Incorporating Medical Supply and Demand into the Index of Physician Maldistribution Improves the Sensitivity to Healthcare Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Data Source
2.3. Outcome Measure
2.4. Other Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistics Bureau, Ministry of Internal Affairs and Communications. Population Estimates. 2019. Available online: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200524&tstat=000000090001&cycle=7&year=20190&month=0&tclass1=000001011679 (accessed on 7 May 2021).
- Ministry of Health, Labour and Welfare. Handbook of Health and Welfare Statistics. 2019. Available online: https://www.mhlw.go.jp/english/database/db-hh/1-2.html (accessed on 28 June 2021).
- Katanoda, K.; Hori, M.; Matsuda, T.; Shibata, A.; Nishino, Y.; Hattori, M.; Soda, M.; Ioka, A.; Sobue, T.; Nishimoto, H. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn. J. Clin. Oncol. 2015, 45, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Okui, T. Age-period-cohort Analysis of Cardiovascular Disease Mortality in Japan, 1995–2018. J. Prev. Med. Public Health 2020, 53, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. Demographic Survey. 1970. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450011&tstat=000001028897&cycle=7&year=19700&tclass1=000001053058&tclass2=000001131643&tclass3=000001131644&tclass4val=0 (accessed on 12 August 2021).
- Ministry of Health, Labour and Welfare. Demographic Survey. 2018. Available online: https://www.e-stat.go.jp/stat-search?page=1&toukei=00450011&year=20180%2C19700 (accessed on 12 August 2021).
- Turin, T.C.; Kokubo, Y.; Murakami, Y.; Higashiyama, A.; Rumana, N.; Watanabe, M.; Okamura, T. Lifetime risk of stroke in Japan. Stroke 2010, 41, 1552–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashima, N.; Arima, H.; Kita, Y.; Fujii, T.; Miyamatsu, N.; Komori, M.; Sugimoto, Y.; Nagata, S.; Miura, K.; Nozaki, K. Incidence, Management and Short-Term Outcome of Stroke in a General Population of 1.4 Million Japanese- Shiga Stroke Registry. Circ. J. 2017, 81, 1636–1646. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Gai, R.T.; Sawada, K.; Takahashi, Y.; Cox, S.E.; Nakayama, T.; Mori, R. Coronary heart disease and stroke disease burden attributable to fruit and vegetable intake in Japan: Projected DALYS to 2060. BMC Public Health 2019, 19, 707. [Google Scholar] [CrossRef] [Green Version]
- Cabinet Office. White Paper on Aging Society. 2020. Available online: https://www8.cao.go.jp/kourei/whitepaper/w-2020/html/zenbun/index.html (accessed on 28 June 2021).
- Hara, K.; Kunisawa, S.; Sasaki, N.; Imanaka, Y. Examining changes in the equity of physician distribution in Japan: A specialty-specific longitudinal study. BMJ Open 2018, 8, e018538. [Google Scholar] [CrossRef] [Green Version]
- Wu, J. Measuring inequalities in the demographical and geographical distribution of physicians in China: Generalist versus specialist. Int. J. Health Plan. Manag. 2018, 33, 860–879. [Google Scholar] [CrossRef]
- Karan, A.; Negandhi, H.; Nair, R.; Sharma, A.; Tiwari, R.; Zodpey, S. Size, composition and distribution of human resource for health in India: New estimates using National Sample Survey and Registry data. BMJ Open 2019, 9, e025979. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.; Sutton, M.; Gravelle, H. Inequity and inequality in the use of health care in England: An empirical investigation. Soc Sci. Med. 2005, 60, 1251–1266. [Google Scholar] [CrossRef]
- Guttmann, A.; Shipman, S.A.; Lam, K.; Goodman, D.C.; Stukel, T.A. Primary care physician supply and children’s health care use, access, and outcomes: Findings from Canada. Pediatrics 2010, 125, 1119–1126. [Google Scholar] [CrossRef]
- Mick, S.S.; Lee, S.Y.; Wodchis, W.P. Variations in geographical distribution of foreign and domestically trained physicians in the United States: ‘safety nets’ or ‘surplus exacerbation’? Soc. Sci. Med. 2000, 50, 185–202. [Google Scholar] [CrossRef] [Green Version]
- Winkelmann, J.; Muench, U.; Maier, C.B. Time trends in the regional distribution of physicians, nurses and midwives in Europe. BMC Health Serv. Res. 2020, 20, 937. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takaki, H. Geographic distribution of physicians in Japan. Lancet 1992, 340, 1391–1393. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Statistics of Doctors, Dentists, and Pharmacists. 2018. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450026&bunya_l=15&tstat=000001135683&cycle=7&year=20180&tclass1=000001135684&tclass2=000001135686&stat_infid=000031889121&tclass3val=0 (accessed on 12 August 2021).
