Preload Dependency of 2D Right Ventricle Speckle Tracking Echocardiography Parameters in Healthy Volunteers: A Prospective Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
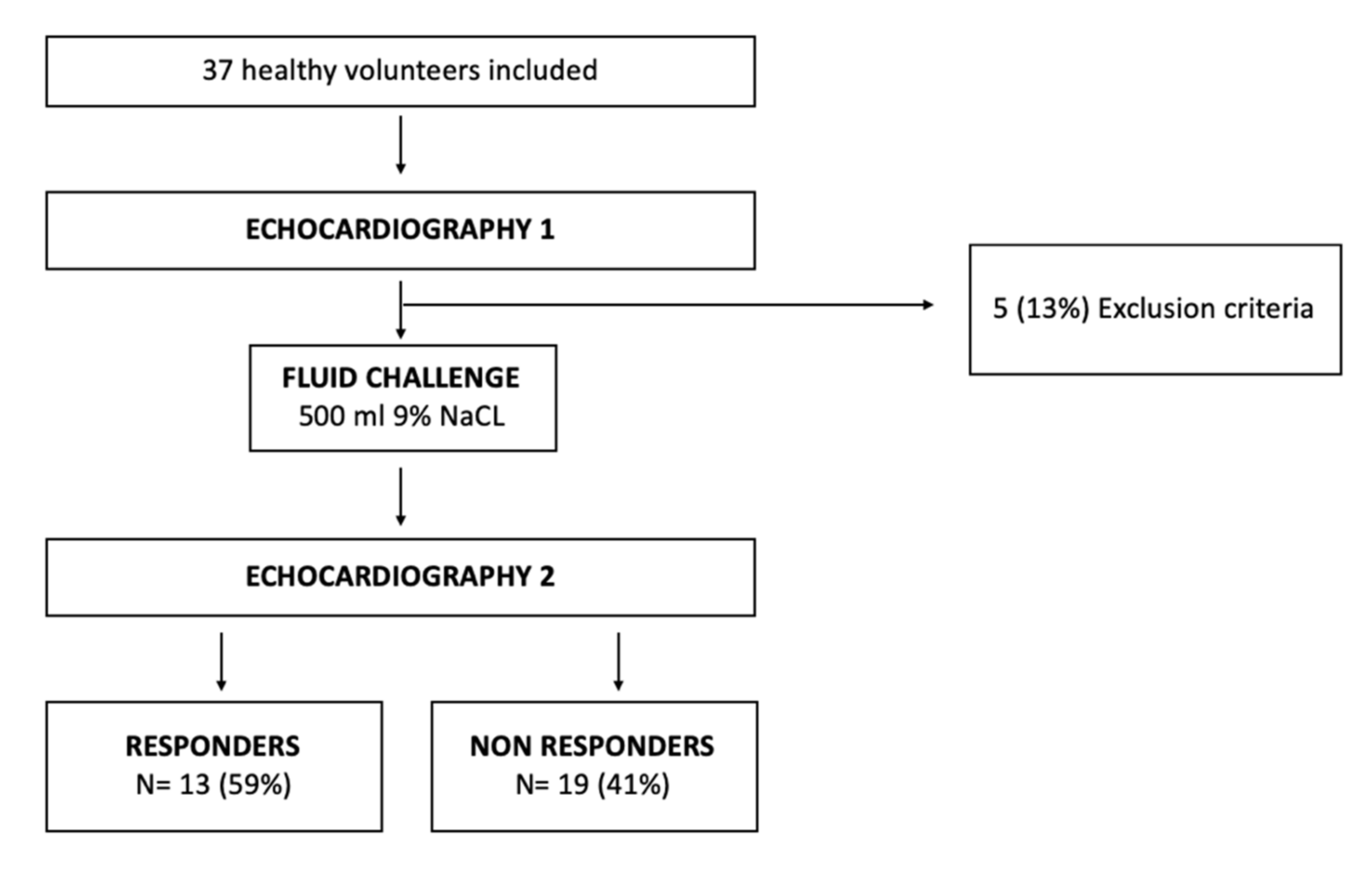
2.1. Study Population
2.2. Definition of Preload Dependency
2.3. Protocol Study
2.4. Echocardiography
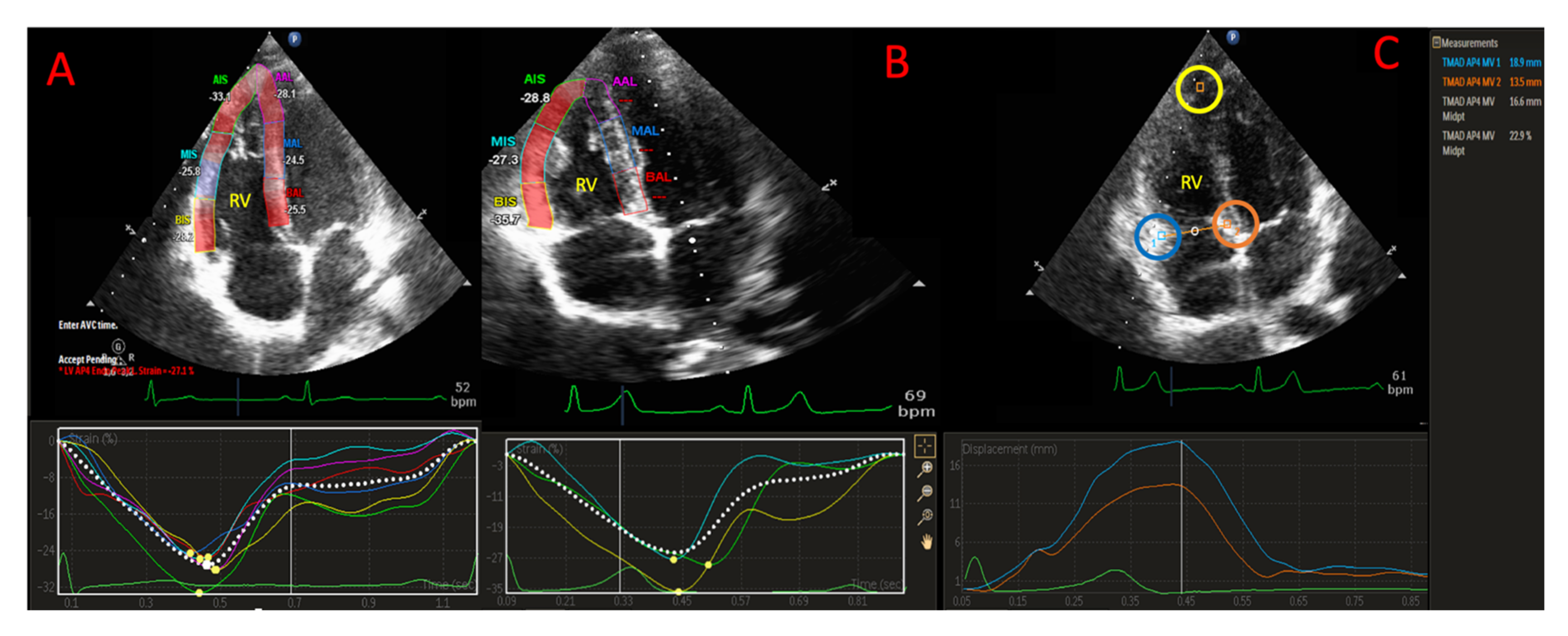
2.5. D-STE Analysis
2.6. RV Strain Analysis
2.7. TAD Analysis
2.8. Statistical Analysis
3. Results
3.1. General Characteristic of the Study Population
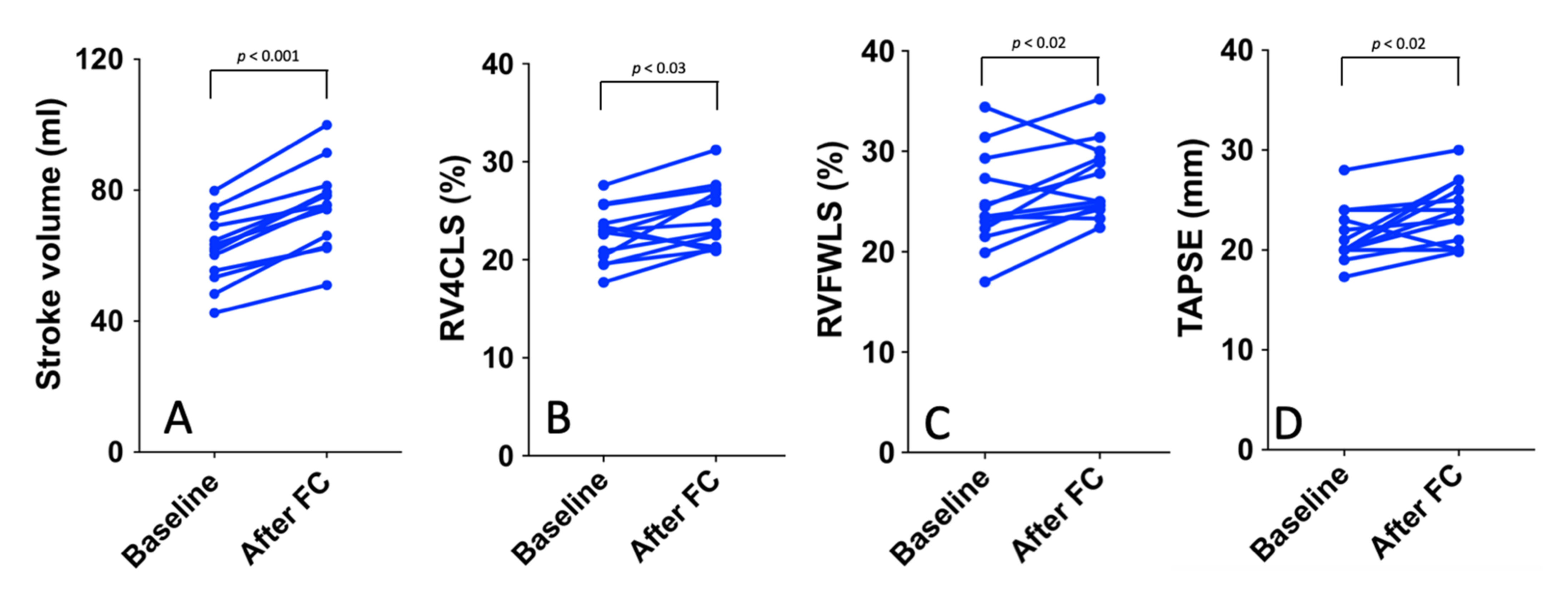
3.2. Preload Dependency
3.3. Preload INDEPENDENCY
3.4. Feasibility and Reproducibility
4. Discussion
4.1. Preaload in Healthy Subject
4.2. Preload Dependency of 2D-STE Parameters in Healthy Volunteers
4.3. Preload Dependency of TAPSE and TAD in Healthy Volunteers
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longobardo, L.; Suma, V.; Jain, R.; Carerj, S.; Zito, C.; Zwicke, D.L.; Khandheria, B.K. Role of Two-Dimensional Speckle-Tracking Echocardiography Strain in the Assessment of Right Ventricular Systolic Function and Comparison with Conventional Parameters. J. Am. Soc. Echocardiogr. 2017, 30, 937–946.e6. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Yang, Y.; Liu, M.; Meng, X.; Shi, Y.; Zhu, W.; Lu, X. Tricuspid annular displacement measured by 2-dimensional speckle tracking echocardiography for predicting right ventricular function in pulmonary hypertension. Medicines 2018, 97, e11710. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Onciul, S.; Peluso, D.; Soriani, N.; Cucchini, U.; Aruta, P.; Romeo, G.; Cavalli, G.; Iliceto, S.; Badano, L.P. Sex- and Method-Specific Reference Values for Right Ventricular Strain by 2-Dimensional Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging 2016, 9, e003866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carluccio, E.; Biagioli, P.; Lauciello, R.; Zuchi, C.; Mengoni, A.; Bardelli, G.; Alunni, G.; Gronda, E.G.; Ambrosio, G. Superior Prognostic Value of Right Ventricular Free Wall Compared to Global Longitudinal Strain in Patients With Heart Failure. J. Am. Soc. Echocardiogr. 2019, 32, 836–844. [Google Scholar] [CrossRef]
