Diagnostic Accuracy and Detection Rate of Glaucoma Screening with Optic Disk Photos, Optical Coherence Tomography Images, and Telemedicine
Abstract
:1. Introduction
2. Methods
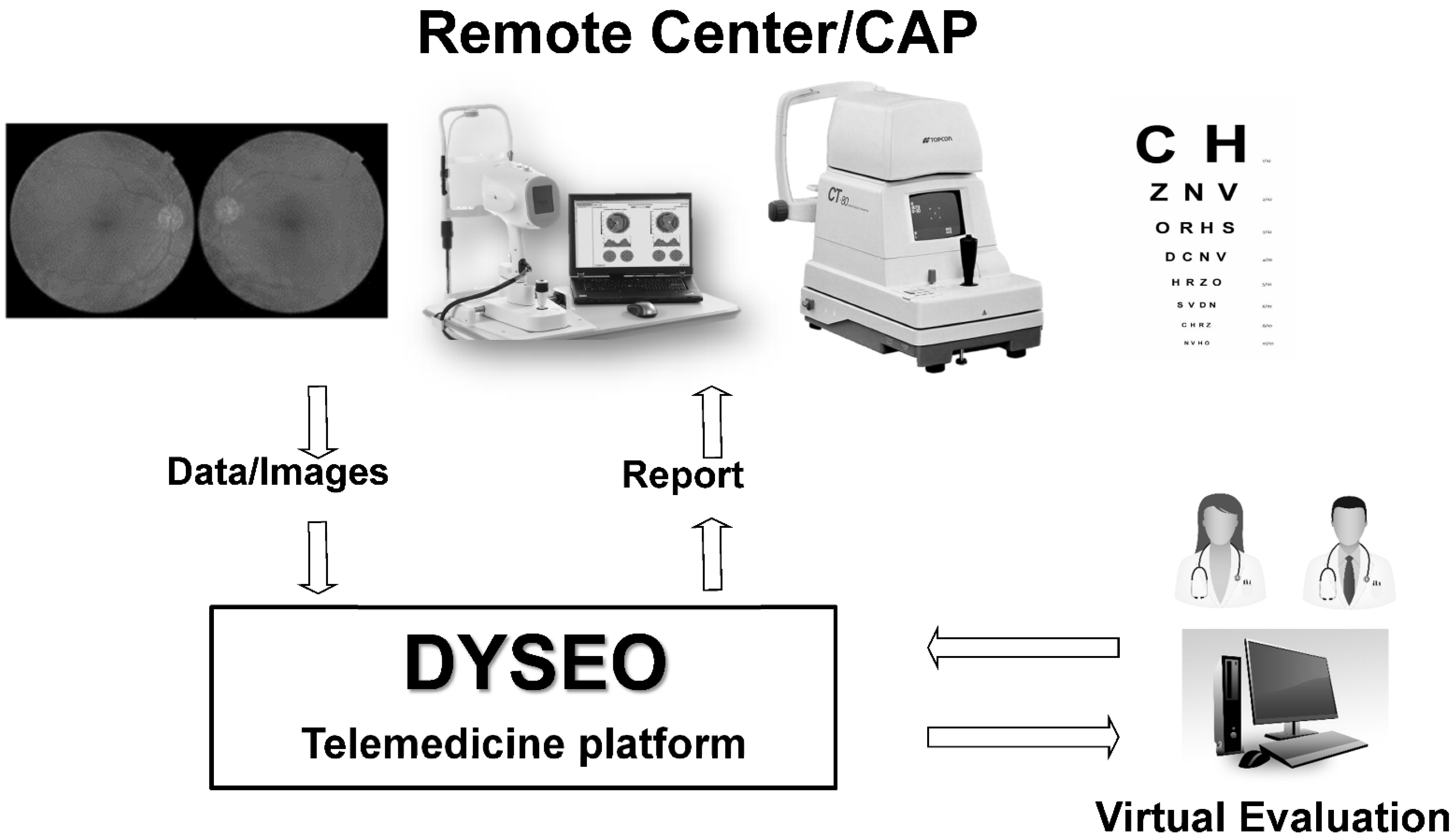
2.1. Telemedicine Platform
2.2. Sample
2.3. Examinations at the Primary Care Center
2.4. Remote Image Evaluation and Grading
2.5. Examination at the Glaucoma Clinic of the Hospital
2.6. Statistical Analysis
2.7. Ethics
3. Results
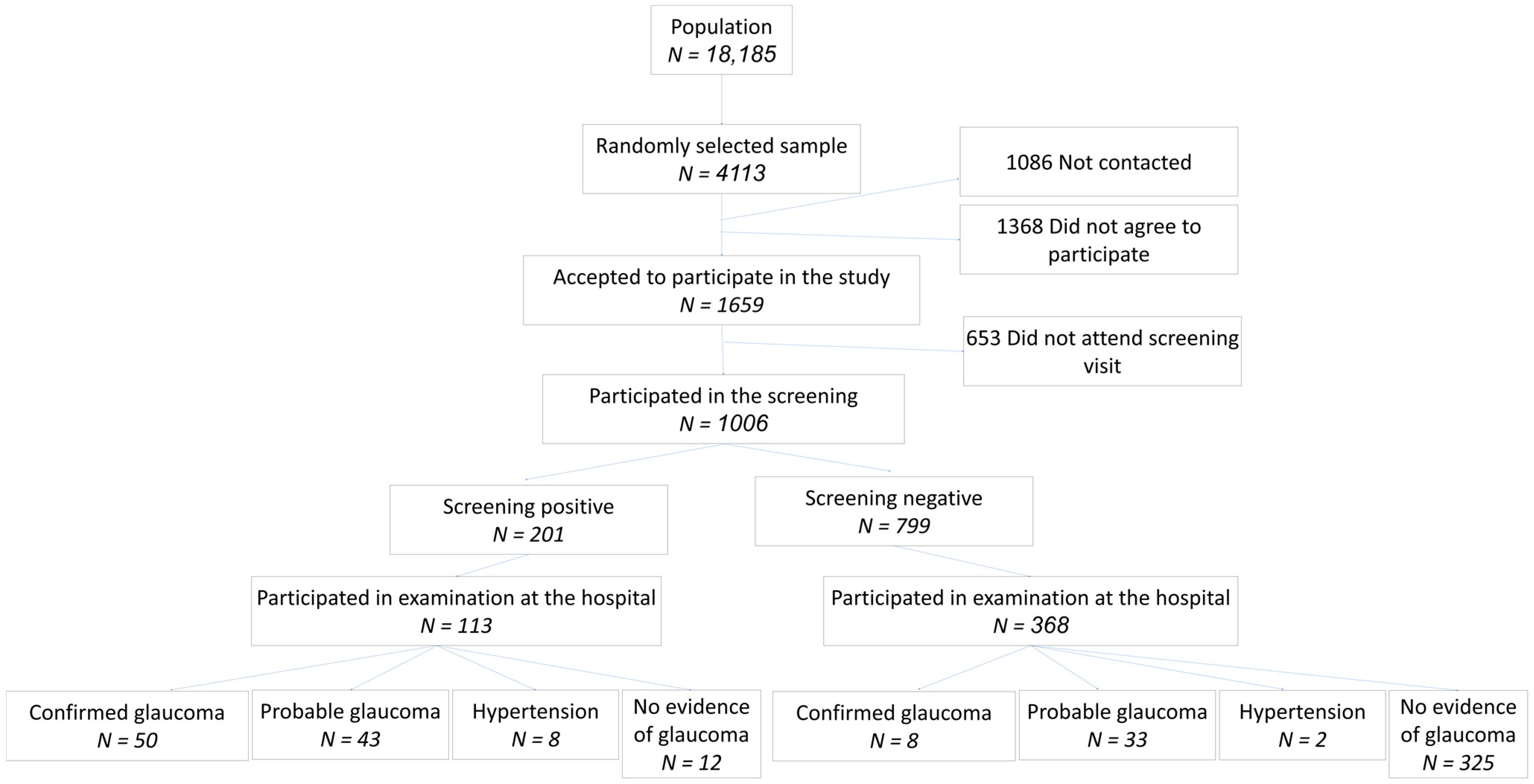
3.1. Study Sample
3.2. Image Quality
3.3. Screening Results
3.4. Detection of Other Diseases
3.5. Examination at Glaucoma Clinic
3.6. Classification Accuracy
3.7. Predisposing Factors for Glaucoma
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Antón, A.; Andrada, M.T.; Mujica, V.; Calle, M.A.; Portela, J.; Mayo, A. Prevalence of Primary Open-Angle Glaucoma in a Spanish Population: The Segovia Study. J. Glaucoma 2004, 13, 371–376. [Google Scholar] [CrossRef]
- Mitchell, P.; Smith, W.; Attebo, K.; Healey, P.R. Prevalence of Open-Angle Glaucoma in Australia: The Blue Mountains Eye Study. Ophthalmology 1996, 103, 1661–1669. [Google Scholar] [CrossRef]
- Klein, B.E.K.; Klein, R.; Sponsel, W.E.; Franke, T.; Cantor, L.B.; Martone, J.; Menage, M.J. Prevalence of Glaucoma: The Beaver Dam Eye Study. Ophthalmology 1992, 99, 1499–1504. [Google Scholar] [CrossRef]
- Burr, J.; Mowatt, G.; Hernández, R.; Siddiqui, M.; Cook, J.; Lourenco, T.; Ramsay, C.; Vale, L.; Fraser, C.; Azuara-Blanco, A.; et al. The Clinical Effectiveness and Cost-Effectiveness of Screening for Open Angle Glaucoma: A Systematic Review and Economic Evaluation. Health Technol. Assess. 2007, 11, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Mowatt, G.; Burr, J.M.; Cook, J.A.; Siddiqui, M.A.R.; Ramsay, C.; Fraser, C.; Azuara-Blanco, A.; Deeks, J.J. Screening Tests for Detecting Open-Angle Glaucoma: Systematic Review and Meta-Analysis. Investig. Opthalmol. Vis. Sci. 2008, 49, 5373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelessi, M.; Lucenteforte, E.; Oddone, F.; Brazzelli, M.; Parravano, M.; Franchi, S.; Ng, S.M.; Virgili, G. Optic Nerve Head and Fibre Layer Imaging for Diagnosing Glaucoma. Cochrane Database Syst. Rev. 2015, 2020, CD008803. [Google Scholar] [CrossRef] [Green Version]
