Radiation-Induced Heart Disease
Abstract
1. Introduction
2. History of Radiation Therapy
3. Pathophysiology of RIHD
4. Types of Radiation Therapy
5. Risk Factors for Reaching the Threshold of RIHD
6. Screening
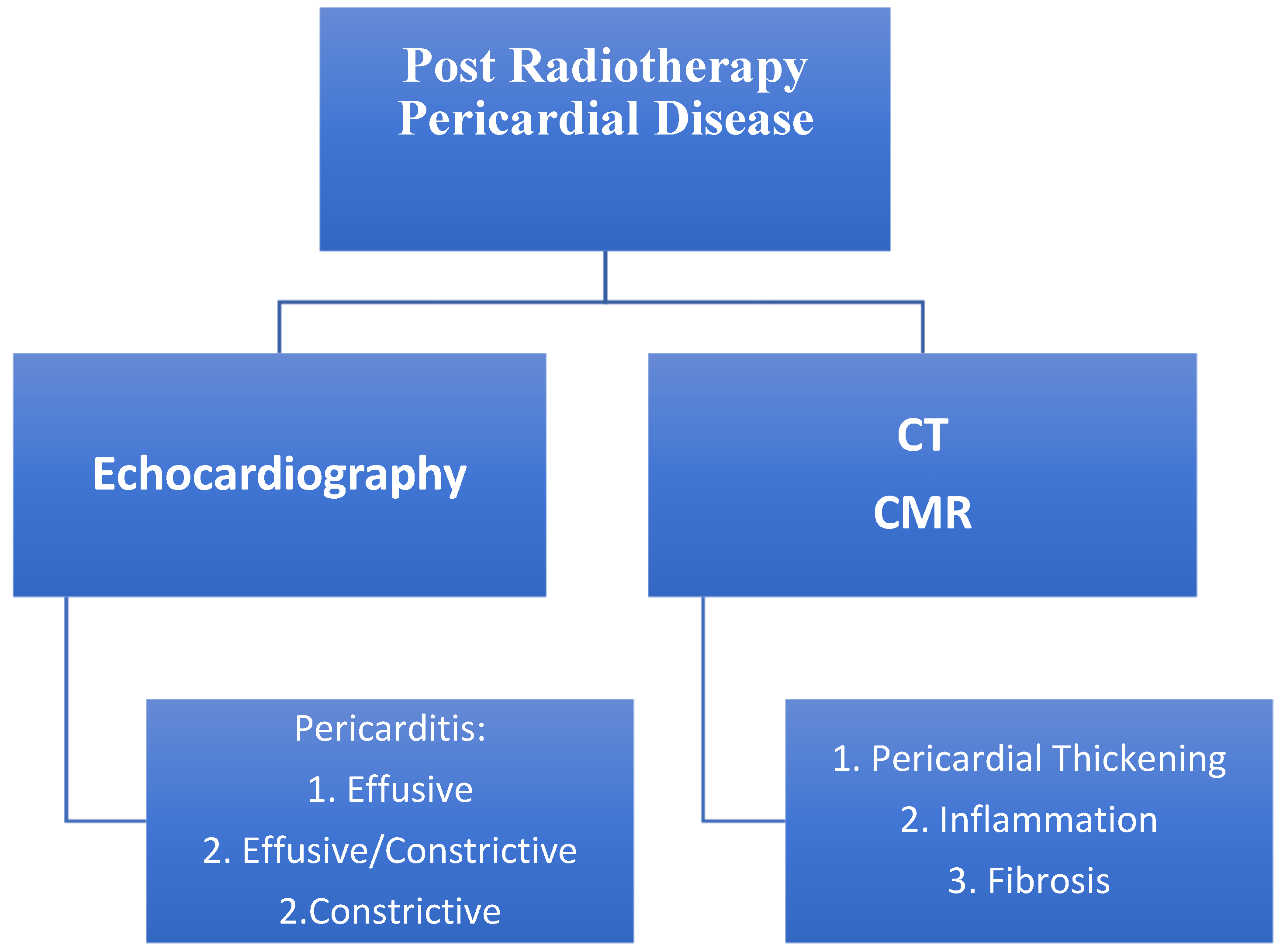
6.1. Pericardial Disease
Evaluation
6.2. Myocardial Dysfunction
Evaluation
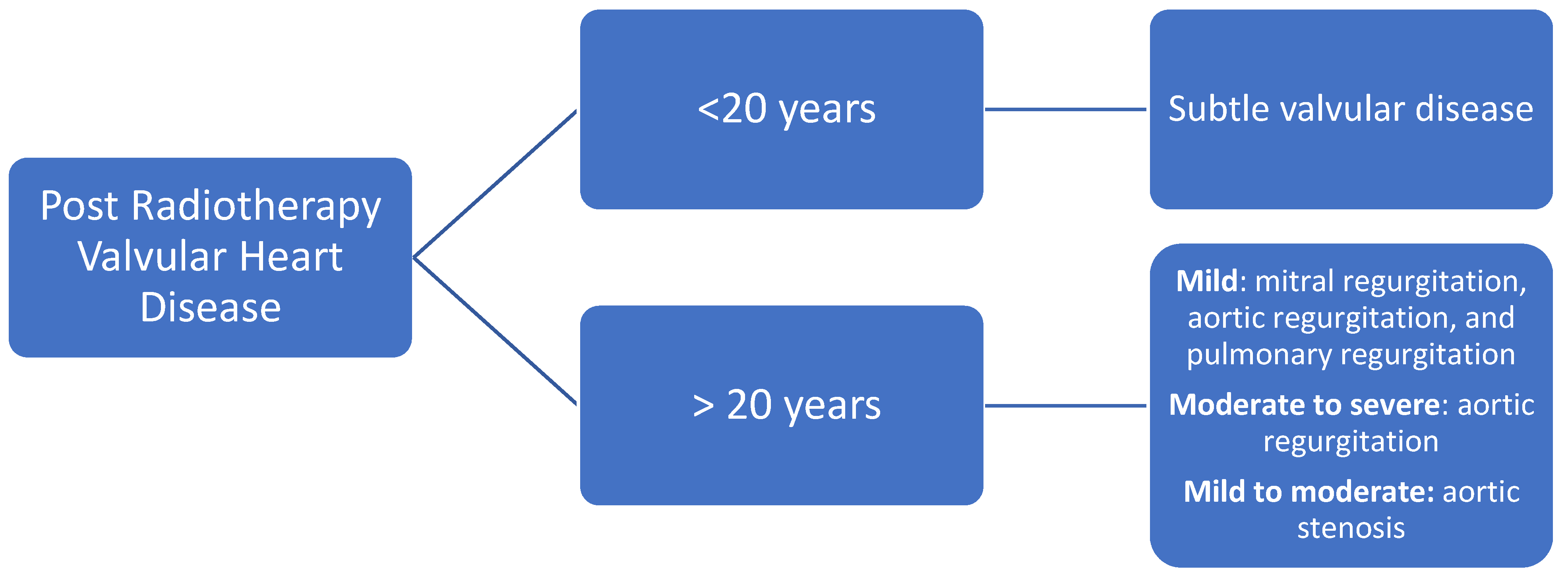
6.3. Valvular Disease
Evaluation
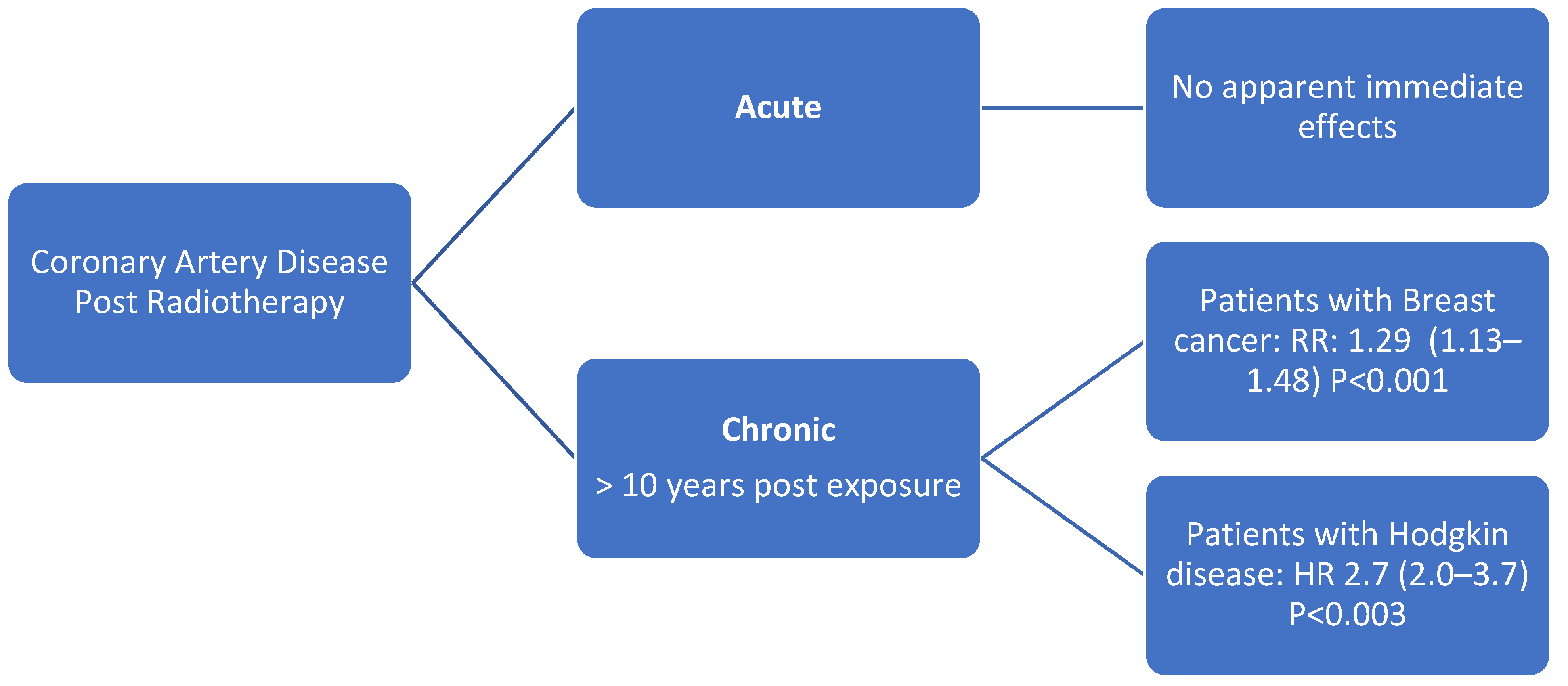
6.4. Coronary Artery Disease
Evaluation
6.5. Carotid Artery Disease
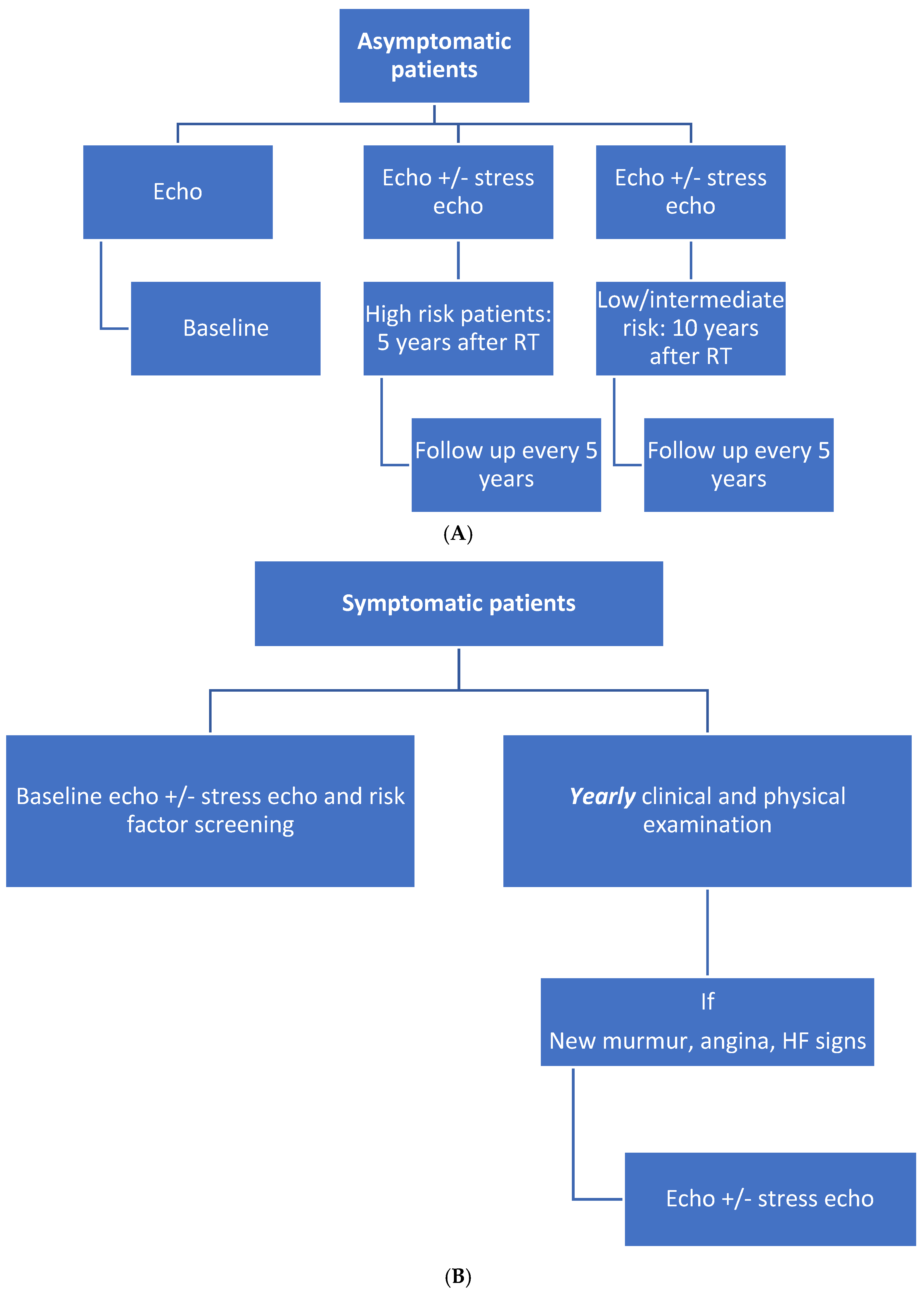
7. Surveillance Protocol after RT
7.1. Asymptomatic Patients
7.2. Symptomatic Patients
8. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hyuna, S.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Nkomo, V.T.; Badano, L.; Bergler, J.; Bogaert, J.; Davin, L.; Cosyns, B.; Coucke, P.; Dulgheru, R.; Edvardsen, T.; et al. Expert Consensus for Multi-Modality Imaging Evaluation of Cardiovascular Complications of Radiotherapy in Adults: A Report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 1013–1032. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The Role of Radiotherapy in Cancer Treatment: Estimating Optimal Utilization from a Review of Evidence-Based Clinical Guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Abe, O.; Abe, R.; Enomoto, K.; Kikuchi, K.; Koyama, H.; Masuda, H.; Nomura, Y.; Sakai, K.; Sugimachi, K.; Tominaga, T.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar]
- Barnett, G.C.; West, C.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.P.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Drost, L.; Yee, C.; Lam, H.; Zhang, L.; Wronski, M.; McCann, C.; Lee, J.; Vesprini, D.; Leung, E.; Chow, E. A Systematic Review of Heart Dose in Breast Radiotherapy. Clin. Breast Cancer 2018, 18, e819–e824. [Google Scholar] [CrossRef] [PubMed]
- Boekel, N.B.; Schaapveld, M.; Gietema, J.A.; Russell, N.S.; Poortmans, P.; Theuws, J.C.; Schinagl, D.A.; Rietveld, D.H.; Versteegh, M.I.; Visser, O.; et al. Cardiovascular Disease Risk in a Large, Population-Based Cohort of Breast Cancer Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients with Lung Cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.A.; Mendenhall, N.P. Novel Radiotherapy Techniques for Breast Cancer. Annu. Rev. Med. 2018, 69, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Tohyama, N.; Kodama, T.; Okabe, N.; Sakai, M.; Konoeda, K. Current Status of Intensity-Modulated Radiation Therapy for Prostate Cancer: History, Clinical Results and Future Directions. Int. J. Urol. 2019, 26, 775–784. [Google Scholar] [CrossRef]
- Takahashi, S. Conformation Radiotherapy. Rotation Techniques as Applied to Radiography and Radiotherapy of Cancer. Acta Radiol. Diagn. 1965, 242, 1–142. [Google Scholar]
