Machine Learning to Calculate Heparin Dose in COVID-19 Patients with Active Cancer
Abstract
:1. Introduction
2. Patients and Methods
2.1. Model Development
2.2. Limitations of the Study
2.3. Performance Evaluation
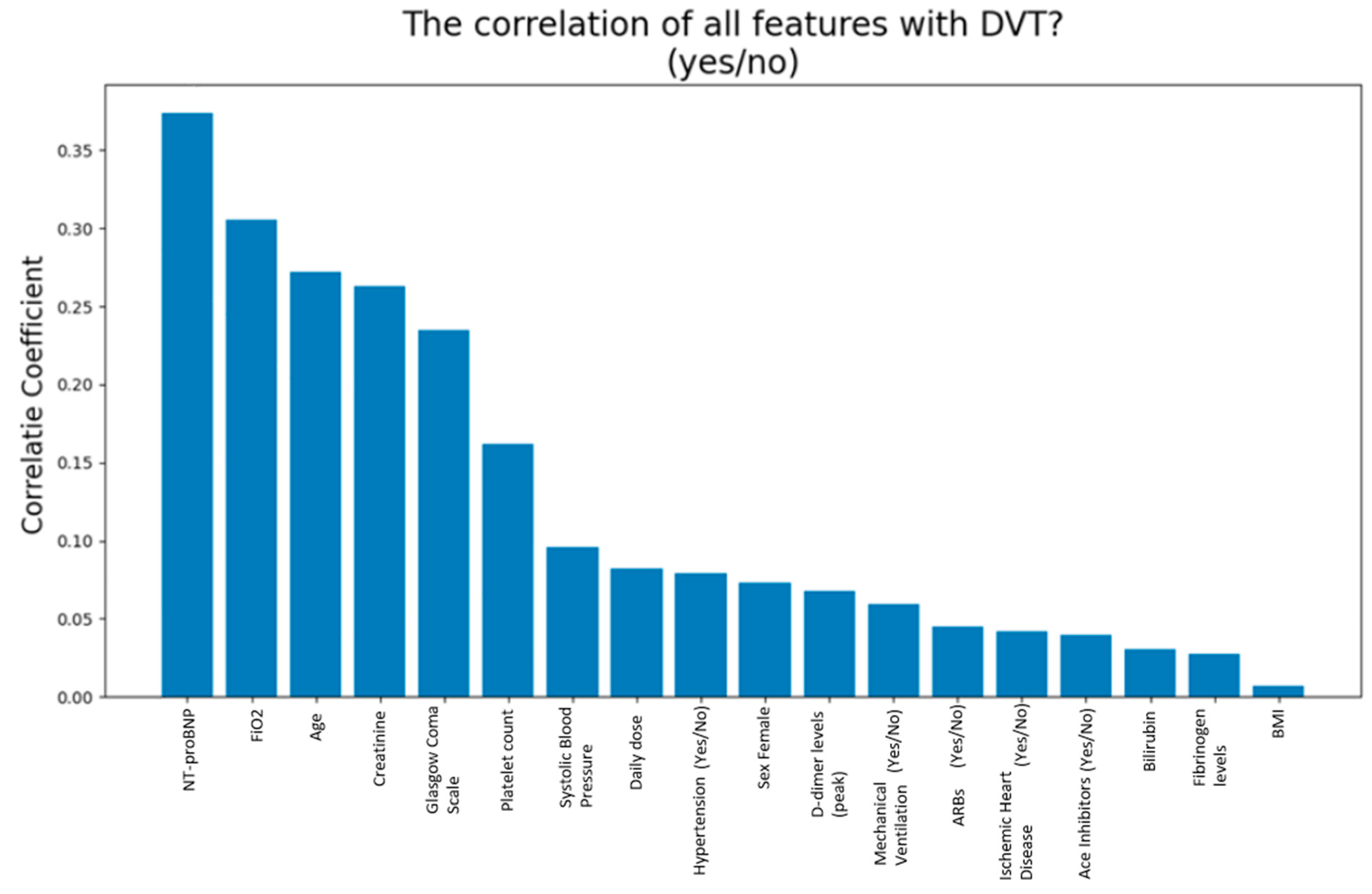
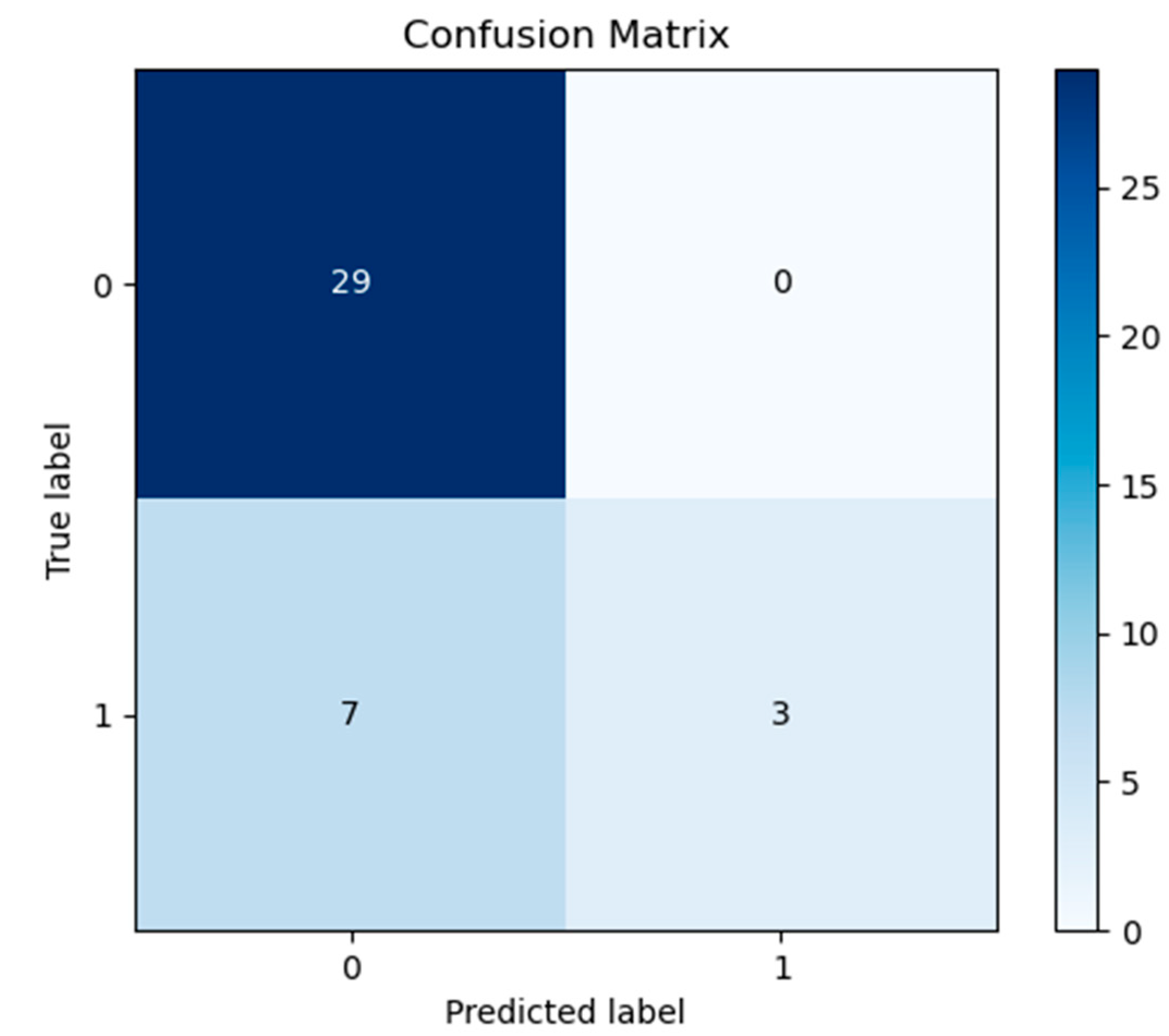
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Limitation
References
- Cattaneo, M.; Bertinato, E.M.; Birocchi, S.; Brizio, C.; Malavolta, D.; Manzoni, M.; Muscarella, G.; Orlandi, M. Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Is the Recommendation to Use High-Dose Heparin for ThromboprophylaxisJustified? Thromb. Haemost. 2020, 120, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- York, N.L.; Kane, C.J.; Smith, C.; Minton, L.A. Care of the patient with an acute pulmonary embolism. Dimens. Crit. Care Nurs. 2015, 34, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier-Decrucq, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary embolism in COVID-19 patients: Awareness of an increased prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Scarcia, M.; Ludovico, G.M.; Fortunato, A.; Fiorentino, A. Patients with cancer in the COVID-19 era: The clinical trial issue. Tumori J. 2020, 106, 271–272. [Google Scholar] [CrossRef]
- Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Candido, S.; Libra, M.; Lerpiriyapong, K.; Cocco, L.; Ramazzotti, G.; Ratti, S.; Follo, M.Y.; et al. Cancer therapy and treatments during COVID-19 era. Adv. Biol. Regul. 2020, 77, 100739. [Google Scholar] [CrossRef]
- Sha, Z.; Chang, K.; Mi, J.; Liang, Z.; Hu, L.; Long, F.; Shi, H.; Lin, Z.; Wang, X.; Pei, X. The impact of the COVID-19 pandemic on lung cancer patients. Ann. Palliat. Med. 2020, 9, 3373–3378. [Google Scholar] [CrossRef]
- Song, K.; Gong, H.; Xu, B.; Dong, X.; Li, L.; Hu, W.; Wang, Q.; Xie, Z.; Rao, Z.; Luo, Z.; et al. Association between recent oncologic treatment and mortality among patients with carcinoma who are hospitalized with COVID-19: A multicenter study. Cancer 2020, 127, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef]