- Fallon, M.; Valero, O.; Pazos, M.; Antón, A. Diagnostic Accuracy of Imaging Devices in Glaucoma: A Meta-Analysis. Surv. Ophthalmol. 2017, 62, 446–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuara-Blanco, A.; Banister, K.; Boachie, C.; Mcmeekin, P.; Gray, J.; Burr, J.; Bourne, R.; Garway-Heath, D.; Batterbury, M.; Hernández, R.; et al. Automated Imaging Technologies for the Diagnosis of Glaucoma: A Comparative Diagnostic Study for the Evaluation of the Diagnostic Accuracy, Performance as Triage Tests and Cost-Effectiveness (GATE Study). Health Technol. Assess. 2016, 20, 1–168. [Google Scholar] [CrossRef] [Green Version]
- Anton, A.; Fallon, M.; Cots, F.; Sebastian, M.A.; Morilla-Grasa, A.; Mojal, S.; Castells, X. Cost and Detection Rate of Glaucoma Screening with Imaging Devices in a Primary Care Center. Clin. Ophthalmol. 2017, 11, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.M.; Jeyaraman, M.; Hodge, W.G.; Hutnik, C.; Costella, J.; Malvankar-Mehta, M.S. The Effectiveness of Teleglaucoma versus In-Patient Examination for Glaucoma Screening: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e113779. [Google Scholar] [CrossRef] [Green Version]
- Beardsley, R.; Law, S.K.; Caprioli, J.; Coleman, A.L.; Nouri-Mahdavi, K.; Hubschman, J.-P.; Schwartz, S.D.; Giaconi, J.A.; Parker, A. Comparison of Outcomes between Endoscopic and Transcleral Cyclophotocoagulation. Vision 2017, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Kurysheva, N.I.; Pechenkina, A.A.; Goncharova, A.S. Examination of Patients with Glaucoma during the COVID-19 Pandemic. Vestn. Oftalmol. 2021, 137, 75–83. [Google Scholar] [CrossRef]
- Heidinger, A.; Falb, T.; Werkl, P.; List, W.; Hoeflechner, L.; Riedl, R.; Ivastinovic, D.; Hommer, A.; Lindner, E. The Impact of Tape Sealing Face Masks on Visual Field Scores in the Era of COVID-19—A Randomized Cross-over Study. J. Glaucoma 2021, 30, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Nolivos, K.; Pazos, M.; Fatti, G.; Herranz, A.; Ayala-Fuentes, M.; Martínez-Prats, E.; Peral, O.; Vega-Lopez, Z.; Monleon-Getino, A.; et al. Interobserver and Intertest Agreement in Telemedicine Glaucoma Screening with Optic Disk Photos and Optical Coherence Tomography. J. Clin. Med. 2021, 10, 3337. [Google Scholar] [CrossRef]