- Hara, K.; Otsubo, T.; Kunisawa, S.; Imanaka, Y. Examining sufficiency and equity in the geographic distribution of physicians in Japan: A longitudinal study. BMJ Open 2017, 7, e013922. [Google Scholar] [CrossRef] [Green Version]
- Tanihara, S.; Kobayashi, Y.; Une, H.; Kawachi, I. Urbanization and physician maldistribution: A longitudinal study in Japan. BMC Health Serv. Res. 2011, 11, 260. [Google Scholar] [CrossRef] [Green Version]
- Ikesu, R.; Miyawaki, A.; Kobayashi, Y. Physician Distribution by Specialty and Practice Setting: Findings in Japan in 2000, 2010 and 2016. Tohoku J. Exp. Med. 2020, 251, 1–8. [Google Scholar] [CrossRef]
- Inoue, K.; Matsumoto, M. Japan’s new postgraduate medical training system. Clin. Teach. 2004, 1, 38–40. [Google Scholar] [CrossRef]
- Saito, H.; Tanimoto, T.; Kami, M.; Suzuki, Y.; Morita, T.; Morita, M.; Yamamoto, K.; Shimada, Y.; Tsubokura, M.; Endo, M. New physician specialty training system impact on distribution of trainees in Japan. Public Health 2020, 182, 143–150. [Google Scholar] [CrossRef]
- Macinko, J.; Starfield, B.; Shi, L. Quantifying the health benefits of primary care physician supply in the United States. Int. J. Health Serv. 2007, 37, 111–126. [Google Scholar] [CrossRef]
- Campos Fernandes, A. A Perspective on the OECD Report “Health at a Glance 2019”. Acta Med. Port. 2020, 33, 4–6. [Google Scholar] [CrossRef]
- Sato, H. Demand, supply and shortages of physicians:a critical analysis on the current government’s method. J. Health Welf. Policy 2020, 3, 39–48. [Google Scholar]
- Ministry of Health, Labour and Welfare. Study Group on Supply and Demand of Medical Staff: Doctor Supply and Demand Subcommittee 4th Interim Report. 2019. Available online: https://www.mhlw.go.jp/content/12601000/000504403.pdf (accessed on 13 August 2021).
- Ministry of Health, Labour and Welfare. Uneven Distribution of Physicians. 2020. Available online: https://www.mhlw.go.jp/content/10801000/000480270.pdf (accessed on 12 December 2021).
- Ministry of Health, Labour and Welfare. Working Conditions of Doctors. 2020. Available online: https://www.mhlw.go.jp/content/10800000/000677264.pdf (accessed on 20 December 2021).
- Ministry of Health, Labour and Welfare. Overview of statistics on physicians, dentists, and pharmacists. 2018. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/ishi/18/dl/gaikyo.pdf (accessed on 20 December 2021).
- Ministry of Health, Labour and Welfare. Patient Survey 2017, Second Volume (Prefectures and Secondary Medical Regions). 2017. Available online: https://www.e-stat.go.jp/dbview?sid=0003313536 (accessed on 20 December 2021).
- Ministry of Health, Labour and Welfare. Current Population Projection as of October 1, 2018. 2018. Available online: https://www.e-stat.go.jp/dbview?sid=0003312316 (accessed on 20 December 2021).
- Ministry of Internal Affairs. Population, Demographics and Number of Households Survey Based on Basic Resident Registers. 2018. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00200241&tstat=000001039591&year=20180 (accessed on 13 August 2021).
