Carbon Monoxide Diffusion Capacity as a Severity Marker in Pulmonary Hypertension
Abstract
:1. Introduction
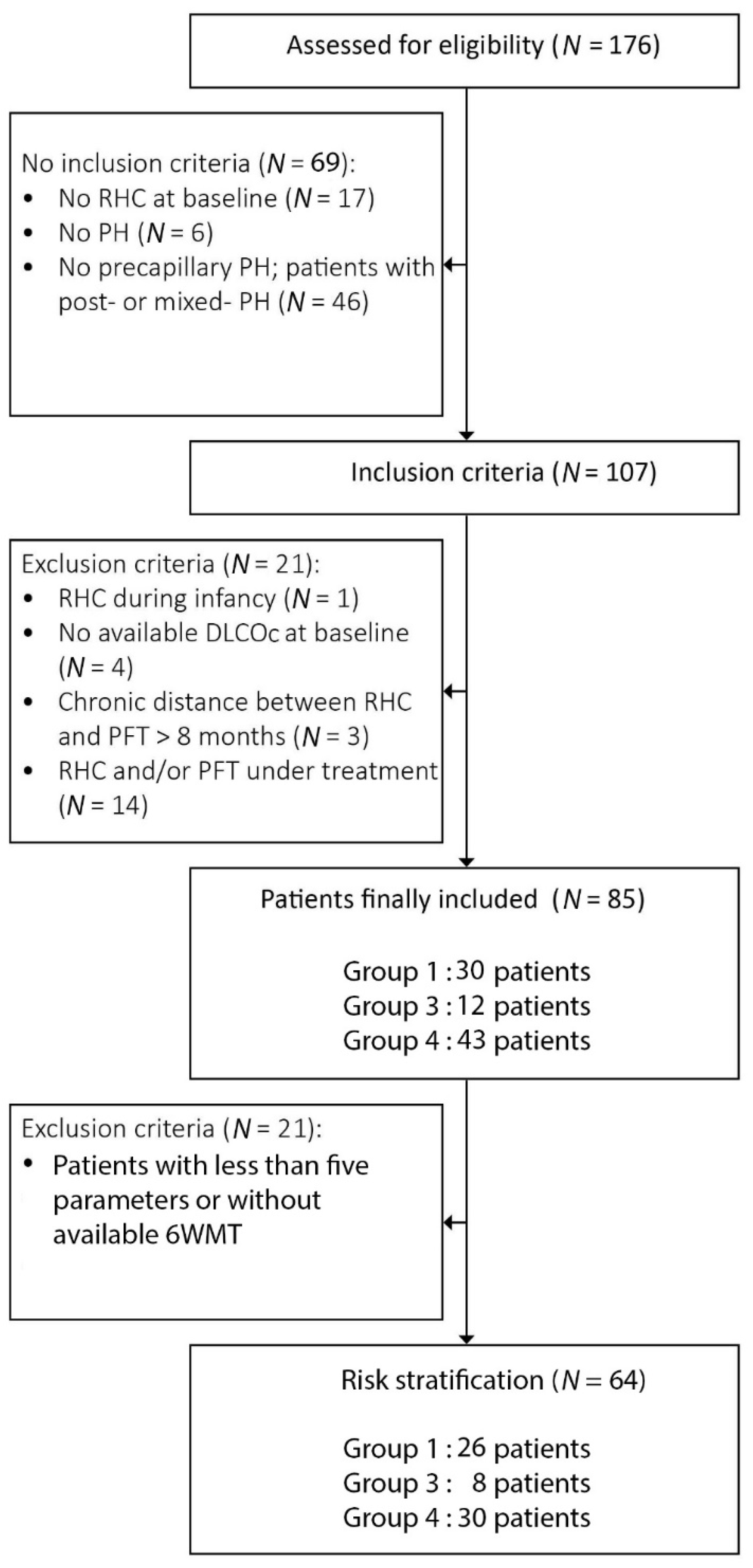
2. Materials and Methods
3. Results
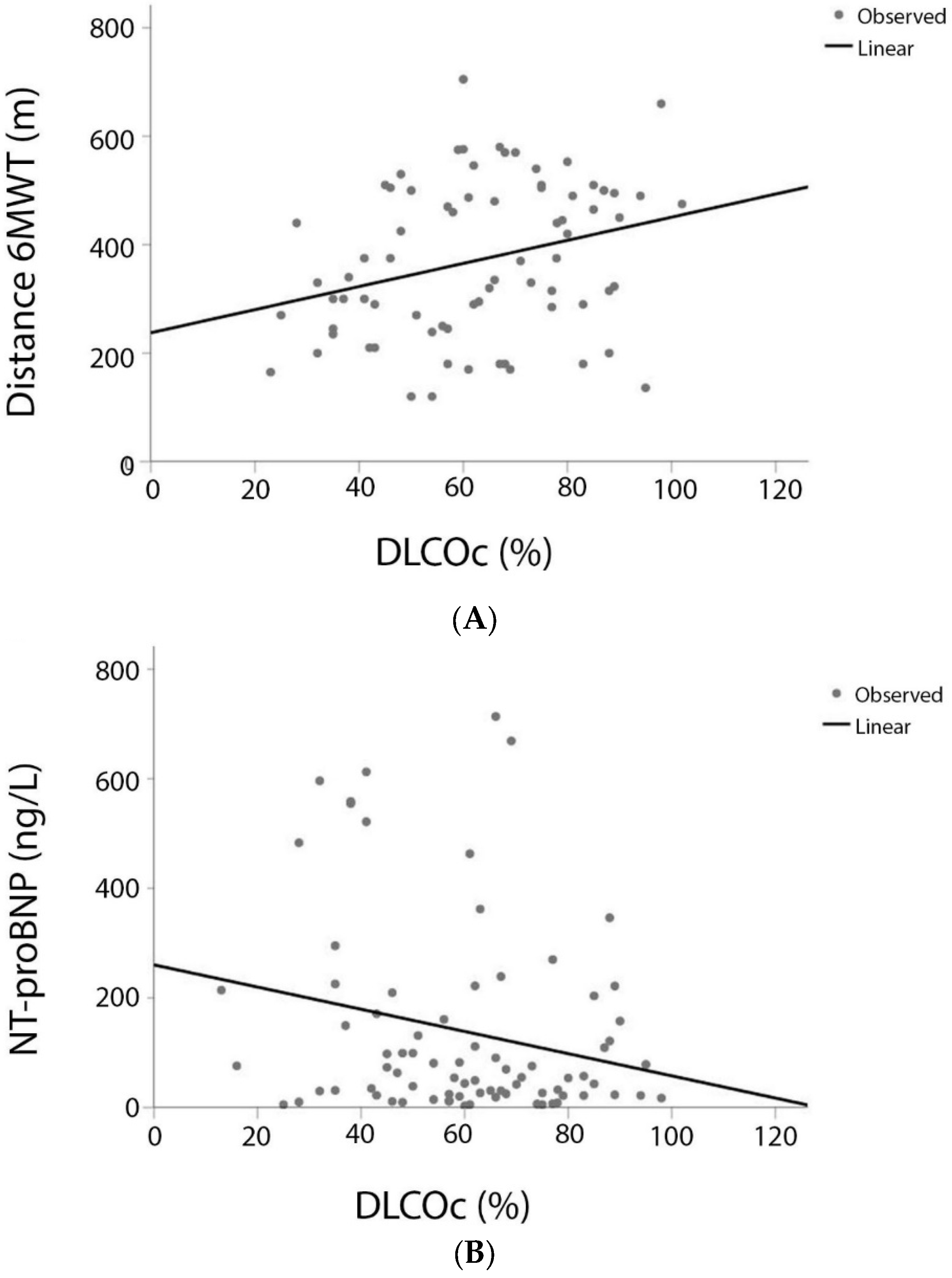
3.1. DLCO and Variables of the COMPERA Score
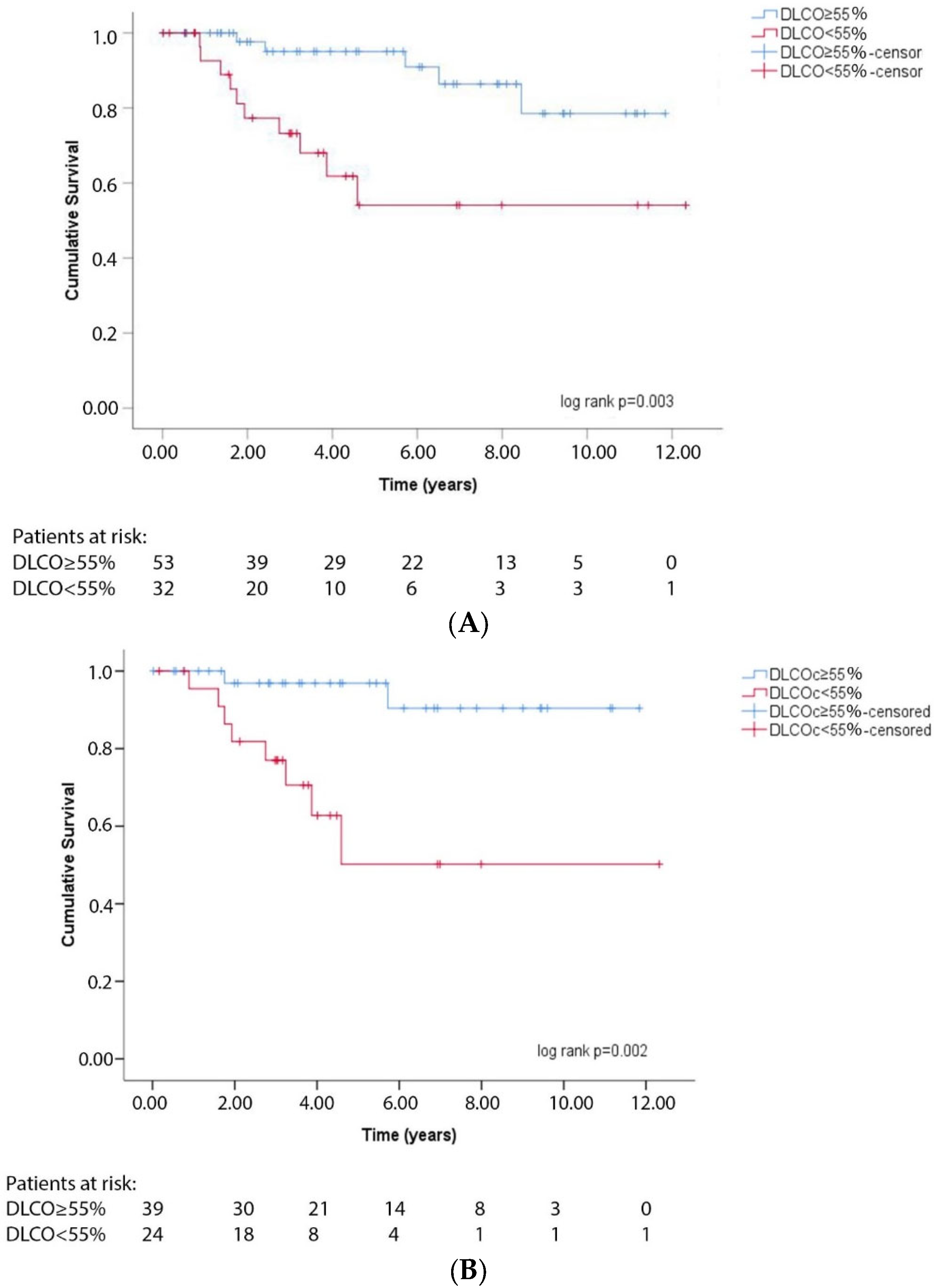
3.2. DLCO and Transplant-Free Survival
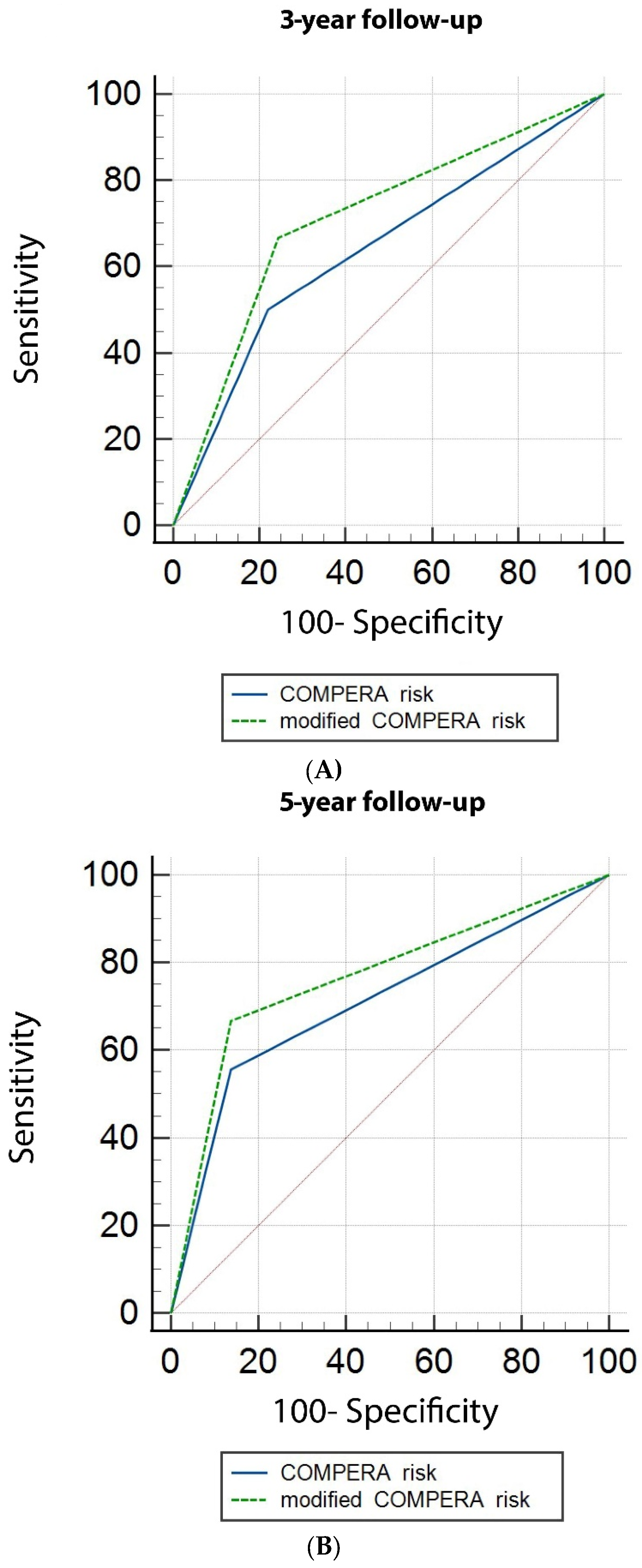
3.3. DLCO and Risk Stratification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Preston, I.R. Right heart catheterisation: Best practice and pitfalls in pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; de Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1700889. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Kylhammar, D.; Kjellström, B.; Hjalmarsson, C.; Jansson, K.; Nisell, M.; Söderberg, S.; Wikström, G.; Radegran, G. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur. Heart J. 2018, 39, 4175–4181. [Google Scholar] [CrossRef]
- Benza, R.L.; Gomberg-Maitland, M.; Elliott, C.G.; Farber, H.W.; Foreman, A.J.; Frost, A.E.; McGoon, M.D.; Pasta, D.J.; Selej, M.; Burger, C.D.; et al. Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest 2019, 156, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Raina, A.; Humbert, M. Risk assessment in pulmonary arterial hypertension. Eur. Respir. Rev. 2016, 25, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.M.B.; Pride, N.B. In defence of the carbon monoxide transfer coefficient KCO (TL/VA). Eur. Respir. J. 2001, 17, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.M.B.; Pride, N.B. Examination of the carbon monoxide diffusing capacity (DLCO) in relation to its KCO and VA components. Am. J. Respir. Crit. Care Med. 2012, 186, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Hyatt, R.E.; Scanlon, P.D.; Nakamura, M. Interpretation of Pulmonary Function Tests, a Practical Guide, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2014; p. 41. [Google Scholar]