- Ahmad, H.; Mor-Avi, V.; Lang, R.M.; Nesser, H.-J.; Weinert, L.; Tsang, W.; Steringer-Mascherbauer, R.; Niel, J.; Salgo, I.S.; Sugeng, L.; et al. Assessment of Right Ventricular Function Using Echocardiographic Speckle Tracking of the Tricuspid Annular Motion: Comparison with Cardiac Magnetic Resonance. Echocardiography 2011, 29, 19–24. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Nafati, C.; Gardette, M.; Leone, M.; Reydellet, L.; Blasco, V.; Lannelongue, A.; Sayagh, F.; Wiramus, S.; Antonini, F.; Albanèse, J.; et al. Use of speckle-tracking strain in preload-dependent patients, need for cautious interpretation! Ann. Intensiv. Care 2018, 8, 29. [Google Scholar] [CrossRef]
- Marik, P.E.; Lemson, J. Fluid responsiveness: An evolution of our understanding. Br. J. Anaesth. 2014, 112, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Delicce, A.V.; Makaryus, A.N. Physiology, Frank Starling Law. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Alonso, P.; Andrés, A.; Miró, V.; Igual, B.; Sánchez, I.; Salvador, A. Diagnostic power of echocardiographic speckle tracking of the tricuspid annular motion to assess right ventricular dysfunction. Int. J. Cardiol. 2014, 172, e218–e219. [Google Scholar] [CrossRef]
- Bunting, K.; Steeds, R.; Slater, L.; Rogers, J.K.; Gkoutos, G.V.; Kotecha, D. A Practical Guide to Assess the Reproducibility of Echocardiographic Measurements. J. Am. Soc. Echocardiogr. 2019, 32, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- García-López, Z.Y.; Vargas-Barrón, J. Evaluation of the global longitudinal strain and segmental strain of the right ventricle with two-dimensional speckle-tracking echocardiography with elevation of the legs. ACME 2019, 89, 15–19. [Google Scholar] [CrossRef]