- Ministry of Health, Labour and Welfare. Basic Statistical Survey on Wage Structure. 2018. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450091&tstat=000001011429&cycle=0&year=20180&tclass1=000001113395&tclass2=000001113397&tclass3=000001113406&tclass4val=0 (accessed on 13 August 2021).
- Ministry of Health, Labour and Welfare. Comprehensive Survey of Living Condition. 2016. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450061&tstat=000001114975 (accessed on 13 August 2021).
- Ministry of Health, Labour and Welfare. Explanation on Major Ratios and Terms Used in Health and Welfare Statistics. (n.d.). Available online: https://www.mhlw.go.jp/english/database/db-hh/dl/appendices-03.pdf (accessed on 12 August 2021).
- Yu, C.Y.; Blaine, T.; Panagos, P.D.; Kansagra, A.P. Demographic Disparities in Proximity to Certified Stroke Care in the United States. Stroke 2021, 52, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.K.; Fang, J.; Chan, C.; Alter, D.A.; Bronskill, S.E.; Hill, M.D.; Manuel, D.G.; Tu, J.V.; Anderson, G.M. Neighborhood income and stroke care and outcomes. Neurology 2012, 79, 1200–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, G.; Luke, A.A.; Elson, L.; Towfighi, A.; Joynt Maddox, K.E. Urban-Rural Inequities in Acute Stroke Care and In-Hospital Mortality. Stroke 2020, 51, 2131–2138. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Matsumoto, M.; Inoue, K.; Kashima, S.; Takeuchi, K. Does the insufficient supply of physicians worsen their urban-rural distribution? A Hiroshima-Nagasaki comparison. Rural Remote Health 2012, 12, 2085. [Google Scholar] [CrossRef]
- Harrell, F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2015. [Google Scholar]
- Kleinbaum, D.G. Applied Regression Analysis and Other Multivariable Methods, 4th ed.; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Hone, T.; Powell-Jackson, T.; Santos, L.M.P.; de Sousa Soares, R.; de Oliveira, F.P.; Sanchez, M.N.; Harris, M.; de Oliveira de Souza Santos, F.; Millett, C. Impact of the Programa Mais medicos (more doctors Programme) on primary care doctor supply and amenable mortality: Quasi-experimental study of 5565 Brazilian municipalities. BMC Health Serv. Res. 2020, 20, 873. [Google Scholar] [CrossRef]
- Watson, D.E.; McGrail, K.M. More doctors or better care? Healthc. Policy 2009, 5, 26–31. [Google Scholar]
- US Department of Health and Human Services. Designation of medically underserved populations and health professional shortage areas—HRSA. Proposed rules. Fed. Regist. 1998, 63, 46538–46555. [Google Scholar]
- Ministry of Housing, Communities and Local Government The English Indices of Deprivation 2019. Research Report. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833947/IoD2019_Research_Report.pdf#:~:text=The%20English%20Indices%20of%20Deprivation%202019%20is%20theforms%20of%20deprivation%20at%20the%20small%20spatial%20scale (accessed on 9 August 2021).