- Blom, J.W.; Vanderschoot, J.P.M.; Oostindier, M.J.; Osanto, S.; Van Der Meer, F.J.M.; Rosendaal, F.R. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. Thromb. Haemost. 2006, 4, 529–535. [Google Scholar] [CrossRef]
- Otten, H.M.M.; Mathijssen, J.; ten Cate, H.; Soesan, M.; Inghels, M.; Richel, D.J.; Prins, M.H. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: An underestimated phenomenon. Arch. Intern. Med. 2004, 164, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Tromboembolismo.pdf (accessed on 24 November 2021).
- Garassino, M.C.; Whisenant, J.G.; Huang, L.-C.; Trama, A.; Torri, V.; Agustoni, F.; Baena, J.; Banna, G.; Berardi, R.; Bettini, A.C.; et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol. 2020, 21, 914–922. [Google Scholar] [CrossRef]
- Park, R.; Lee, S.A.; Kim, S.Y.; De Melo, A.C.; Kasi, A. Association of active oncologic treatment and risk of death in cancer patients with COVID-19: A systematic review and meta-analysis of patient data. Acta Oncol. 2020, 60, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, P.; Tufano, A.; Cardillo, G.; Imbalzano, E.; Amitrano, M.; Lodigiani, C.; Bellizzi, A.; Camporese, G.; Cavalli, A.; De Stefano, C.; et al. The Impact of Risk-Adjusted Heparin Regimens on the Outcome of Patients with COVID-19 Infection. A Prospective Cohort Study. Viruses 2021, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; Bastoni, D.; Ioannilli, E.; Vercelli, A.; Magnacavallo, A. Deep Vein Thrombosis and Pulmonary Embolism: Two Complications of COVID-19 Pneumonia? Eur. J. Case Rep. Intern. Med. 2020, 7, 001646. [Google Scholar] [CrossRef]
- Cosgun, E.; Limdi, N.A.; Duarte, C.W. High-dimensional pharmacogenetic prediction of a continuous trait using machine learning techniques with application to warfarin dose prediction in African Americans. Bioinformatics 2011, 27, 1384–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [Green