- Sano, H.; Tanaka, H.; Motoji, Y.; Mukai, J.; Suto, M.; Takada, H.; Soga, F.; Hatani, Y.; Matsuzoe, H.; Hatazawa, K.; et al. Echocardiography during preload stress for evaluation of right ventricular contractile reserve and exercise capacity in pulmonary hypertension. Echocardiography 2018, 35, 1997–2004. [Google Scholar] [CrossRef]
- Susilovic-Grabovac, Z.; Obad, A.; Duplančić, D.; Banić, I.; Brusoni, D.; Agostoni, P.; Vuković, I.; Dujic, Z.; Bakovic, D. 2D speckle tracking echocardiography of the right ventricle free wall in SCUBA divers after single open sea dive. Clin. Exp. Pharmacol. Physiol. 2017, 45, 234–240. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.; Murphy, D.J. Right Ventricular Function in Cardiovascular Disease, Part I: Anatomy, Physiology, Aging, and Functional Assessment of the Right Ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef]
- Tan, Y.; Manouras, A.; Lund, L.H.; Venkateshvaran, A. Feasibility and accuracy of tricuspid annular displacement assessed by speckle tracking echocardiography and Doppler tissue imaging. Echocardiography 2019, 36, 2004–2009. [Google Scholar] [CrossRef]
- Beyls, C.; Bohbot, Y.; Huette, P.; Booz, T.; Daumin, C.; Abou-Arab, O.; Mahjoub, Y. Usefulness of Right Ventricular Longitudinal Shortening Fraction to Detect Right Ventricular Dysfunction in Acute Cor Pulmonale Related to COVID-19. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3594–3603. [Google Scholar] [CrossRef]
- Shen, T.; Picard, M.H.; Hua, L.; Burns, S.M.; Andrawes, M.N. Assessment of Tricuspid Annular Motion by Speckle Tracking in Anesthetized Patients Using Transesophageal Echocardiography. Anesth. Analg. 2018, 126, 62–67. [Google Scholar] [CrossRef]