- Santana Baskar, P.; Cordato, D.; Wardman, D.; Bhaskar, S. In-hospital acute stroke workflow in acute stroke—Systems-based approaches. Acta Neurol. Scand. 2021, 143, 111–120. [Google Scholar] [CrossRef]
- Hart, L.G.; Larson, E.H.; Lishner, D.M. Rural definitions for health policy and research. Am. J. Public Health 2005, 95, 1149–1155. [Google Scholar] [CrossRef]
- Matsumoto, K.; Seto, K.; Hayata, E.; Fujita, S.; Hatakeyama, Y.; Onishi, R.; Hasegawa, T. The geographical maldistribution of obstetricians and gynecologists in Japan. PLoS ONE 2021, 16, e0245385. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Wang, W.; Hughes, D.R.; Duszak, R., Jr. A County-Level Analysis of the US Radiologist Workforce: Physician Supply and Subspecialty Characteristics. J. Am. Coll. Radiol. 2018, 15, 601–606. [Google Scholar] [CrossRef]
- Filler, G.; Piedboeuf, B.; Paediatric Chairs of Canada. Variability of the pediatric subspecialty workforce in Canada. J. Pediatr. 2010, 157, 844–847.e1. [Google Scholar] [CrossRef]
- Gholami, M.; Fawad, I.; Shadan, S.; Rowaiee, R.; Ghanem, H.; Hassan Khamis, A.; Ho, S.B. COVID-19 and healthcare workers: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 335–346. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Glob. Health 2020, 5, e003097. [Google Scholar] [CrossRef]
- Wu, T.; Jia, X.; Shi, H.; Niu, J.; Yin, X.; Xie, J.; Wang, X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 91–98. [Google Scholar] [CrossRef]
- Danet Danet, A. Psychological impact of COVID-19 pandemic in Western frontline healthcare professionals. A systematic review. Med. Clin. 2021, 156, 449–458. [Google Scholar] [CrossRef]