- Koh, V.; Tham, Y.-C.; Cheung, C.Y.; Mani, B.; Wong, T.Y.; Aung, T.; Cheng, C.-Y. Diagnostic Accuracy of Macular Ganglion Cell-Inner Plexiform Layer Thickness for Glaucoma Detection in a Population-Based Study: Comparison with Optic Nerve Head Imaging Parameters. PLoS ONE 2018, 13, e0199134. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, S.; Takeda, H.; Higashide, T.; Sasaki, T.; Sugiyama, K. A Pilot Study to Detect Glaucoma with Confocal Scanning Laser Ophthalmoscopy Compared with Nonmydriatic Stereoscopic Photography in a Community Health Screening. J. Glaucoma 2007, 16, 531–538. [Google Scholar] [CrossRef]
- Gaasterland, D.E.; Ederer, F.; Beck, A.; Costarides, A.; Leef, D.; Closek, J.; Banks, J.; Jackson, S.; Moore, K.; Vela, A.; et al. The Advanced Glaucoma Intervention Study (AGIS): 7. The Relationship between Control of Intraocular Pressure and Visual Field Deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- Nakano, T.; Hayashi, T.; Nakagawa, T.; Honda, T.; Owada, S.; Endo, H.; Tatemichi, M. Applicability of Automatic Spectral Domain Optical Coherence Tomography for Glaucoma Mass Screening. Clin. Ophthalmol. 2017, 11, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramyashri, S.; Rao, H.L.; Jonnadula, G.B.; Addepalli, U.K.; Choudhari, N.; Senthil, S.; Garudadri, C. Determinants of Optical Coherence Tomography Parameters in a Population-Based Study. Am. J. Ophthalmol. 2021, 224, 163–171. [Google Scholar] [CrossRef]
- Tham, Y.C.; Chee, M.L.; Dai, W.; Lim, Z.W.; Majithia, S.; Siantar, R.; Thakur, S.; Rim, T.; Cheung, C.Y.; Sabanayagam, C.; et al. Profiles of Ganglion Cell-Inner Plexiform Layer Thickness in a Multi-Ethnic Asian Population: The Singapore Epidemiology of Eye Diseases Study. Ophthalmology 2020, 127, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Banister, K.; Boachie, C.; Bourne, R.; Cook, J.; Burr, J.M.; Ramsay, C.; Garway-Heath, D.; Gray, J.; McMeekin, P.; Hernández, R.; et