Effectiveness of S-O Clip-Assisted Colorectal Endoscopic Submucosal Dissection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. ESD Preparation
2.3. Conventional Colonic ESD
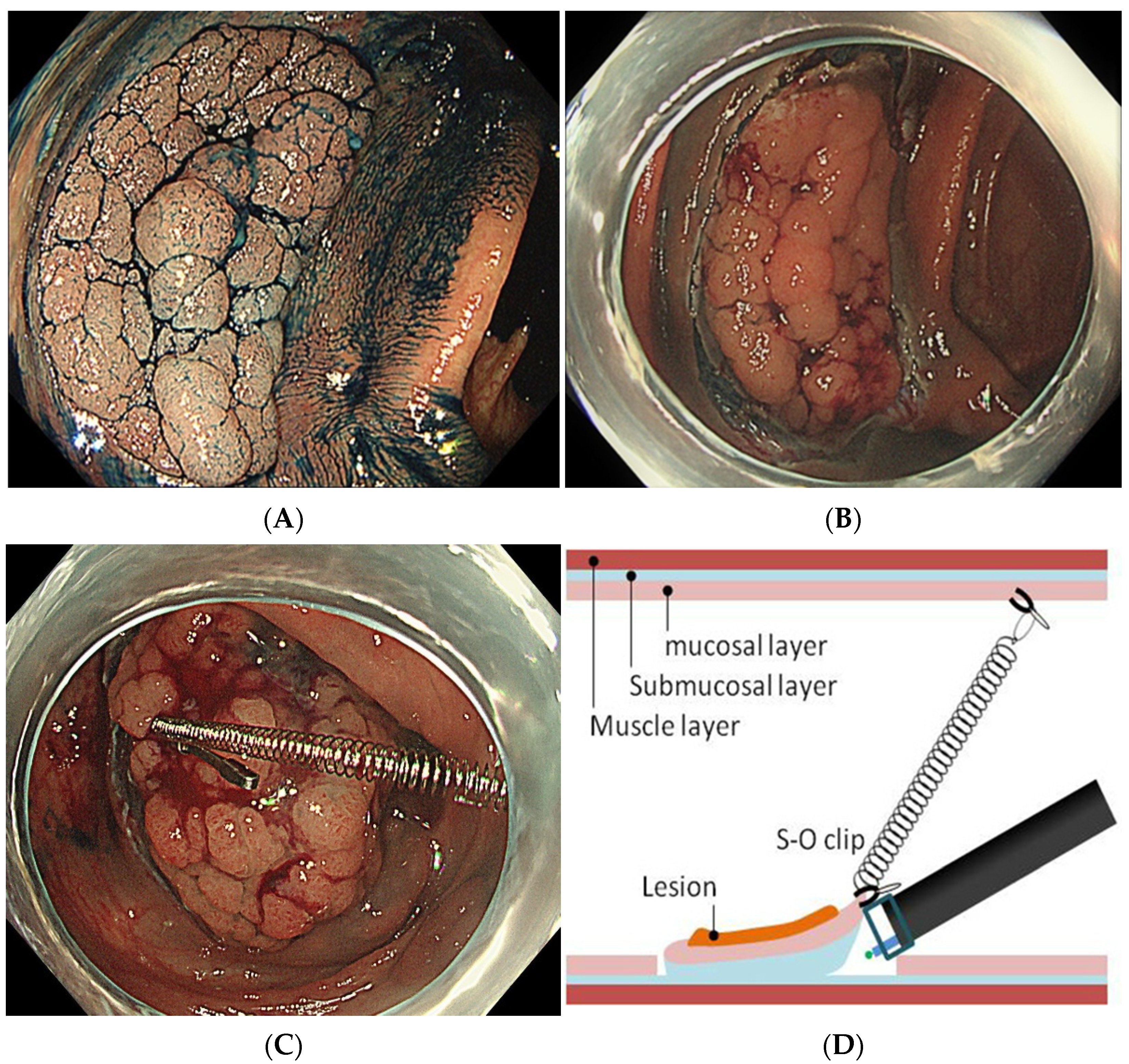
2.4. S-O Clip-Assisted ESD
2.5. Evaluation of Therapeutic Efficacy and Complications
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shigita, K.; Oka, S.; Tanaka, S.; Sumimoto, K.; Hirano, D.; Tamaru, Y.; Ninomiya, Y.; Asayama, N.; Hayashi, N.; Shimamoto, F.; et al. Long-term outcomes after endoscopic submucosal dissection for superficial colorectal tumors. Gastrointest. Endosc. 2017, 85, 546–553. [Google Scholar] [CrossRef]
- Cao, Y.; Liao, C.; Tan, A.; Gao, Y.; Mo, Z.; Gao, F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009, 41, 751–757. [Google Scholar] [CrossRef]
- Tanaka, S.; Oka, S.; Kaneko, I.; Hirata, M.; Mouri, R.; Kanao, H.; Yoshida, S.; Chayama, K. Endoscopic submucosal dissection for colorectal neoplasia: Possibility of standardization. Gastrointest. Endosc. 2007, 66, 100–107. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, J.B.; Choi, Y.S.; Lee, S.H.; Lee, D.H.; Kim, D.S.; Youk, E.G. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg. Endosc. 2012, 26, 1587–1594. [Google Scholar] [CrossRef]
- Mizushima, T.; Kato, M.; Iwanaga, I.; Sato, F.; Kubo, K.; Ehira, N.; Uebayashi, M.; Ono, S.; Nakagawa, M.; Mabe, K.; et al. Technical difficulty according to location, and risk factors for perforation, in endoscopic submucosal dissection of colorectal tumors. Surg. Endosc. 2015, 29, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, J.B.; Lee, S.H.; Kim, D.S.; Lee, D.H.; Lee, D.S.; Youk, E.G. Endoscopic submucosal dissection for colorectal tumors--1000 colorectal ESD cases: One specialized institute’s experiences. Surg. Endosc. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Uraoka, T.; Yamaguchi, Y.; Hotta, K.; Sakamoto, N.; Ikematsu, H.; Fukuzawa, M.; Kobayashi, N.; Nasu, J.; Michida, T.; et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest. Endosc. 2010, 72, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Ono, S.; Ohki, D.; Takeuchi, C.; Yakabi, S.; Kataoka, Y.; Saito, I.; Sakaguchi, Y.; Minatsuki, C.; Tsuji, Y.; et al. Recent development of techniques and devices in colorectal endoscopic submucosal dissection. Clin. Endosc. 2017, 50, 562–568. [Google Scholar] [CrossRef]
- Sakamoto, N.; Osada, T.; Shibuya, T.; Beppu, K.; Matsumoto, K.; Mori, H.; Kawabe, M.; Nagahara, A.; Otaka, M.; Ogihara, T.; et al. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest. Endosc. 2009, 69, 1370–1374. [Google Scholar] [CrossRef]