- Beyls, C.; Bohbot, Y.; Huette, P.; Abou-Arab, O.; Mahjoub, Y. Tricuspid Longitudinal Annular Displacement for the Assessment of Right Ventricular Systolic Dysfunction during Prone Positioning in Patients with COVID-19. J. Am. Soc. Echocardiogr. 2020, 33, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Myatchin, I.; Abraham, P.; Malbrain, M.L. Bio-electrical impedance analysis in critically ill patients: Are we ready for prime time? J. Clin. Monit. 2019, 34, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Vitarelli, A.; Mangieri, E.; Terzano, C.; Gaudio, C.; Salsano, F.; Rosato, E.; Capotosto, L.; D’Orazio, S.; Azzano, A.; Truscelli, G.; et al. Three-Dimensional Echocardiography and 2D-3D Speckle-Tracking Imaging in Chronic Pulmonary Hypertension: Diagnostic Accuracy in Detecting Hemodynamic Signs of Right Ventricular (RV) Failure. J. Am. Heart Assoc. 2015, 4, e001584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.; Dobson, G.; Dawson, D.; Charalampopoulos, A.; Grapsa, J.; Nihoyannopoulos, P. Three-Dimensional Speckle Tracking of the Right Ventricle. J. Am. Coll. Cardiol. 2014, 64, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erley, J.; Tanacli, R.; Genovese, D.; Tapaskar, N.; Rashedi, N.; Bucius, P.; Kawaji, K.; Karagodin, I.; Lang, R.M.; Kelle, S.; et al. Myocardial strain analysis of the right ventricle: Comparison of different cardiovascular magnetic resonance and echocardiographic techniques. J. Cardiovasc. Magn. Reson. 2020, 22, 51. [Google Scholar] [CrossRef] [PubMed]
| Overall Population (n = 32) | Baseline | After FC | p-Value |
|---|---|---|---|
| Age (years) | 26 (24–28) | - | - |
| BMI (Kg/cm2) | 22 (20–24) | - | - |
| Male, n (%) | 28 (87) | - | - |
| Haemodynamic parameters | |||
| HR (bpm) | 64 (58–71) | 60 (54–69) | 0.03 |
| SAP (mmHg) | 119 (115–128) | 116 (110–121) | 0.02 |
| MAP (mmHg) | 84 (76–90) | 81 (75–89) | 0.18 |
| DAP (mmHg) | 68 (64–77) | 70 (62–75) | 0.71 |
| Overall Population (n = 32) | Baseline | After FC | p-Value |
|---|---|---|---|
| LV systolic parameters | |||
| LVEF (%) | 61 (58–68) | 60 (55–66) | 0.18 |
| LV end diastolic volume (mL) | 113 (94–118) | 111 (94–121) | 0.29 |