- Frost, A.; Badesch, D.; Gibbs, J.S.R.; Gopalan, D.; Khanna, D.; Manes, A.; Oudiz, R.; Satoh, T.; Torres, F.; Torbicki, A. Diagnosis of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801904. [Google Scholar] [CrossRef] [Green Version]
- Coghlan, J.G.; Denton, C.P.; Grünig, E.; Bonderman, D.; Distler, O.; Khanna, D.; Müller-Ladner, U.; Pope, J.E.; Vonk, M.C.; Doelberg, M.; et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann. Rheum. Dis. 2014, 73, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allanore, Y.; Borderie, D.; Avouac, J.; Zerkak, D.; Meune, C.; Hachulla, E.; Mouthon, L.; Guillevin, L.; Meyer, O.; Ekindjian, O.G.; et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008, 58, 284–291. [Google Scholar] [CrossRef]
- Khanna, D.; Gladue, H.; Channick, R.; Chung, L.; Distler, O.; Furst, D.; Hachulla, E.; Humbert, M.; Langleben, D.; Mathai, S.; et al. Recommendations for Screening and Detection of Connective-Tissue Disease Associated Pulmonary Arterial Hypertension. Arthritis Rheum. 2013, 65, 3194–3201. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Shah, S.J.; Thenappan, T.; Archer, S.L.; Rich, S.; Gomberg-Maitland, M. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J. Heart Lung Transplant. 2010, 29, 181–187. [Google Scholar] [CrossRef]
- Trip, P.; Nossent, E.J.; De Man, F.S.; van den Berk, I.; Boonstra, A.; Groepenhoff, H.; Leter, E.M.; Westerhof, N.; Grünberg, K.; Bogaard, H.; et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: Patient characteristics and treatment responses. Eur. Respir. J. 2013, 42, 1575–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benza, R.L.; Gomberg-Maitland, M.; Miller, D.P.; Frost, A.; Frantz, R.; Foreman, A.; Badesch, D.B.; McGoon, M.D. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012, 141, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Farber, H.W.; Frost, A.; Ghofrani, H.A.; Gomez-Sanchez, M.A.; Langleben, D.; Rosenkranz, S.; Busse, D.; Meier, C.; Nikkho, S.; et al. REVEAL risk scores applied to riociguat-treated patients in PATENT-2: Impact of changes in risk score on survival. J. Heart Lung Transplant. 2018, 37, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.F.; Chabot, F.; et al. Pulmonary arterial hypertension in France: Results from a national registry. Am. J. Respir. Crit. Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Eur. Respir. J. 1993, 6, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; Maclntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeper, M.M.; Pausch, C.; Olsson, K.M.; Huscher, D.; Pittrow, D.; Grünig, E.; Staehler, G.; Dario Vizza, C.; Gall, H.; Distler, O.; et al. COMPERA 2.0: A refined 4-strata risk assessment model for pulmonary arterial hypertension. Eur. Resp. J. 2021. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Kang, K.Y.; Jeon, C.H.; Choi, S.J.; Yoon, B.Y.; Choi, C.; Lee, C.H.; Suh, C.H.; Lee, C.W.; Cho, C.S.; Nam, E.J.; et al. Survival and prognostic factors in patients with connective tissue disease-associated pulmonary hypertension diagnosed by echocardiography: Results from a Korean nationwide registry. Int. J. Rheum. Dis. 2017, 20, 1227–1236. [Google Scholar] [CrossRef]
- Ramjug, S.; Hussain, N.; Hurdman, J.; Billings, C.; Charalampopoulos, A.; Elliot, C.A.; Kiely, D.G.; Sabroe, I.; Rajaram, S.; Swift, A.J.; et al. Idiopathic and Systemic Sclerosis-Associated Pulmonary Arterial Hypertension: A Comparison of Demographic, Hemodynamic, and MRI Characteristics and Outcomes. Chest 2017, 152, 92–102. [Google Scholar] [CrossRef]