- Sakamoto, N.; Osada, T.; Shibuya, T.; Beppu, K.; Matsumoto, K.; Shimada, Y.; Konno, A.; Kurosawa, A.; Nagahara, A.; Ohkusa, T.; et al. The facilitation of a new traction device (S-O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy 2008, 40 (Suppl. S2), E94–E95. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Naito, Y.; Kugai, M.; Inoue, K.; Wakabayashi, N.; Yagi, N.; Yanagisawa, A.; Yoshikawa, T. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J. Gastroenterol. 2010, 16, 4180–4186. [Google Scholar] [CrossRef]
- Yamashina, T.; Takeuchi, Y.; Nagai, K.; Matsuura, N.; Ito, T.; Fujii, M.; Hanaoka, N.; Higashino, K.; Uedo, N.; Ishihara, R.; et al. Scissor-type knife significantly improves self-completion rate of colorectal endoscopic submucosal dissection: Single-center prospective randomized trial. Dig. Endosc. 2017, 29, 322–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujinami, H.; Hosokawa, A.; Ogawa, K.; Nishikawa, J.; Kajiura, S.; Ando, T.; Ueda, A.; Yoshita, H.; Sugiyama, T. Endoscopic submucosal dissection for superficial esophageal neoplasms using the stag beetle knife. Dis. Esophagus 2014, 27, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Oka, S.; Chayama, K. Colorectal endoscopic submucosal dissection: Present status and future perspective, including its differentiation from endoscopic mucosal resection. J. Gastroenterol. 2008, 43, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Itoh, T.; Horiki, N.; Matsuda, M.; Setoyama, T.; Suzuki, S.; Uemura, M.; Iizuka, Y.; Fukuda, K.; Suzuki, K.; et al. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife for large superficial colorectal neoplasia including ileocecal lesions. Surg. Endosc. 2010, 24, 1941–1947. [Google Scholar] [CrossRef]
- Lee, B.I. Debates on colorectal endoscopic submucosal dissection—Traction for effective dissection: Gravity is enough. Clin. Endosc. 2013, 46, 467–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopini, F.; Saito, Y.; Bella, A.; Gotoda, T.; Rigato, P.; Elisei, W.; Montagnese, F.; Iacopini, G.; Costamagna, G. Colorectal endoscopic submucosal dissection: Predictors and neoplasm-related gradients of difficulty. Endosc. Int. Open 2017, 5, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Imaeda, H.; Hosoe, N.; Ida, Y.; Kashiwagi, K.; Morohoshi, Y.; Suganuma, K.; Nagakubo, S.; Komatsu, K.; Suzuki, H.; Saito, Y.; et al. Novel technique of endoscopic submucosal dissection using an external grasping forceps for superficial gastric neoplasia. Dig. Endosc. 2009, 21, 122–127. [Google Scholar] [CrossRef]
- Imaeda, H.; Hosoe, N.; Ida, Y.; Nakamizo, H.; Kashiwagi, K.; Kanai, T.; Iwao, Y.; Hibi, T.; Ogata, H. Novel technique of endoscopic submucosal dissection by using external forceps for early rectal cancer (with videos). Gastrointest. Endosc. 2012, 75, 1253–1257. [Google Scholar] [CrossRef]