Version]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Chekroud, A.M.; Zotti, R.J.; Shehzad, Z.; Gueorguieva, R.; Johnson, M.K.; Trivedi, M.H.; Cannon, T.D.; Krystal, J.H.; Corlett, P.R. Cross-trial prediction of treatment outcome in depression: A machine learning approach. Lancet Psychiatry 2016, 3, 243–250. [Google Scholar] [CrossRef]
- Waljee, A.K.; Wallace, B.; Cohen-Mekelburg, S.; Liu, Y.; Liu, B.; Sauder, K.; Stidham, R.W.; Zhu, J.; Higgins, P.D.R. Development and Validation of Machine Learning Models in Prediction of Remission in Patients with Moderate to Severe Crohn Disease. JAMA Netw. Open 2019, 2, e193721. [Google Scholar] [CrossRef]
- Kagiyama, N.; Piccirilli, M.; Yanamala, N.; Shrestha, S.; Farjo, P.D.; Casaclang-Verzosa, G.; Tarhuni, W.M.; Nezarat, N.; Budoff, M.J.; Narula, J.; et al. Machine Learning Assessment of Left Ventricular Diastolic Function Based on Electrocardiographic Features. J. Am. Coll. Cardiol. 2020, 76, 930–941. [Google Scholar] [CrossRef]
- Baskaran, L.; Ying, X.; Xu, Z.; Al’Aref, S.J.; Lee, B.C.; Lee, S.-E.; Danad, I.; Park, H.-B.; Bathina, R.; Baggiano, A.; et al. Machine learning insight into the role of imaging and clinical variables for the prediction of obstructive coronary artery disease and revascularization: An exploratory analysis of the CONSERVE study. PLoS ONE 2020, 15, e0233791. [Google Scholar] [CrossRef]
- Du, R.; Tsougenis, E.D.; Ho, J.W.K.; Chan, J.K.Y.; Chiu, K.W.H.; Fang, B.X.H.; Ng, M.Y.; Leung, S.-T.; Lo, C.S.Y.; Wong, H.-Y.F.; et al. Machine learning application for the prediction of SARS-CoV-2 infection using blood tests and chest radiograph. Sci. Rep. 2021, 11, 14250. [Google Scholar] [CrossRef] [PubMed]
- Burdick, H.; Lam, C.; Mataraso, S.; Siefkas, A.; Braden, G.; Dellinger, R.P.; McCoy, A.; Vincent, J.-L.; Green-Saxena, A.; Barnes, G.; et al. Prediction of respiratory decompensation in COVID-19 patients using machine learning: The READY trial. Comput. Biol. Med. 2020, 124, 103949. [Google Scholar] [CrossRef]