- Lewis, R.A.; Thompson, A.A.; Billings, C.G.; Charalampopoulos, A.; Elliot, C.; Hamilton, N.; Hill, C.; Hurdman, J.; Rajaram, S.; Sabroe, I.; et al. Mild parenchymal lung disease and/or low diffusion capacity impacts survival and treatment response in patients diagnosed with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2020, 55, 2000041. [Google Scholar] [CrossRef]
- Rose, L.; Prins, K.W.; Archer, S.L.; Pritzker, M.; Weir, E.K.; Misialek, J.R.; Thenappan, T. Survival in pulmonary hypertension due to chronic lung disease: Influence of low diffusion capacity of the lungs for carbon monoxide. J. Heart Lung Transplant. 2019, 38, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, A.; Kolb, T.M.; Damico, R.L.; Hassoun, P.M.; McCormack, M.C.; Mathai, S.C. Diffusing capacity is an independent predictor of outcomes in pulmonary hypertension associated with COPD. Chest 2020, 158, 722–734. [Google Scholar] [CrossRef]
- Suda, R.; Tanabe, N.; Ishida, K.; Kato, F.; Urushibara, T.; Sekine, A.; Nishimura, R.; Jujo, T.; Sugiura, T.; Shigeta, A.; et al. Prognostic and pathophysiological marker for patients with chronic thromboembolic pulmonary hypertension: Usefulness of diffusing capacity for carbon monoxide at diagnosis. Respirology 2017, 22, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, Y.; Jaïs, X.; Jevnikar, M.; Boucly, A.; Weatherald, J.; Brenot, P.; Planche, O.; Parent, F.; Savale, L.; Fadel, E.; et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2019, 38, 833–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, S.; Mergenthaler, N.; Lange, T.J. The prognostic value of DLCO and pulmonary blood flow in patients with pulmonary hypertension. Pulm. Circ. 2019, 9, 2045894019894531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeper, M.M.; Pausch, C.; Grünig, E.; Klose, H.; Staehler, G.; Huscher, D.; Pittrow, D.; Olsson, K.M.; Vizza, C.D.; Gall, H.; et al. Idiopathic pulmonay arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J. Heart Lung Transplant. 