- Brahme, A. Optimization of stationary and moving beam radiation therapy techniques. Radiother. Oncol. 1988, 12, 129–140. [Google Scholar] [CrossRef]
- Abshire, D.; Lang, M.K. The Evolution of Radiation Therapy in Treating Cancer. Semin. Oncol. Nurs. 2018, 34, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, A.; Schönecker, S.; Simonetto, C.; Eidemüller, M.; Pazos, M.; Reitz, D.; Rottler, M.; Freislederer, P.; Braun, M.; Würstlein, R.; et al. Heart sparing radiotherapy in breast cancer: The importance of baseline cardiac risks. Radiat. Oncol. 2020, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Kirova, Y.; Tallet, A.; Aznar, M.; Loap, P.; Bouali, A.; Bourgier, C. Radio-induced cardiotoxicity: From physiopathology and risk factors to adaptation of radiotherapy treatment planning and recommended cardiac follow-up. Cancer/Radiothérapie 2020, 24, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Yeung, R.; Conroy, L.; Long, K.; Walrath, D.; Li, H.; Smith, W.; Hudson, A.; Phan, T. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat. Oncol. 2015, 10, 200. [Google Scholar] [CrossRef]
- Remouchamps, V.M.; Vicini, F.A.; Sharpe, M.B.; Kestin, L.L.; Martinez, A.A.; Wong, J.W. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int. J. Radiat. Oncol. 2003, 55, 392–406. [Google Scholar] [CrossRef]
- Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37. 2007, Volume 103, pp. 1–332. Available online: https://www.icrp.org/publication.asp?id=ICRP%20Publication%20103 (accessed on 2 December 2021).
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Smtyh, M.L.; Knight, K.A.; Aarons, Y.K.; Wasiak, J. The Cardiac Dose-Sparing Benefits of Deep Inspiration Breath-Hold in Left Breast Irradiation: A Systematic Review. J. Med. Radiat. Sci. 2015, 62, 66–73. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, S.; Moses, A.; Nallathambi, C.; Mahata, A.; Mandal, S.; Achari, R.; Mallick, I.; Shrimali, R.K.; Bhattacharyya, T.; et al. Resource requirements and reduction in cardiac mortality from deep inspiration breath hold (DIBH) radiation therapy for left sided breast cancer patients: A prospective service development analysis. Pract. Radiat. Oncol. 2018, 8, 382–387. [Google Scholar] [CrossRef]
- Hull, M.C.; Morris, C.G.; Pepine, C.J.; Mendenhall, N.P. Valvular Dysfunction and Carotid, Subclavian, and Coronary Artery Disease in Survivors of Hodgkin Lymphoma Treated with Radiation Therapy. JAMA 2003, 290, 2831–2837. [Google Scholar] [CrossRef]
- Veinot, J.P.; Edwards, W.D. Pathology of Radiation-Induced Heart Disease: A Surgical and Autopsy Study of 27 Cases. Hum. Pathol. 1996, 27, 766–773. [Google Scholar] [CrossRef]