- Samama, M.M.; Cohen, A.T.; Darmon, J.-Y.; Desjardins, L.; Eldor, A.; Janbon, C.; Leizorovicz, A.; Nguyen, H.; Olsson, C.-G.; Turpie, A.G.; et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N. Engl. J. Med. 1999, 341, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.R.; Yu, H.; Gou, J.Z.; Li, X.X.; Sun, Y.; Li, J.X.; He, J.X.; Liu, L. Histopathologic Findings in the Explant Lungs of a Patient With COVID-19 Treated with Bilateral Orthotopic Lung Transplant. Transplantation 2020, 104, e329–e331. [Google Scholar] [CrossRef]
- Bick, R.L. Alterations of hemostasis associated with malignancy: Etiology, pathophysiology, diagnosis and management. Semin. Thromb. Hemost. 1978, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Grilz, E.; Posch, F.; Nopp, S.; Königsbrügge, O.; Lang, I.M.; Klimek, P.; Thurner, S.; Pabinger, I.; Ay, C. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer-a nationwide analysis. Eur. Hear. J. 2021, 42, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Moore, E.E.; Moore, H.; Thomas, S.; Lune, S.V.; Zimmer, D.; Dynako, J.; Hake, D.; Crowell, Z.; McCauley, R.; et al. Use of Viscoelastography in Malignancy-Associated Coagulopathy and Thrombosis: A Review. Semin. Thromb. Hemost. 2019, 45, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalia, T.; Lahan, S.; Ranka, S.; Acharya, P.; Gautam, A.; Goyal, A.; Mastoris, I.; Sauer, A.; Shah, Z. Impact of congestive heart failure and role of cardiac biomarkers in COVID-19 patients: A systematic review and meta-analysis. Indian Hear. J. 2020, 73, 91–98. [Google Scholar] [CrossRef]
- Wungu, C.D.K.; Khaerunnisa, S.; Putri, E.A.C.; Hidayati, H.B.; Qurnianingsih, E.; Lukitasari, L.; Humairah, I.; Soetjipto. Meta-analysis of cardiac markers for predictive factors on severity and mortality of COVID-19. Int. J. Infect. Dis. 2021, 105, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Kumar, A.; Patel, D.; Puri, R.; Kalra, A.; Kapadia, S.R.; Reed, G.W. Meta-analysis Comparing Outcomes in Patients with and Without Cardiac Injury and Coronavirus Disease 2019 (COVID 19). Am. J. Cardiol. 