2020, 39, 1435–1444. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Vonk-Noordegraaf, A. Is there a vanishing pulmonary capillary syndrome? Lancet Respir. Med. 2017, 5, 676–678. [Google Scholar] [CrossRef]
- Gall, H.; Felix, J.F.; Schneck, F.K.; Milger, K.; Sommer, N.; Voswinckel, R.; Franco, O.H.; Hofman, A.; Schermuly, R.T.; Weissmann, N.; et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J. Heart Lung Transplant. 2017, 36, 957–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelniker, T.A.; Huscher, D.; Vonk-Noordegraaf, A.; Ewert, R.; Lange, T.; Klose, H.; Dumitrescu, D.; Halank, M.; Held, M.; Gall, H.; et al. The 6MWT as a prognostic tool in pulmonary arterial hypertension: Results from the COMPERA registry. Clin. Res. Cardiol. 2018, 107, 460–470. [Google Scholar] [CrossRef]
- Farkhooy, A.; Bellocchia, M.; Hedenström, H.; Libertucci, D.; Bucca, C.; Janson, C.; Solidoro, P.; Malinovschi, A. Lung function in relation to six-minute walk test in pulmonary hypertension. Eur. Clin. Respir. J. 2020, 7, 1745492. [Google Scholar] [CrossRef] [Green Version]
- Berghaus, T.M.; Kutsch, J.; Faul, C.; von Scheidt, W.; Schwaiblmair, M. The association of N-terminal pro-brain-type natriuretic peptide with hemodynamics and functional capacity in therapy-naive precapillary pulmonary hypertension: Results from a cohort study. BMC Pulm. Med. 2017, 17, 167. [Google Scholar] [CrossRef] [Green Version]
- Szturmowicz, M.; Kacprzak, A.; Franczuk, M.; Burakowska, B.; Kurzyna, M.; Fijalkowska, A.; Skoczylas, A.; Wesolowski, S.; Kus, J.; Torbicki, A. Low DLCO in idiopathic pulmonary arterial hypertension-clinical correlates and prognostic significance. Pneumonol. Alergol. Pol. 2016, 84, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Swedberg, K.; Cleland, J.; Dargie, H.; Drexler, H.; Follath, F.; Komajda, M.; Tavazzi, L.; Smiseth, O.A.; Gavazzi, A.; Haverich, A.; et al. Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005). Eur. Heart J. 2005, 26, 1115–1140. [Google Scholar] [CrossRef] [Green Version]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Cheng Jing, Z.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef]
- Kazimierczyk, R.; Kazimierczyk, E.; Knapp, M.; Sobkowicz, B.; Malek, L.A.; Blaszczak, P.; Ptaszynska-Kopczynska, K.; Grzywna, R.; Kaminski, K.A. Echocardiographic assessment of right ventricular-arterial coupling in predicting prognosis of pulmonary arterial hypertension patients. J. Clin. Med. 2021, 10, 2995. [Google Scholar] [CrossRef]
- Boucly, A.; Savale, L.; Jaïs, X.; Bauer, F.; Bergot, E.; Bertoletti, L.; Beurnier, A.; Bourdin, A.; Bouvaist, H.; Bulifon, S.; et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2021, 204, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Awdish, R.; Cajigas, H. Definition, epidemiology and registries of pulmonary hypertension. Heart Fail. Rev. 2016, 21, 223–228. [Google Scholar] [CrossRef]
- Humbert, M.; Farber, H.W.; Ghofrani, H.A.; Benza, R.L.; Busse, D.; Meier, C.; Hoeper, M.M. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1802004. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Staehler, G.; Gall, H.; Grünig, E.; Held, M.; Halank, M.; Klose, H.; Vonk-Noordegraaf, A.; Rosenkranz, S.; Pepke-Zaba, J.; et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur. Respir. J. 2018, 52, 1800248. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, A.; Tello, K.; Faber, M.; Sommer, N.; Kuhnert, S.; Seeger, W.; Grimminger, F.; Ardeschir Ghofrani, H.; Richter, M.J.; Gall, H. Risk assessment in severe pulmonary hypertension due to interstitial lung disease. J. Heart Lung Transplant. 2020, 39, 1118–1125. [Google Scholar] [CrossRef]