| LV end systolic volume (mL) | 43 (34–49) | 45 (34–52) | 0.21 |
| Stroke volume (mL s−1) | 72 (61–79) | 75 (65–80) | 0.07 |
| CO (L min−1) | 4.6 (3.9–5.2) | 4.2 (3.7–5.3) | 0.52 |
| LV diastolic functional parameters | |||
| E wave (cm s−1) | 84 (74–94) | 88 (78–95) | 0.09 |
| A wave (cm s−1) | 42 (38–54) | 47 (40–59) | 0.16 |
| E/A ratio | 1.8 (1.6–2.2) | 1.8 (1.7–2.1) | 0.93 |
| Lateral E/e’ | 4.2 (3.8–5) | 4.5 (4.1–5.4) | 0.22 |
| Deceleration time (ms) | 204 (168–266) | 202 (68–269) | 0.7 |
| LA volume index (mL m−2) | 18 (20–23) | 21 (19–23) | 0.92 |
| RV Parameters | |||
| RVOT PSAX distal dimension (mm) | 27 (24–32) | 30 (27–33) | 0.46 |
| RVOT PLAX proximal dimension (mm) | 31 (26–34) | 31 (27–34) | 0.11 |
| RV basal dimension (mm) | 40 (36–44) | 39 (37–42) | 0.78 |
| RV mid-cavity dimension (mm) | 3.6 (3.5–4) | 3.8 (3.7–4) | 0.01 |
| RV longitudinal dimension (mm) | 8.3 (8–8.5) | 8.3 (7.9–8.5) | 0.82 |
| RV EDA (mL) | 21 (16–23) | 21 (18–34) | 0.16 |
| RV ESA (mL) | 11 (9–15) | 12 (10–15) | 0.62 |
| RA volume indexed to BSA (mL/m2) | 23 (19–27) | 24 (22–29) | 0.63 |
| IVC collapsibility index (%) | 35.5 (28–55) | 24 (22–30) | 0.001 |
| RV Systolic Function Parameters | |||
| TAPSE (mm) | 23 (20–27) | 25 (21–28) | 0.06 |
| RV- S’ (cm/s−1) | 15 (11–16) | 14 (13–16) | 0.9 |
| RV FAC (%) | 42 (33–48) | 42 (37–46) | 0.7 |
| IVA (m s−2) | 3.2 (2.5–3.7) | 3 (2.5–3.5) | 0.29 |
| 2D STE RV Strain | |||
| RVFWLS (%) | −24.3 (22.5–28.9) | −24.9 (23.4–29) | 0.34 |
| RV4CLS (%) | −22.6 (20.7–25.4) | −23.5 (22.0–26.1) | 0.05 |
| TAD parameters | |||
| 22.7 (20.5–25) | 24.6 (21.3–26.3) | 0.08 |
| 15.2 (13.7–17.3) | 16.1 (13.1–19.5) | 0.44 | |
| 24.2 (22.2–27) | 25 (21–29.4) | 0.3 |
| Responder Group (n = 13) | Baseline | After FC | p-Value |
|---|---|---|---|
| Hemodynamic parameters | |||
| HR (bpm) | 64 (57–74) | 61 (56–67) | 0.09 |
| SAP (mmHg) | 119 (113–125) | 112 (107–117) | 0.25 |