- Adams, M.J.; Lipshultz, S.E.; Schwartz, C.; Fajardo, L.F.; Coen, V.; Constine, L.S. Radiation-associated cardiovascular disease: Manifestations and management. Semin. Radiat. Oncol. 2003, 13, 346–356. [Google Scholar] [CrossRef]
- Van Der Pal, H.J.; Van Dalen, E.C.; Van Delden, E.; Van Dijk, I.W.; Kok, W.E.; Geskus, R.B.; Sieswerda, E.; Oldenburger, F.; Koning, C.C.; Van Leeuwen, F.E.; et al. High Risk of Symptomatic Cardiac Events in Childhood Cancer Survivors. J. Clin. Oncol. 2012, 30, 1429–1437. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-Related Heart Disease: Current Knowledge and Future Prospects. Int. J. Radiat. Oncol. 2010, 76, 656–665. [Google Scholar] [CrossRef]
- Raghunathan, D.; Khilji, M.I.; Hassan, S.A.; Yusuf, S.W. Radiation-Induced Cardiovascular Disease. Curr. Atheroscler. Rep. 2017, 19, 22. [Google Scholar] [CrossRef]
- Upadhaya, B.R.; Chakravarti, R.N.; Wahi, P.L. Post-Irradiation Vascular Injury and Accelerated Development of Experimental Atherosclerosis. Indian J. Med. Res. 1972, 60, 403–408. [Google Scholar]
- Liao, J.K. Endothelium and acute coronary syndromes. Clin. Chem. 1998, 44, 1799–1808. [Google Scholar] [CrossRef]
- Orit, K.Z.; Zagar, T.M.; Oldan, J.D.; Matney, J.; Jones, E.L.; Das, S.; Jensen, B.C.; Zellars, R.C.; Wong, T.Z.; Marks, L.B. Early Cardiac Perfusion Defects after Left-Sided Radiation Therapy for Breast Cancer: Is There a Volume Response? Breast Cancer Res. Treat. 2017, 164, 253–262. [Google Scholar]
- Marks, L.B.; Yu, X.; Prosnitz, R.G.; Zhou, S.-M.; Hardenbergh, P.H.; Blazing, M.; Hollis, D.; Lind, P.; Tisch, A.; Wong, T.Z.; et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int. J. Radiat. Oncol. 2005, 63, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, A.J.; Higgins, C.D.; Smith, P.; Cunningham, D.; Hancock, B.W.; Horwich, A.; Hoskin, P.; Lister, A.; Radford, J.; Rohatiner, A.Z.S.; et al. Myocardial Infarction Mortality Risk After Treatment for Hodgkin Disease: A Collaborative British Cohort Study. J. Natl. Cancer Inst. 2007, 99, 206–214. [Google Scholar] [CrossRef]
- Diamantopoulos, S.; Platoni, K.; Dilvoi, M.; Nazos, I.; Geropantas, K.; Maravelis, G.; Tolia, M.; Beli, I.; Efstathopoulos, E.; Pantelakos, P.; et al. Clinical implementation of total skin electron beam (TSEB) therapy: A review of the relevant literature. Phys. Med. 2011, 27, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Yoo, S.; Song, Y.; Kim, E.; Shin, J.; Han, S.; Jung, W.; Nam, S.; Lee, R.; Lee, K.; et al. Analysis of Neutron Production in Passively Scattered Ion-Beam Therapy. Radiat. Prot. Dosim. 2017, 175, 297–303. [Google Scholar] [CrossRef]
- Kamran, S.C.; Light, J.O.; Efstathiou, J.A. Proton Versus Photon-Based Radiation Therapy for Prostate Cancer: Emerging Evidence and Considerations in the Era of Value-Based Cancer Care. Prostate Cancer Prostatic Dis. 2019, 22, 509–521. [Google Scholar] [CrossRef]