- Hall, P.L. Mitigating the Impact of Reemergence From a Pandemic on Healthcare. Mil. Med. 2021, 186, 259–262. [Google Scholar] [CrossRef]
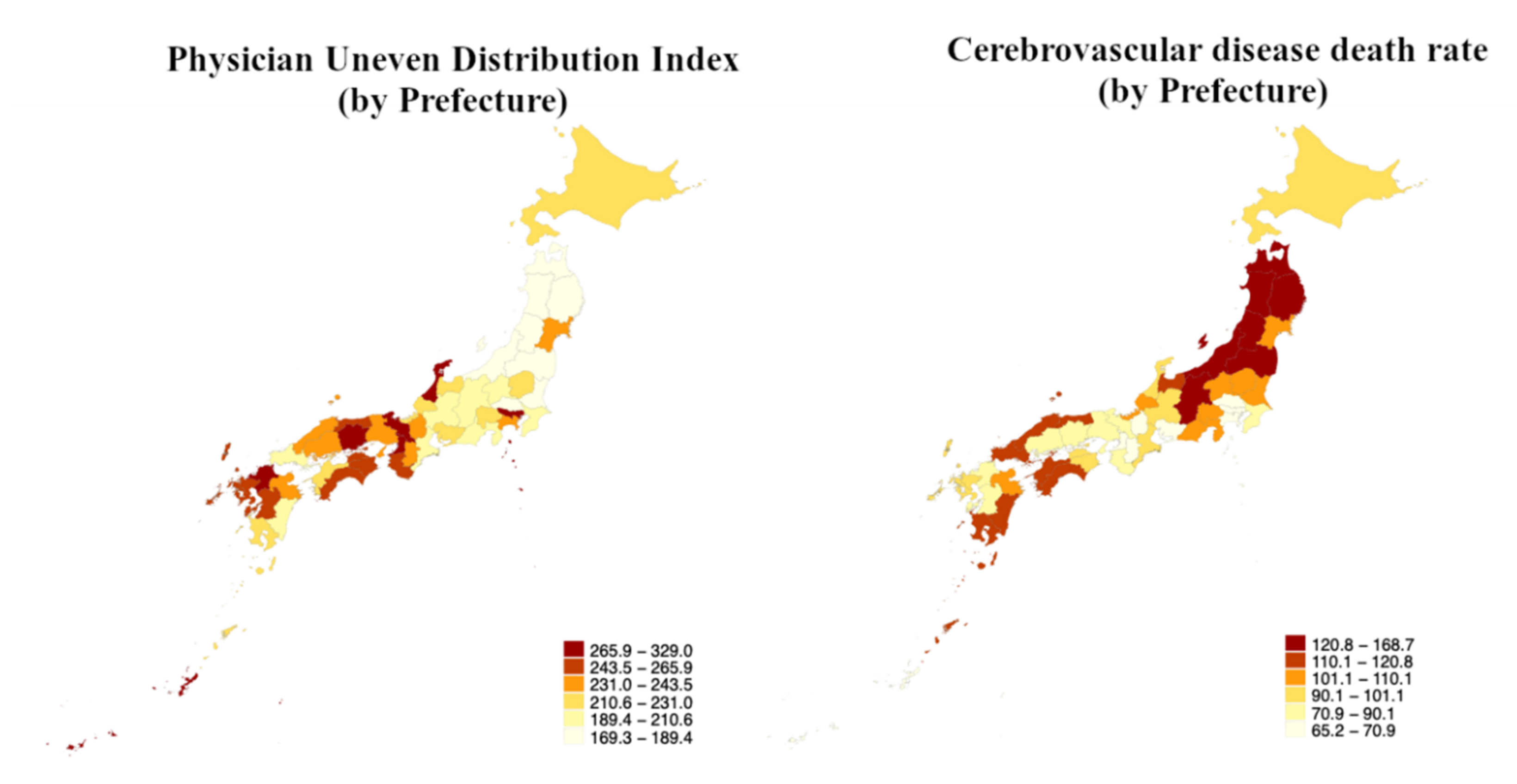
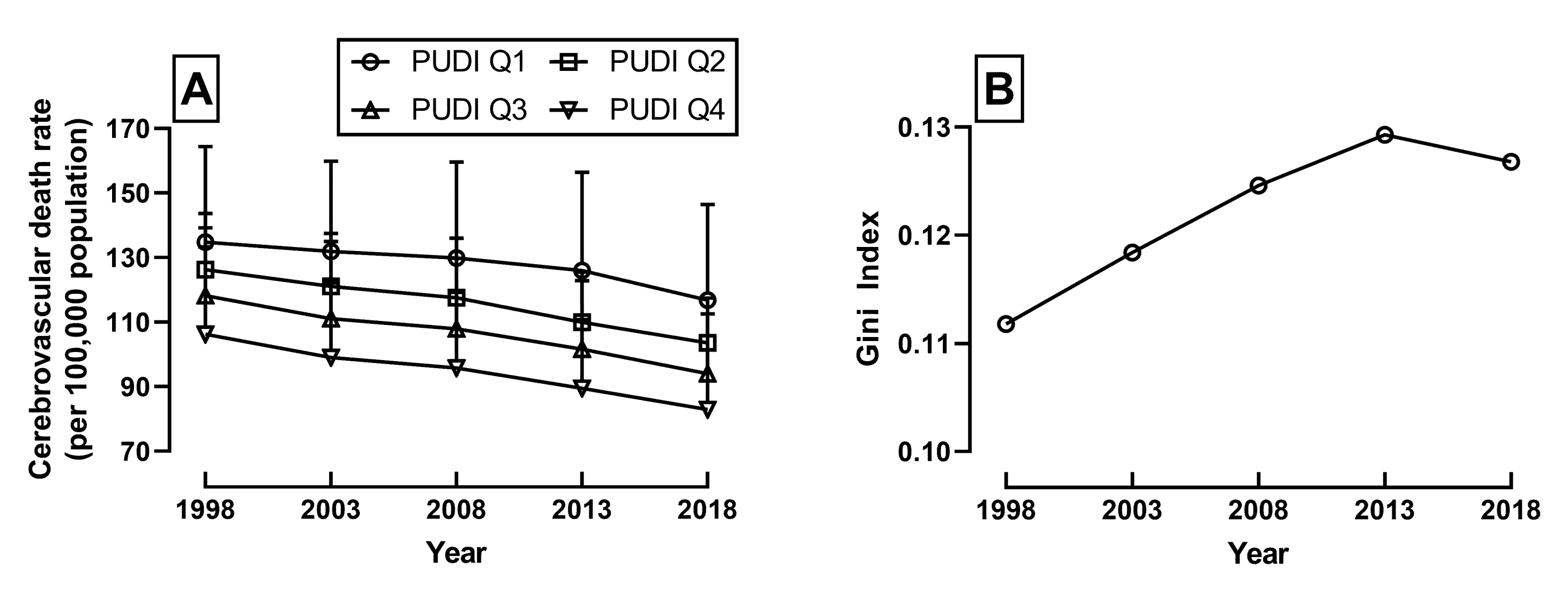
| PUDI = Q1 (n = 12) | PUDI = Q2 (n = 12) | PUDI = Q3 (n = 12) | PUDI = Q4 (n = 11) | |
|---|---|---|---|---|
| CeVD death rate (n/100,000) | 118 (31) | 104 (14) | 92 (19) | 85 (16) |
| Population density (n/km2) | 0.44 (0.57) | 0.34 (0.37) | 0.61 (1.02) | 1.35 (2.17) |
| Annual mean income (JPY *, ×10,000/year) | 271 (24) | 279 (20) | 284 (27) | 288 (39) |
| Prevalence of hypertension (%) | 13.4 (1.8) | 12.1 (1.3) | 12.3 (1.9) | 11.9 (1.4) |
| Model 0, β (95%CI) | Model 1 β (95%CI) | Model 2, β (95%CI) | Model 3, β (95%CI) | |
|---|---|---|---|---|
| PUDI | −0.34 *** (−0.49–−0.19) | −0.25 ** (−0.40–−0.10) | −0.24 *** (−0.36–−0.12) | −0.19 ** (−0.30–−0.07) |
| Population density | −7.11 ** (−11.75–−2.46) | 3.44 (−2.09–8.97) | 2.13 (−3.02–7.27) | |
| Annual mean income | −0.60 *** (−0.83 –−0.36) | −0.40 ** (−0.66–−0.15) | ||
| Prevalence of hypertension | 476.4 ** (153.72–799.05) | |||
| n | 47 | 47 | 47 | 46 |
| Adjusted R-sq | 0.29 | 0.41 | 0.62 | 0.69 |
| Model 0, β (95%CI) | Model 1, β (95%CI) | Model 2, β (95%CI) | Model 3, β (95%CI) | |
|---|---|---|---|---|
| NPPP * | −0.07 (−0.24–0.10) | −0.06 (−0.20–0.09) | −0.12 (−0.24–0.00) | −0.09 (−0.20–0.02) |
| Population density | −9.95 *** (−14.71–−5.19) | 1.85 (−4.31–8.01) | 0.68 (−4.79–6.16) | |
| Annual mean income | −0.66 *** (−0.92–−0.39) | −0.40 ** (−0.68–−0.12) | ||