al. Can Automated Imaging for Optic Disc and Retinal Nerve Fiber Layer Analysis Aid Glaucoma Detection? Ophthalmology 2016, 123, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springelkamp, H.; Lee, K.; Wolfs, R.C.W.; Buitendijk, G.H.S.; Ramdas, W.D.; Hofman, A.; Vingerling, J.R.; Klaver, C.C.W.; Abràmoff, M.D.; Jansonius, N.M. Population-Based Evaluation of Retinal Nerve Fiber Layer, Retinal Ganglion Cell Layer, and Inner Plexiform Layer as a Diagnostic Tool for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8428–8438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Categorical Variable | Categories | Total Group (n = 1006) | Suspects (n = 201) (19.9%) | Non-Suspects (n = 799) (79.4%) | p-Value |
|---|---|---|---|---|---|
| Gender | Female | 523 (51.9) | 94 (46.8) | 426 (52.9) | 0.2503 |
| Male | 483 (48.1) | 107 (53.2) | 373 (47.1) | ||
| Age | <65 | 425 (42.2) | 64 (31.8) | 360 (45.1) | <0.001 |
| 65–74 | 386 (38.4) | 81 (40.3) | 305 (38.2) | ||
| >74 | 195 (19.4) | 56 (27.9) | 134 (16.7) | ||
| Visual acuity | Low (<0.2) | 63 (6.3) | 31 (15.4) | 33 (4.1) | <0.001 |
| Medium ([0.2–0.5]) | 166 (16.5) | 41 (20.4) | 119 (14.8) | ||
| High (>0.5) | 777 (77.2) | 129 (64.2) | 647 (81.1) | ||
| Intraocular pressure | [0–21] | 979 (97.3) | 178 (88.6) | 795 (99.5) | <0.001 |
| >21 | 27 (2.7) | 23 (11.4) | 4 (0.5) | ||
| Family history of glaucoma | yes | 102 (10.1) | 13 (6.5) | 89 (11.1) | 0.0504 |
| Personal record of glaucoma | yes | 61 (5.1) | 24 (11.9) | 34 (4.3) | <0.001 |
| Degree of Visual Field Damage | Cases of Confirmed or Probable Glaucoma |
|---|---|
| Advanced (MD < −11.9 dB) | 10 (10.7%) |
| Moderate (MD −11.9 to −6 dB) | 15 (16.1%) |
| Initial (MD > −6 dB) | 58 (62.3%) |
| No reliable visual field available | 10 (10.7%) |
| Total | 93 |
| Sensitivity | Specificity | Negative Predictive Value | Positive Predictive Value | |
|---|---|---|---|---|
| Definition 1 | ||||
| Sample | 0.862 | 0.821 | 0.970 | 0.467 |
| <64 Years | 0.684 | 0.874 | 0.949 | 0.448 |
| 65–74 Years | 0.895 | 0.773 | 0.981 | 0.362 |