- Bruno, G.; di Stefano, C.; Mutter, R.W.; Laack, N.N.; Kahila, M.M.; Whitaker, T.J.; Pellikka, P.A.; Pumper, G.M.; Herrmann, J.; Villarraga, H.R. P5228epicardial Versus Endocardial Strain and Strain Rate Evaluation in Oncological Patients Undergoing a New Radiotherapeutic Treatment: Proton Beam Versus Photon (X-Ray) Radiotherapy. Eur. Heart J. 2017, 38, ehx493.P5228. [Google Scholar] [CrossRef][Green Version]
- Kammerer, E.; Le Guevelou, J.; Chaikh, A.; Danhier, S.; Geffrelot, J.; Levy, C.; Saloux, E.; Habrand, J.-L.; Thariat, J. Proton therapy for locally advanced breast cancer: A systematic review of the literature. Cancer Treat. Rev. 2018, 63, 19–27. [Google Scholar] [CrossRef]
- Patel, S.H.; Wang, Z.; Wong, W.W.; Murad, M.H.; Buckey, C.R.; Mohammed, K.; Alahdab, F.; Altayar, O.; Nabhan, M.; Schild, S.E.; et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1027–1038. [Google Scholar] [CrossRef]
- Furlow, B. Dosimetric promise versus cost: Critics question proton therapy. Lancet Oncol. 2013, 14, 805–806. [Google Scholar] [CrossRef]
- Frankart, A.J.; Nagarajan, R.; Pater, L. The impact of proton therapy on cardiotoxicity following radiation treatment. J. Thromb. Thrombolysis 2021, 51, 877–883. [Google Scholar] [CrossRef]
- Cordova-Madera, S.N.; Garcia-Bello, L.; Bruno, G.; Di Stefano, C.; A Quintero-Martinez, J.; Laack, N.; Corbin, K.; Whitaker, T.; Pellikka, P.; Herrmann, J.; et al. Comprehensive strain analysis in oncological patients undergoing thoracic radiotherapy: 1-year follow-up of a prospective study comparing proton vs. photon beam therapy. Eur. Heart J. 2021, 42, ehab724.024. [Google Scholar] [CrossRef]
- Tseng, Y.D.; Cutter, D.J.; Plastaras, J.; Parikh, R.R.; Cahlon, O.; Chuong, M.D.; Dedeckova, K.; Khan, M.K.; Lin, S.-Y.; McGee, L.A.; et al. Evidence-based Review on the Use of Proton Therapy in Lymphoma from the Particle Therapy Cooperative Group (PTCOG) Lymphoma Subcommittee. Int. J. Radiat. Oncol. 2017, 99, 825–842. [Google Scholar] [CrossRef]
- Zhang, R.; Howell, R.M.; Taddei, P.; Giebeler, A.; Mahajan, A.; Newhauser, W.D. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiother. Oncol. 2014, 113, 84–88. [Google Scholar] [CrossRef]
- Cheng, Y.; Nie, X.; Ji, C.; Lin, X.; Liu, L.; Chen, X.; Yao, H.; Wu, S. Long-Term Cardiovascular Risk After Radiotherapy in Women with Breast Cancer. J. Am. Heart Assoc. 2017, 6, e005633. [Google Scholar] [CrossRef]