- Lankeit, M.; Krieg, V.; Hobohm, L.; Kölmel, S.; Liebetrau, C.; Konstantinides, S.; Hamm, C.W.; Mayer, E.; Wiedenroth, C.B.; Guth, S. Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2018, 37, 250–258. [Google Scholar] [CrossRef]
- Quadery, S.R.; Swift, A.J.; Billings, C.G.; Thompson, A.A.R.; Elliot, C.A.; Hurdman, J.; Charalampopoulos, A.; Sabroe, I.; Armstrong, I.J.; Hamilton, N.; et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2018, 52, 1800589. [Google Scholar] [CrossRef]
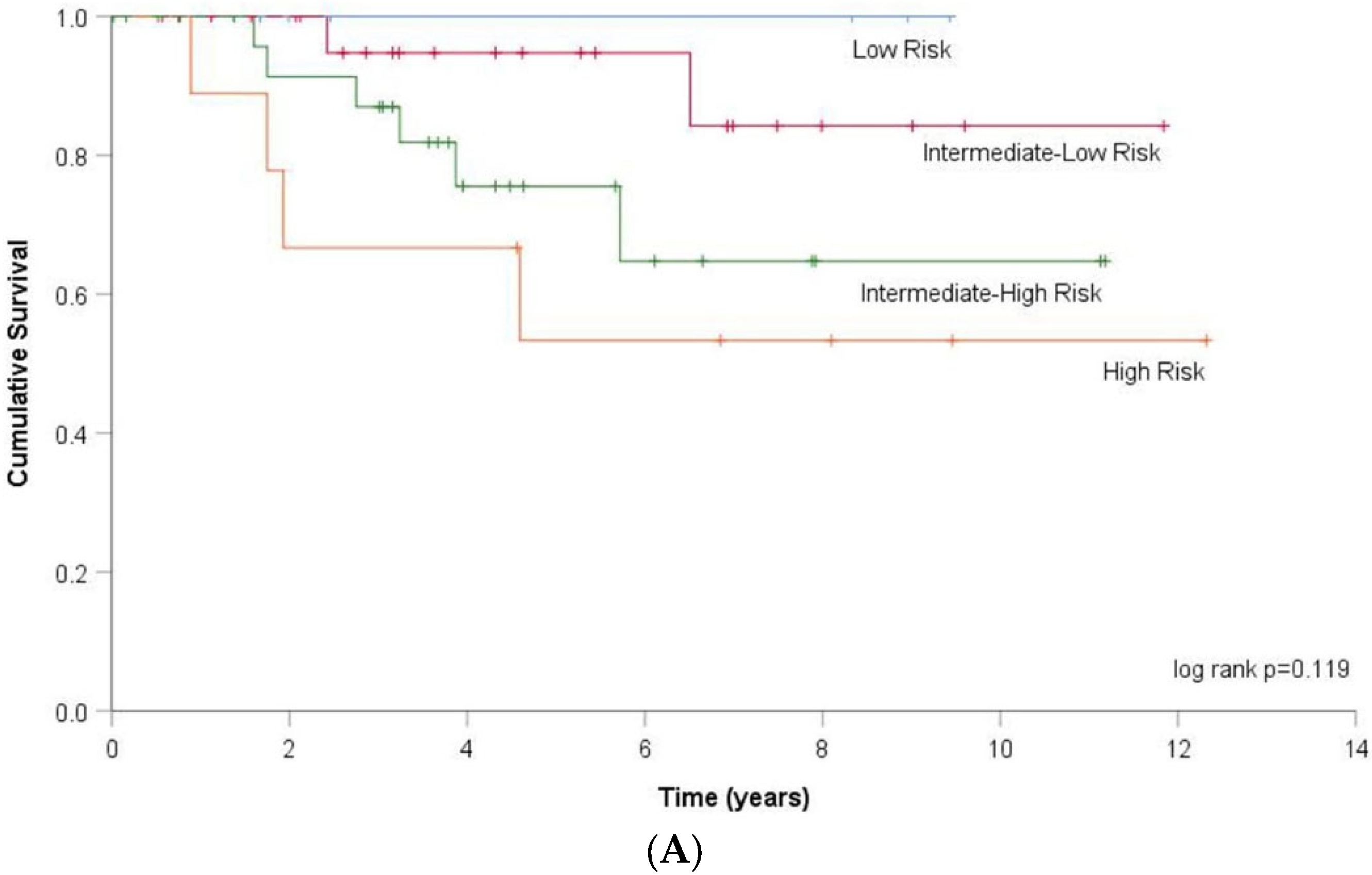
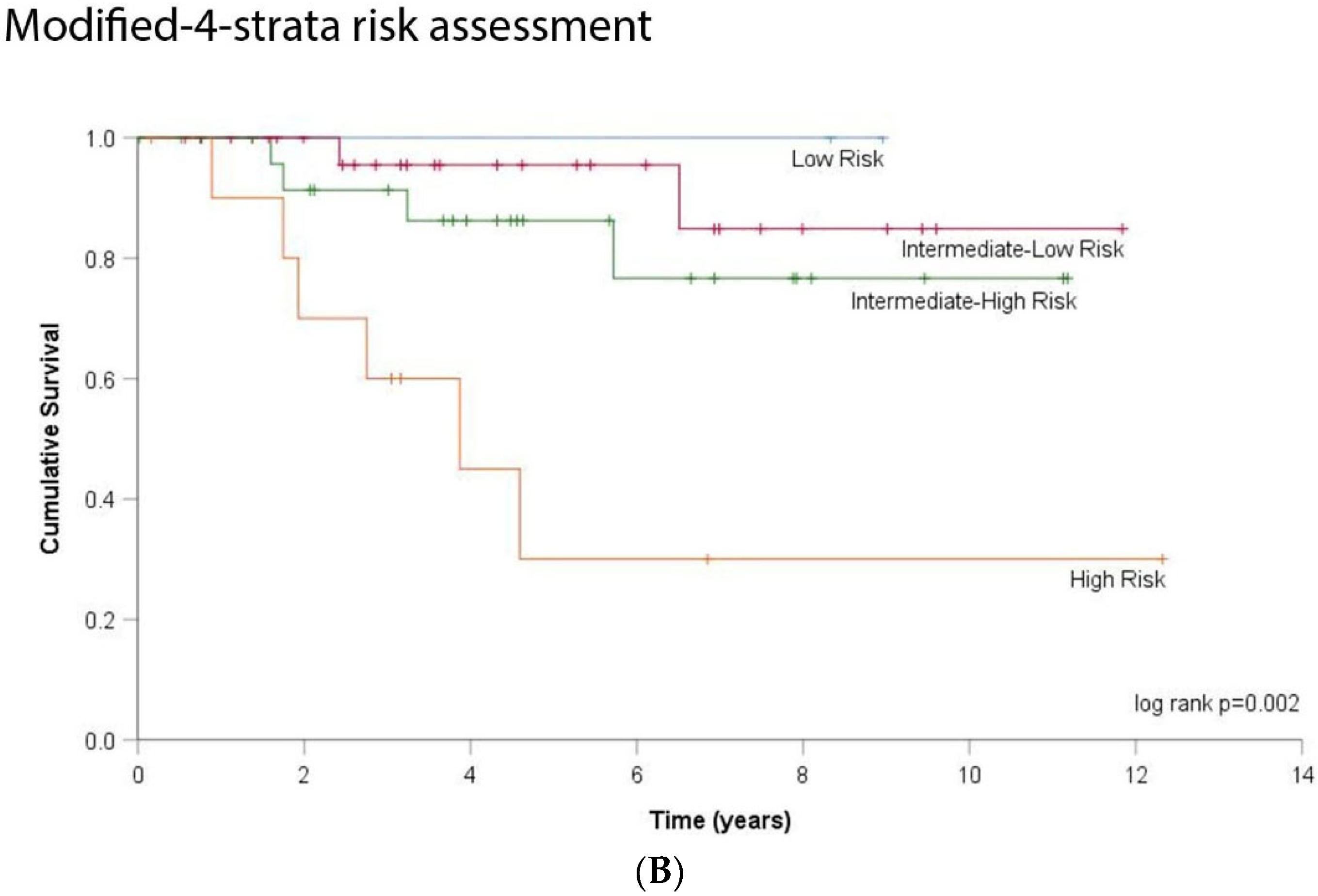
| Data (N, %) | DLCOc < 55% N = 32 | DLCOc ≥ 55% N = 53 | p | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age at diagnosis (range) | 85 (100) | 65 (32–89) | 61 (23–89) | 0.196 |
| Female sex (N = 47, 53%) | 17 (53%) | 30 (57%) | 0.824 | |
| Dyspnea NYHA III-IV (N, %) | 82 (96) | 25 (78) | 27 (51) | 0.005 |
| Smoking status (N, %) | 84 (99) | |||
| Never smokers (N, %) | 11 (34) | 25 (47) | 0.364 | |
| Pulmonary function | ||||
| Median DLCOc (%) (range) | 85 (100) | 41.5 (13–54) | 71 (56–102) | |
| Median Va (%) (range) | 79 (93) | 72 (41–100) | 88 (64–124) | <0.001 |
| Median KCO (%) (range) | 83 (98) | 59 (18–102) | 85 (50–122) | <0.001 |
| Median FEV1 (%) (range) | 83 (98) | 71 (22–128) | 92.5 (37–142) | <0.001 |
| Haemodynamics | ||||
| mPAP (mmHg) (range) | 85 (100) | 44 (28–88) | 42 (27–83) | 0.552 |
| sPAP (mmHg) (range) | 83 (98) | 69 (41–142) | 75 (35–146) | 0.437 |
| CI (L/min/m2) (range) | 76 (89) | 2.48 (1.4–3.39) | 2.5 (1.45–6) | 0.365 |
| mRAP (mmHg) (range) | 72 (85) | 9 (1–18) | 6 (0–16) | 0.042 |
| PAWP (mmHg) (range) | 79 (93) | 11 (6–15) | 10 (2–15) | 0.150 |
| 6MWT | ||||
| Distance (m) (range) | 74 (87.5) | 300 (120–530) | 445 (136–705) | 0.015 |