| MAP (mmHg) | 79 (75–87) | 77 (73–84) | 0.41 |
| DAP (mmHg) | 65 (62–68) | 67 (60–72) | 0.93 |
| LV systolic parameters | |||
| LVEF (%) | 59 (55–68) | 59 (57–64) | 0.65 |
| LV end diastolic volume (mL) | 112 (88–116) | 113 (94–125) | 0.24 |
| LV end systolic volume (mL) | 43 (32–51) | 45 (34–52) | 0.59 |
| Aortic VTI (cm/s−1) | 18 (16–20) | 22 (19–25) | 0.001 |
| Stroke Volume (mL) | 62 (54–70) | 75 (64–80) | 0.001 |
| RV Function | |||
| TAPSE (mm) | 20 (20–23.5) | 24 (21–29) | 0.02 |
| RV- S’ (cm s−1) | 15 (11–16) | 14 (13–16) | 0.44 |
| RV FAC (%) | 42 (36–48) | 43 (37–46) | 0.31 |
| IVA (m s−2) | 3.1 (2.5–3.5) | 3.0 (2.6–3.6) | 0.44 |
| RV Strain | |||
| RVFWSL (%) | −23.5 (22.3–27.3) | −25.0 (24.0–29.6) | 0.03 |
| RV4CSL (%) | −22.8 (20.4–30.7) | −23.7 (21.2–27.0) | 0.02 |
| TMAD | |||
| 22 (20–24) | 24 (18–26) | 0.27 |
| 15 (14–18) | 16 (13–20) | 0.43 | |
| 24 (22–27) | 25 (20–30) | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyls, C.; Bohbot, Y.; Caboche, M.; Huette, P.; Haye, G.; Dupont, H.; Mahjoub, Y.; Osama, A.-A. Preload Dependency of 2D Right Ventricle Speckle Tracking Echocardiography Parameters in Healthy Volunteers: A Prospective Pilot Study. J. Clin. Med. 2022, 11, 19. https://doi.org/10.3390/jcm11010019
Beyls C, Bohbot Y, Caboche M, Huette P, Haye G, Dupont H, Mahjoub Y, Osama A-A. Preload Dependency of 2D Right Ventricle Speckle Tracking Echocardiography Parameters in Healthy Volunteers: A Prospective Pilot Study. Journal of Clinical Medicine. 2022; 11(1):19. https://doi.org/10.3390/jcm11010019
Chicago/Turabian StyleBeyls, Christophe, Yohann Bohbot, Matthieu Caboche, Pierre Huette, Guillaume Haye, Hervé Dupont, Yazine Mahjoub, and Abou-Arab Osama. 2022. "Preload Dependency of 2D Right Ventricle Speckle Tracking Echocardiography Parameters in Healthy Volunteers: A Prospective Pilot Study" Journal of Clinical Medicine 11, no. 1: 19. https://doi.org/10.3390/jcm11010019