- Hancock, S.L.; Tucker, M.A.; Hoppe, R.T. Factors Affecting Late Mortality from Heart Disease after Treatment of Hodgkin’s Disease. JAMA 1993, 270, 1949–1955. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Hardenbergh, P.H.; Gelman, R.; Blanks, D.; Hauptman, P.; Recht, A.; Hayes, D.F.; Harris, J.; Henderson, I.C. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J. Clin. Oncol. 1998, 16, 3493–3501. [Google Scholar] [CrossRef]
- Hooning, M.J.; Botma, A.; Aleman, B.M.P.; Baaijens, M.H.A.; Bartelink, H.; Klijn, J.G.M.; Taylor, C.W.; Van Leeuwen, F.E. Long-Term Risk of Cardiovascular Disease in 10-Year Survivors of Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.; Krol, A.D.; Petersen, E.J.; Raemaekers, J.M.; Kok, W.E.; Aleman, B.M.; van Leeuwen, F.E. Cardiovascular Disease after Hodgkin Lymphoma Treatment: 40-Year Disease Risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- King, V.; Constine, L.S.; Clark, D.; Schwartz, R.G.; Muhs, A.G.; Henzler, M.; Hutson, A.; Rubin, P. Symptomatic Coronary Artery Disease after Mantle Irradiation for Hodgkin’s Disease. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 881–889. [Google Scholar] [CrossRef]
- Boero, I.J.; Paravati, A.J.; Triplett, D.P.; Hwang, L.; Matsuno, R.K.; Gillespie, E.; Yashar, C.M.; Moiseenko, V.; Einck, J.P.; Mell, L.K.; et al. Modern Radiation Therapy and Cardiac Outcomes in Breast Cancer. Int. J. Radiat. Oncol. 2016, 94, 700–708. [Google Scholar] [CrossRef]
- Tamulevicius, P.; Wang, M.; Iliakis, G. Homology-Directed Repair is Required for the Development of Radioresistance during S Phase: Interplay between Double-Strand Break Repair and Checkpoint Response. Radiat. Res. 2007, 167, 1–11. [Google Scholar] [CrossRef]
- Virani, S.A.; Dent, S.; Brezden-Masley, C.; Clarke, B.; Davis, M.K.; Jassal, D.S.; Johnson, C.; Lemieux, J.; Paterson, I.; Sebag, I.A. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can. J. Cardiol. 2016, 32, 831–841. [Google Scholar] [CrossRef]
- Baker, J.E.; Moulder, J.E.; Hopewell, J.W. Radiation as a Risk Factor for Cardiovascular Disease. Antioxid. Redox Signal. 2011, 15, 1945–1956. [Google Scholar] [CrossRef]
- Lee, P.J.; Mallik, R. Cardiovascular Effects of Radiation Therapy: Practical Approach to Radiation Therapy-Induced Heart Disease. Cardiol. Rev. 2005, 13, 80–86. [Google Scholar] [CrossRef]
- Brosius, F.C., 3rd; Waller, B.F.; Roberts, W.C. Radiation Heart Disease. Analysis of 16 Young (Aged 15 to 33 Years) Necropsy Patients Who Received over 3500 Rads to the Heart. Am. J. Med. 1981, 70, 519–530. [Google Scholar] [CrossRef]
- Filopei, J.; Frishman, W. Radiation-Induced Heart Disease. Cardiol. Rev. 2012, 20, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, C.; Mariani, J.; Wheeler, G.; Kaye, D.M. Cardiac Complications of Thoracic Irradiation. J. Am. Coll. Cardiol. 2013, 61, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Andreis, A.; Imazio, M.; Casula, M.; Avondo, S.; Brucato, A. Recurrent pericarditis: An update on diagnosis and management. Intern. Emerg. Med. 2021, 16, 551–558. [Google Scholar] [CrossRef]