- Chen, P.J.; Chu, H.C.; Chang, W.K.; Hsieh, T.Y.; Chao, Y.C. Endoscopic submucosal dissection with internal traction for early gastric cancer (with video). Gastrointest. Endosc. 2008, 67, 128–132. [Google Scholar] [CrossRef]
- Okamoto, K.; Muguruma, N.; Kitamura, S.; Kimura, T.; Takayama, T. Endoscopic submucosal dissection for large colorectal tumors using a cross-counter technique and a novel large-diameter balloon overtube. Dig. Endosc. 2012, 24 (Suppl. S1), 96–99. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nagahara, A.; Ueyama, H.; Konuma, H.; Morimoto, T.; Sasaki, H.; Hayashi, T.; Shibuya, T.; Sakamoto, N.; Osada, T.; et al. Development and clinical usability of a new traction device “medical ring” for endoscopic submucosal dissection of early gastric cancer. Surg. Endosc. 2013, 27, 3444–3451. [Google Scholar] [CrossRef] [Green Version]
- Parra-Blanco, A.; Nicolas, D.; Arnau, M.R.; Gimeno-Garcia, A.Z.; Rodrigo, L.; Quintero, E. Gastric endoscopic submucosal dissection assisted by a new traction method: The clip-band technique. A feasibility study in a porcine model (with video). Gastrointest. Endosc. 2011, 74, 1137–1141. [Google Scholar] [CrossRef]
- Mori, H.; Kobara, H.; Nishiyama, N.; Fujihara, S.; Matsunaga, T.; Masaki, T. Novel effective and repeatedly available ring-thread counter traction for safer colorectal endoscopic submucosal dissection. Surg. Endosc. 2017, 31, 3040–3047. [Google Scholar] [CrossRef] [Green Version]
- Ritsuno, H.; Sakamoto, N.; Osada, T.; Goto, S.P.; Murakami, T.; Ueyama, H.; Mori, H.; Matsumoto, K.; Beppu, K.; Shibuya, T.; et al. Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S-O clip. Surg. Endosc. 2014, 28, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M. Internal traction method using a spring-and-loop with clip (S-O clip) allows countertraction in gastric endoscopic submucosal dissection. Surg. Endosc. 2020, 34, 3722–3733. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Nishiyama, H.; Yamaguchi, N.; Fukuda, E.; Ishii, H.; Ikeda, K.; Ohnita, K.; Nakao, S.; Shikuwa, S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2009, 41, 679–683. [Google Scholar] [CrossRef]




| CO Group (n = 47) | SO Group (n = 80) | p-Value | |
|---|---|---|---|
| Male/Female, n | 32/15 | 47/33 | 0.345 |
| Mean age (range), years | 65.5 (38–80) | 69.7 (39–89) | 0.531 |
| Lesion size, mean ± SD (range), mm | 29.4 ± 9.1 (20–48) | 30.6 ± 7.5 (20–50) | 0.272 |
| Lesion location | 0.685 | ||
| Right colon, n | 35 | 56 | |
| Left colon, n | 12 | 24 |
| CO Group (n = 47) | SO Group (n = 80) | p-Value | |
|---|---|---|---|
| Surgery duration, mean ± SD (range), min | 73.9 ± 43.5 (31–226) | 52.3 ± 21.8 (16–113) | 0.0006 * |
