; Padhani, A.R.; Grimm, R. Quantitative WB-MRI with ADC histogram analysis for response assessment in diffuse bone disease. Magn. Flash 2017, 69, 32–37. [Google Scholar]
- Dalili, D.; Joshi, P.V.; Grimm, R.; Padhani, A.R. Evolution of the malignant bone marrow with successful therapy—Quantitative analysis with whole body diffusion-weighted MRI. Magn. Flash 2017, 69, 43–48. [Google Scholar]
- Dalili, D.; Padhani, A.R.; Grimm, R. Quantitative WB-MRI with ADC histogram analysis for demonstrating complex response of bone marrow metastatic disease. Magn. Flash 2017, 69, 38–42. [Google Scholar]
- Wen, Q.; Jalilian, L.; Lupo, J.M.; Molinaro, A.M.; Chang, S.M.; Clarke, J.; Prados, M.; Nelson, S.J. Comparison of ADC metrics and their association with outcome for patients with newly diagnosed glioblastoma being treated with radiation therapy, temozolomide, erlotinib and bevacizumab. J. Neurooncol. 2015, 121, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardor, Y.; Pfeffer, R.; Spiegelmann, R.; Roth, Y.; Maier, S.E.; Nissim, O.; Berger, R.; Glicksman, A.; Baram, J.; Orenstein, A.; et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J. Clin. Oncol. 2003, 21, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Dalah, E.; Erickson, B.; Oshima, K.; Schott, D.; Hall, W.A.; Paulson, E.; Tai, A.; Knechtges, P.; Li, X.A. Correlation of ADC With Pathological Treatment Response for Radiation Therapy of Pancreatic Cancer. Transl. Oncol. 2018, 11, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Subhawong, T.K.; Jacobs, M.A.; Fayad, L.M. Diffusion-weighted MR imaging for characterizing musculoskeletal lesions. Radiographics 2014, 34, 1163–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Patient | Sex | Age (yr) | Target Lesion Location | Primary Cancer Type | Radiation Dose (cGy) | Follow-Up Time (Months) | Sum of Greatest Diameters * (cm) | Last FU Image | Disease Status | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre RT | Post RT | |||||||||
| 1 | F | 72 | Sacrum | Thyroid cancer | 5000 | 36 | 6.6 | 5.9 | CT | Non-PD |
| 2 | M | 80 | Pelvic bone | Lung cancer | 4500 | 7 | 10.9 | 13.2 | CT | Non-PD |
| 3 | M | 72 | Humerus | Hepatocellular carcinoma | 4300 | 15 | 7.0 | 6.0 | CT | Non-PD |
| 4 | F | 65 | Hand | Renal cell carcinoma | 4500 | 22 | 3.7 | 4.2 | XR | Non-PD |
| 5 | F | 45 | Pelvic bone | Breast cancer | 3750 | 63 | 3.0 | 2.1 | CT | Non-PD |
| 6 | M | 66 | Foot | Renal cell carcinoma | 4000 | 5 | 5.2 | 5.7 | CT | Non-PD |
| 7 | M | 70 | Femur | Thyroid cancer | 4500 | 52 | 3.0 | 2.9 | MR | Non-PD |
| 8 | M | 66 | Clavicle | Hepatocellular carcinoma | 5000 | 33 | 6.0 | 6.0 | CT | Non-PD |
| 9 | F | 63 | Pelvic bone | Lung cancer | 2500 | 7 | 3.8 | 4.5 | CT | Non-PD |
| 10 | M | 51 | Foot | Hepatocellular carcinoma | 3500 | 37 | 11.1 | 6.6 | MR | Non-PD |
| 11 | M | 59 | Sacrum | Lung cancer | 2000 | 5 | 10.7 | 12.4 | MR | Non-PD |
| 12 | M | 52 | Pelvic bone | Cholangiocarcinoma | 2400 | 17 | 14.6 | 15.2 | XR | Non-PD |
| 13 | F | 44 | Femur | Rhabdomyosarcoma | 3000 | 27 | 5.7 | 2.7 | CT | Non-PD |
| 14 | M | 56 | Pelvic bone | Hepatocellular carcinoma | 3600 | 22 | 3.9 | 4.2 | CT | Non-PD |
| 15 | F | 65 | Sacrum | Thymic cancer | 2800 | 1 | 6.6 | 8.8 | MR | Non-PD |
| 16 | F | 51 | Humerus | Lung cancer | 2500 | 21 | 5.4 | 1.4 | MR | Non-PD |