- Reuss, C.S.; Wilansky, S.M.; Lester, S.J.; Lusk, J.L.; Grill, D.E.; Oh, J.K.; Tajik, A.J. Using Mitral ‘Annulus Reversus’ to Diagnose Constrictive Pericarditis. Eur. J. Echocardiogr. 2009, 10, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Pivetta, E.; Restrepo, S.P.; Sormani, P.; Pedrotti, P.; Quarta, G.; Brucato, A.; Bubbico, E.; Corso, M.D.; Milazzo, A.; et al. Usefulness of Cardiac Magnetic Resonance for Recurrent Pericarditis. Am. J. Cardiol. 2020, 125, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, J.G.; Bonaventura, A.; Vecchié, A.; Wohlford, G.F.; Mauro, A.G.; Jordan, J.H.; Grizzard, J.D.; Montecucco, F.; Berrocal, D.H.; Brucato, A.; et al. Management of Acute and Recurrent pericarditis: Jacc State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA 2015, 314, 1498–1506. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Sami, S.; Daher, I.N. Radiation-Induced Heart Disease: A Clinical Update. Cardiol. Res. Pract. 2011, 2011, 317659. [Google Scholar] [CrossRef]
- Jurcut, R.; Ector, J.; Erven, K.; Choi, H.F.; Voigt, J. Radiotherapy Effects on Systolic Myocardial Function Detected by Strain Rate Imaging in a Left-Breast Cancer Patient. Eur. Heart J. 2007, 28, 2966. [Google Scholar] [CrossRef][Green Version]
- Villarraga, H.R.; Herrmann, J.; Nkomo, V.T. Cardio-Oncology: Role of Echocardiography. Prog. Cardiovasc. Dis. 2014, 57, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Flachskampf, F.; Lancellotti, P.; Badano, L.; Aguilar, R.; Monaghan, M.; Zamorano, J.; Nihoyannopoulos, P.; on behalf of the European Association of Echocardiography. European Association of Echocardiography Recommendations for Standardization of Performance, Digital Storage and Reporting of Echocardiographic Studies. Eur. J. Echocardiogr. 2008, 9, 438–448. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated With Anthracyclines, Taxanes, and Trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Tang, S.; Byth, K.; Stefani, L.; Lo, Q.; Otton, J.; Jameson, M.; Tran, D.; Batumalai, V.; Holloway, L.; et al. Segmental Cardiac Radiation Dose Determines Magnitude of Regional Cardiac Dysfunction. J. Am. Heart Assoc. 2021, 10, e019476. [Google Scholar] [CrossRef]
- Lo, Q.; Hee, L.; Batumalai, V.; Allman, C.; MacDonald, P.; Delaney, G.P.; Lonergan, D.; Thomas, L. Subclinical Cardiac Dysfunction Detected by Strain Imaging During Breast Irradiation with Persistent Changes 6 Weeks After Treatment. Int. J. Radiat. Oncol. 2015, 92, 268–276. [Google Scholar] [CrossRef]