| Resting SpO2 (%) (range) | 74 (87.5) | 93 (65–98) | 94 (83–98) | 0.542 |
| Min SpO2 (%) (range) | 75 (88) | 83 (44–100) | 87 (68–96) | 0.017 |
| Serum biomarkers | ||||
| NT-proBNP (ng/L) (range) | 80 (94) | 978 (52–6127) | 466.5 (32–7137) | 0.072 |
| Survival | ||||||
|---|---|---|---|---|---|---|
| All Patients (N = 85) | ||||||
| Univariate Analysis | Multivariate Analysis * | |||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| DLCOc | 0.939 | 0.908–0.971 | <0.001 | 0.932 | 0.882–0.986 | 0.013 |
| DLCOc < 55% | 4.676 | 1.583–13.817 | 0.005 | 5.665 | 1.196–26.842 | 0.029 |
| Patients in intermediate- and high-risk category (N = 63) | ||||||
| Univariate analysis | Multivariate analysis ** | |||||
| DLCOc | 0.931 | 0.890–0.975 | 0.002 | 0.934 | 0.890–0.980 | 0.005 |
| DLCOc < 55% | 8.407 | 1.755–40.261 | 0.008 | 7.082 | 1.449–34.604 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamanti, E.; Karava, V.; Yerly, P.; Aubert, J.D. Carbon Monoxide Diffusion Capacity as a Severity Marker in Pulmonary Hypertension. J. Clin. Med. 2022, 11, 132. https://doi.org/10.3390/jcm11010132
Diamanti E, Karava V, Yerly P, Aubert JD. Carbon Monoxide Diffusion Capacity as a Severity Marker in Pulmonary Hypertension. Journal of Clinical Medicine. 2022; 11(1):132. https://doi.org/10.3390/jcm11010132
Chicago/Turabian StyleDiamanti, Eleni, Vasiliki Karava, Patrick Yerly, and John David Aubert. 2022. "Carbon Monoxide Diffusion Capacity as a Severity Marker in Pulmonary Hypertension" Journal of Clinical Medicine 11, no. 1: 132. https://doi.org/10.3390/jcm11010132
APA StyleDiamanti, E., Karava, V., Yerly, P., & Aubert, J. D. (2022). Carbon Monoxide Diffusion Capacity as a Severity Marker in Pulmonary Hypertension. Journal of Clinical Medicine, 11(1), 132. https://doi.org/10.3390/jcm11010132