| 17 | F | 62 | Pelvic bone | Thyroid cancer | 3000 | 16 | 5.9 | 6.0 | MR | Non-PD |
| 18 | F | 56 | Femur | Breast cancer | 3000 | 16 | 6.9 | 9.0 | MR | PD |
| 19 | F | 72 | Femur | Thyroid cancer | 5000 | 32 | 3.1 | 3.9 | MR | PD |
| 20 | M | 48 | Pelvic bone | Hepatocellular carcinoma | 5000 | 12 | 3.5 | 6.3 | MR | PD |
| 21 | F | 60 | Pelvic bone | Thyroid cancer | 4500 | 50 | 3.1 | 3.9 | MR | PD |
| 22 | M | 67 | Pelvic bone | Nasopharyngeal cancer | 2000 | 3 | 3.2 | 5.0 | MR | PD |
| 23 | M | 55 | Pelvic bone | Lung cancer | 3000 | 3 | 15.8 | 19.8 | XR | PD |
| 24 | F | 57 | Pelvic bone | Breast cancer | 5400 | 43 | 4.4 | 6.2 | CT | PD |
| 25 | M | 63 | Pelvic bone | Hepatocellular carcinoma | 3000 | 5 | 5.9 | 8.7 | MR | PD |
| 26 | M | 63 | Pelvic bone | Hepatocellular carcinoma | 5000 | 1 | 3.8 | 5.5 | MR | PD |
| 27 | M | 57 | Pelvic bone | Hepatocellular carcinoma | 5000 | 2 | 4.1 | 5.4 | MR | PD |
| 28 | M | 56 | Femur | Hepatocellular carcinoma | 4000 | 5 | 9.7 | 14.6 | MR | PD |
| Baseline MRI | Post-RT MRI | p Value | |
|---|---|---|---|
| ADC parameters | |||
| Minimum (×10−3 mm2/s) * | 0.37 (0.00–0.99) | 0.53 (0.00–1.61) | 0.120 |
| Maximum (×10−3 mm2/s) # | 1.68 (0.85–2.55) | 2.18 (1.08–3.18) | <0.001 |
| Mean (×10−3 mm2/s) # | 0.95 (0.62–1.58) | 1.33 (0.61–2.05) | <0.001 |
| Median (×10−3 mm2/s) # | 0.94 (0.61–1.64) | 1.31 (0.58–2.07) | <0.001 |
| Standard deviation * | 0.19 (0.07–0.48) | 0.31 (0.07–0.89) | <0.001 |
| Skewness # | 0.29 (−0.86–3.28) | 0.10 (−0.98–1.74) | 0.329 |
| Kurtosis * | 4.57 (2.49–19.19) | 3.3 (1.42–7.67) | 0.051 |
| Conventional parameters | |||
| Maximum diameter (cm) * | 4.1 (1.5–10.5) | 4.4 (1.6–13.0) | 0.018 |
| Volume * (cm3) | 17.4 (0.8–111.0) | 23 (0.9–203.9) | 0.001 |
| HR | 95% CI | p Value | |
|---|---|---|---|
| Age | 0.997 | 0.934–1.063 | 0.918 |
| Cancer type | 0.815 | 0.075–8.856 | 0.367 |
| RT dose | 1.123 | 0.573–2.198 | 0.736 |
| Metastatic site * | 2.100 | 0.550–8.020 | 0.278 |
| RADCmean < 1.41 | 3.817 | 1.088–13.514 | 0.036 |
| RADCSD < 1.03 | 3.311 | 0.924–11.905 | 0.066 |
| RADCskewness < 0.56 | 6.211 | 0.732–52.632 | 0.094 |
| RADCkurtosis < 0.73 | 0.361 | 0.077– 1.684 | 0.195 |
| RTumor diameter ≥ 1.17 | 5.802 | 1.604–20.989 | 0.007 |
| RTumor volume ≥ 1.55 | 5.155 | 1.357–19.582 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yoon, Y.C.; Lee, J.H.; Kim, H.S. Which Parameter Influences Local Disease-Free Survival after Radiation Therapy Due to Osteolytic Metastasis? A Retrospective Study with Pre- and Post-Radiation Therapy MRI including Diffusion-Weighted Images. J. Clin. Med. 2022, 11, 106. https://doi.org/10.3390/jcm11010106
Lee J, Yoon YC, Lee JH, Kim HS. Which Parameter Influences Local Disease-Free Survival after Radiation Therapy Due to Osteolytic Metastasis? A Retrospective Study with Pre- and Post-Radiation Therapy MRI including Diffusion-Weighted Images. Journal of Clinical Medicine. 2022; 11(1):106. https://doi.org/10.3390/jcm11010106
Chicago/Turabian StyleLee, Jiyeong, Young Cheol Yoon, Ji Hyun Lee, and Hyun Su Kim. 2022. "Which Parameter Influences Local Disease-Free Survival after Radiation Therapy Due to Osteolytic Metastasis? A Retrospective Study with Pre- and Post-Radiation Therapy MRI including Diffusion-Weighted Images" Journal of Clinical Medicine 11, no. 1: 106. https://doi.org/10.3390/jcm11010106