- Cao, L.; Cai, G.; Chang, C.; Miao, A.Y.; Yu, X.L.; Yang, Z.Z.; Ma, J.L.; Zhang, Q.; Wu, J.; Guo, X.M.; et al. Diastolic Dysfunction Occurs Early in Her2-Positive Breast Cancer Patients Treated Concurrently with Radiation Therapy and Trastuzumab. Oncologist 2015, 20, 605–614. [Google Scholar] [CrossRef]
- Henein, M.Y.; Lindqvist, P. Diastolic function assessment by echocardiography: A practical manual for clinical use and future applications. Echocardiography 2020, 37, 1908–1918. [Google Scholar] [CrossRef]
- Sritharan, H.P.; Delaney, G.; Lo, Q.; Batumalai, V.; Xuan, W.; Thomas, L. Evaluation of traditional and novel echocardiographic methods of cardiac diastolic dysfunction post radiotherapy in breast cancer. Int. J. Cardiol. 2017, 243, 204–208. [Google Scholar] [CrossRef]
- Cutter, D.J.; Schaapveld, M.; Darby, S.C.; Hauptmann, M.; Van Nimwegen, F.A.; Krol, S.; Janus, C.P.M.; Van Leeuwen, F.E.; Aleman, B.M.P. Risk for Valvular Heart Disease After Treatment for Hodgkin Lymphoma. J. Natl. Cancer Inst. 2015, 107, djv008. [Google Scholar] [CrossRef]
- Hering, D.; Faber, L.; Horstkotte, D. Echocardiographic features of Radiation-Associated valvular disease. Am. J. Cardiol. 2003, 92, 226–230. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 Acc/Aha Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Veeragandham, R.S.; Goldin, M.D. Surgical Management of Radiation-Induced Heart Disease. Ann. Thorac. Surg. 1998, 65, 1014–1019. [Google Scholar] [CrossRef]
- Kleikamp, G.; Schnepper, U.; Körfer, R. Coronary Artery and Aortic Valve Disease as a Long-Term Sequel of Mediastinal and Thoracic Irradiation. Thorac. Cardiovasc. Surg. 1997, 45, 27–31. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Schnittger, I.; Strauss, H.W.; Vagelos, R.H.; Lee, B.K.; Mariscal, C.S.; Tate, D.J.; Horning, S.J.; Hoppe, R.T.; Hancock, S.L. Screening for Coronary Artery Disease after Mediastinal Irradiation for Hodgkin’s Disease. J. Clin. Oncol. 2007, 25, 43–49. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Parsaee, M.; Maleki, M. The Role of Echocardiography in Coronary Artery Disease and Acute Myocardial Infarction. J. Tehran Univ. Heart Cent. 2013, 8, 1–13. [Google Scholar]
- Rademaker, J.; Schoöder, H.; Ariaratnam, N.S.; Strauss, H.W.; Yahalom, J.; Steingart, R.; Oeffinger, K.C. Coronary Artery Disease after Radiation Therapy for Hodgkin’s Lymphoma: Coronary Ct Angiography Findings and Calcium Scores in Nine Asymptomatic Patients. Am. J. Roentgenol. 2008, 191, 32–37. [Google Scholar] [CrossRef]
- Jordan, J.H.; Todd, R.M.; Vasu, S.; Hundley, W.G. Cardiovascular Magnetic Resonance in the Oncology Patient. JACC Cardiovasc. Imaging 2018, 11, 1150–1172. [Google Scholar] [CrossRef]