| Prevalence of hypertension | 597.40 *** (258.86–935.92) | |||
| n | 47 | 47 | 47 | 46 |
| Adjusted R-sq | −0.01 | 0.27 | 0.52 | 0.64 |
| Cerebral Hemorrhage CeVD Rate, β (95%CI) | Cerebral Infarction CeVD Rate, β (95%CI) | |
|---|---|---|
| PUDI | −0.11 ** (−0.18–−0.03) | −0.06 ** (−0.11–0.02) |
| Population density | 1.58 (−1.65–4.81) | 0.60 (−1.45–2.65) |
| Annual mean income | −0.29 ** (−0.45–−0.13) | −0.09 (−0.19–0.02) |
| Prevalence of hypertension | 307.46 ** (104.92–510.01) | 135.93 * (7.38–264.48) |
| n | 46 | 46 |
| Adjusted R-sq | 0.71 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayama, A.; Poudyal, H. Incorporating Medical Supply and Demand into the Index of Physician Maldistribution Improves the Sensitivity to Healthcare Outcomes. J. Clin. Med. 2022, 11, 155. https://doi.org/10.3390/jcm11010155
Takayama A, Poudyal H. Incorporating Medical Supply and Demand into the Index of Physician Maldistribution Improves the Sensitivity to Healthcare Outcomes. Journal of Clinical Medicine. 2022; 11(1):155. https://doi.org/10.3390/jcm11010155
Chicago/Turabian StyleTakayama, Atsushi, and Hemant Poudyal. 2022. "Incorporating Medical Supply and Demand into the Index of Physician Maldistribution Improves the Sensitivity to Healthcare Outcomes" Journal of Clinical Medicine 11, no. 1: 155. https://doi.org/10.3390/jcm11010155
APA StyleTakayama, A., & Poudyal, H. (2022). Incorporating Medical Supply and Demand into the Index of Physician Maldistribution Improves the Sensitivity to Healthcare Outcomes. Journal of Clinical Medicine, 11(1), 155. https://doi.org/10.3390/jcm11010155