| Lesion area, mean ± SD (range), mm2 | 616.8 ± 576.8 (235.6–1507.9) | 660.6 ± 333.6 (259.2–1696.4) | 0.227 |
| Dissection time, mean ± SD (range), min | 49.7 ± 37.1 (17–189) | 31.9 ± 16.4 (7–82) | <0.001 * |
| Dissection speed, mean ± SD (range), mm2/min | 14.8 ± 8.7 (4.1–50.1) | 24.4 ± 12.9 (5.5–70.6) | 0.0014 * |
| En bloc resection rate, % (n) | 80.9 (38/47) | 98.8 (79/80) | <0.001 * |
| Perforation rate, % (n) | 4.3 (2/47) | 1.3 (1/80) | 0.554 |
| Hemorrhage rate, % (n) | 0 (0/47) | 2.5 (2/80) | 0.530 |
| CO Group | SO Group | p-Value | |
|---|---|---|---|
| Right colon, n | 35 | 56 | |
| Surgery duration, mean ± SD (range), min | 78.1 ± 48.0 (33–226) | 52.2 ± 21.3 (16–113) | 0.0054 * |
| Lesion area, mean ± SD (range), mm2 | 648.4 ± 660.4 (235.6–1507.9) | 685.5 ± 324.3 (259.1–1445.1) | 0.1220 |
| Dissection time, mean ± SD (range), min | 51.5 ± 40.9 (17–189) | 30.7 ± 15.2 (7–64) | 0.0019 * |
| Dissection speed, mean ± SD (range), mm2/min | 14.9 ± 9.1 (4.0–50.1) | 25.4 ± 11.7 (9.5–61.7) | <0.001 * |
| En bloc resection rate, % (n) | 77.1 (27/35) | 98.2 (55/56) | 0.0018 * |
| Left colon, n | 12 | 24 | |
| Surgery duration, mean ± SD (range), min | 61.5 ± 24.1 (31–121) | 51.9± 18.2 (26–112) | 0.3139 |
| Lesion area, mean ± SD (range), mm2 | 524.6 ± 175.4 (314.1–824.6) | 563.0 ± 291.4 (311.0–1696.4) | 0.9464 |
| Dissection time, mean ± SD (range), min | 44.4 ± 23.4 (20–100) | 33.3 ± 18.1 (14–82) | 0.1488 |
| Dissection speed, mean ± SD (range), mm2/min | 14.0 ± 8.0 (5.7–32.1) | 22.0 ± 15.5 (5.4–70.6) | 0.1587 |
| En bloc resection rate, % (n) | 91.7 (11/12) | 100 (24/24) | 0.3333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujinami, H.; Teramoto, A.; Takahashi, S.; Ando, T.; Kajiura, S.; Yasuda, I. Effectiveness of S-O Clip-Assisted Colorectal Endoscopic Submucosal Dissection. J. Clin. Med. 2022, 11, 141. https://doi.org/10.3390/jcm11010141
Fujinami H, Teramoto A, Takahashi S, Ando T, Kajiura S, Yasuda I. Effectiveness of S-O Clip-Assisted Colorectal Endoscopic Submucosal Dissection. Journal of Clinical Medicine. 2022; 11(1):141. https://doi.org/10.3390/jcm11010141
Chicago/Turabian StyleFujinami, Haruka, Akira Teramoto, Saeko Takahashi, Takayuki Ando, Shinya Kajiura, and Ichiro Yasuda. 2022. "Effectiveness of S-O Clip-Assisted Colorectal Endoscopic Submucosal Dissection" Journal of Clinical Medicine 11, no. 1: 141. https://doi.org/10.3390/jcm11010141
APA StyleFujinami, H., Teramoto, A., Takahashi, S., Ando, T., Kajiura, S., & Yasuda, I. (2022). Effectiveness of S-O Clip-Assisted Colorectal Endoscopic Submucosal Dissection. Journal of Clinical Medicine, 11(1), 141. https://doi.org/10.3390/jcm11010141