- Novo, G.; Santoro, C.; Manno, G.; Di Lisi, D.; Esposito, R.; Mandoli, G.E.; Evola, V.; Pastore, M.C.; Sperlongano, S.; D’Andrea, A.; et al. Usefulness of Stress Echocardiography in the Management of Patients Treated with Anticancer Drugs. J. Am. Soc. Echocardiogr. 2021, 34, 107–116. [Google Scholar] [CrossRef]
- Picano, E.; Ciampi, Q.; Citro, R.; D’Andrea, A.; Scali, M.C.; Cortigiani, L.; Olivotto, I.; Mori, F.; Galderisi, M.; Costantino, M.F.; et al. Stress Echo 2020: The International Stress Echo Study in Ischemic and Non-Ischemic Heart Disease. Cardiovasc. Ultrasound 2017, 15, 3. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Genders, T.S.S.; Pugliese, F.; Mollet, N.R.; Meijboom, W.B.; Weustink, A.C.; van Mieghem, C.A.G.; de Feyter, P.J.; Hunink, M.G.M. Incremental value of the CT coronary calcium score for the prediction of coronary artery disease. Eur. Radiol. 2010, 20, 2331–2340. [Google Scholar] [CrossRef]
- Chang, M.; Suh, J.; Kirtani, V.; Dobrescu, A.; Haas, J.; Zeldis, S.; Shayani, S.; Hindenburg, A.A. Coronary Calcium Scanning in Patients after Adjuvant Radiation for Early Breast Cancer and Ductal Carcinoma In situ. Front. Oncol. 2013, 3, 253. [Google Scholar] [CrossRef]
- Kupeli, S. Risks and diagnosis of coronary artery disease in Hodgkin lymphoma survivors. World J. Cardiol. 2014, 6, 555–561. [Google Scholar] [CrossRef]
- Machann, W.; Beer, M.; Breunig, M.; Störk, S.; Angermann, C.; Seufert, I.; Schwab, F.; Kölbl, O.; Flentje, M.; Vordermark, D. Cardiac Magnetic Resonance Imaging Findings in 20-Year Survivors of Mediastinal Radiotherapy for Hodgkin’s Disease. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1117–1123. [Google Scholar] [CrossRef]
- Committee Members; Klocke, F.J.; Baird, M.G.; Lorell, B.H.; Bateman, T.M.; Messer, J.V.; Berman, D.S.; O’Gara, P.T.; Carabello, B.A.; Russell, R.O. Acc/Aha/Asnc Guidelines for the Clinical Use of Cardiac Radionuclide Imaging—Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Acc/Aha/Asnc Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J. Am. Coll. Cardiol. 2003, 42, 1318–1333. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Martinez, J.A.; Cordova-Madera, S.N.; Villarraga, H.R. Radiation-Induced Heart Disease. J. Clin. Med. 2022, 11, 146. https://doi.org/10.3390/jcm11010146
Quintero-Martinez JA, Cordova-Madera SN, Villarraga HR. Radiation-Induced Heart Disease. Journal of Clinical Medicine. 2022; 11(1):146. https://doi.org/10.3390/jcm11010146
Chicago/Turabian StyleQuintero-Martinez, Juan A., Sandra N. Cordova-Madera, and Hector R. Villarraga. 2022. "Radiation-Induced Heart Disease" Journal of Clinical Medicine 11, no. 1: 146. https://doi.org/10.3390/jcm11010146
APA StyleQuintero-Martinez, J. A., Cordova-Madera, S. N., & Villarraga, H. R. (2022). Radiation-Induced Heart Disease. Journal of Clinical Medicine, 11(1), 146. https://doi.org/10.3390/jcm11010146