| >74 Years | 1.000 | 0.814 | 0.975 | 0.614 |
| Initial glaucoma | 0.813 | 0.834 | 0.972 | 0.382 |
| Moderate glaucoma | 0.857 | 0.800 | 0.888 | 0.750 |
| Advanced glaucoma | 1.000 | 0.888 | 0.874 | 0.815 |
| Definition 2 | ||||
| Sample | 0.694 | 0.942 | 0.848 | 0.869 |
| <64 years | 0.587 | 0.980 | 0.838 | 0.931 |
| 65–74 years | 0.720 | 0.891 | 0.865 | 0.765 |
| >74 years | 0.789 | 0.976 | 0.833 | 0.968 |
| Initial glaucoma | 0.673 | 0.948 | 0.848 | 0.853 |
| Moderate glaucoma | 0.714 | 0.923 | 0.667 | 0.938 |
| Advanced glaucoma | 1.000 | 1.000 | 0.868 | 0.898 |
| Definition 3 | ||||
| Sample | 0.701 | 0.974 | 0.840 | 0.944 |
| <64 years | 0.571 | 0.990 | 0.821 | 0.966 |
| 65–74 years | 0.754 | 0.957 | 0.865 | 0.915 |
| >74 years | 0.789 | 0.976 | 0.833 | 0.968 |
| Initial glaucoma | 0.643 | 0.973 | 0.839 | 0.926 |
| Moderate glaucoma | 0.727 | 1.000 | 0.625 | 0.933 |
| Advanced glaucoma | 1.000 | 1.000 | 0.868 | 0.898 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton, A.; Nolivos, K.; Pazos, M.; Fatti, G.; Ayala, M.E.; Martínez-Prats, E.; Peral, O.; Poposki, V.; Tsiroukis, E.; Morilla-Grasa, A.; et al. Diagnostic Accuracy and Detection Rate of Glaucoma Screening with Optic Disk Photos, Optical Coherence Tomography Images, and Telemedicine. J. Clin. Med. 2022, 11, 216. https://doi.org/10.3390/jcm11010216
Anton A, Nolivos K, Pazos M, Fatti G, Ayala ME, Martínez-Prats E, Peral O, Poposki V, Tsiroukis E, Morilla-Grasa A, et al. Diagnostic Accuracy and Detection Rate of Glaucoma Screening with Optic Disk Photos, Optical Coherence Tomography Images, and Telemedicine. Journal of Clinical Medicine. 2022; 11(1):216. https://doi.org/10.3390/jcm11010216
Chicago/Turabian StyleAnton, Alfonso, Karen Nolivos, Marta Pazos, Gianluca Fatti, Miriam Eleonora Ayala, Elena Martínez-Prats, Oscar Peral, Vladimir Poposki, Evangelos Tsiroukis, Antonio Morilla-Grasa, and et al. 2022. "Diagnostic Accuracy and Detection Rate of Glaucoma Screening with Optic Disk Photos, Optical Coherence Tomography Images, and Telemedicine" Journal of Clinical Medicine 11, no. 1: 216. https://doi.org/10.3390/jcm11010216