2020, 141, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; Niola, M.; Napoli, C. Can COVID 2019 induce a specific cardiovascular damage or it exacerbates pre-existing cardiovascular diseases? Pathol. Res. Pract. 2020, 216, 153086. [Google Scholar] [CrossRef] [PubMed]
- Unudurthi, S.D.; Luthra, P.; Bose, R.J.; McCarthy, J.R.; Kontaridis, M.I. Cardiac inflammation in COVID-19: Lessons from heart failure. Life Sci. 2020, 260, 118482. [Google Scholar] [CrossRef] [PubMed]
- de Falco, R.; Vargas, M.; Palma, D.; Savoia, M.; Miscioscia, A.; Pinchera, B.; Vano, M.; Servillo, G.; Gentile, I.; Fortunato, G. B-Type Natriuretic Peptides and High-Sensitive Troponin I as COVID-19 Survival Factors: Which One Is the Best Performer? J. Clin. Med. 2021, 10, 2726. [Google Scholar] [CrossRef]


| All Patients n = 131 | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age (years) | 71 | 15 | 18 | 100 |
| BMI (kg/m2) | 24.35 | 3.09 | 16.53 | 33.3 |
| D-dimer (ng/mL) | 1.89 | 1.71 | 0.27 | 9.3 |
| Platelet count (mm3) | 251.28 | 104.51 | 31 | 490 |
| Fibrinogen (mg/dL) | 494.59 | 149.88 | 152 | 991 |
| Daily dose | 0.5 | 0.29 | 0.3 | 3.2 |
| Creatinine (mg/dL) | 0.97 | 0.56 | 0.3 | 3.1 |
| FiO2 (%) | 34.9 | 17.81 | 21 | 80 |
| Bilirubin (mg/dL) | 0.58 | 0.26 | 0.16 | 1.31 |
| GCS. | 12.91 | 2.53 | 3 | 15 |
| SBP (mmHg) | 122.56 | 16.16 | 68 | 160 |
| NT-ProBNP | 1541.87 | 4489.72 | 17 | 33,873 |
| All Patients (n = 131) | |
|---|---|
| Mechanical Ventilation | |
| Yes | 40 (31%) |
| No | 91 (69%) |
| Hypertension | |
| Yes | 75 (57%) |
| No | 56 (43%) |
| Coronary Artery Disease | |
| Yes | 15 (11%) |
| No | 116 (89%) |
| Ace Inhibitors | |
| Yes | 21 (16%) |
| No | 110 (84%) |
| Arbs | |
| Yes | 37 (29%) |
| No | 94 (71%) |
| Sex Female | |
| Yes | 65 (49%) |
| No | 66 (51%) |
| All Patients n = 131 | VTE | (n = 30) | Not VTE | (n = 101) | |||
|---|---|---|---|---|---|---|---|
| Mean | Median | DS | Mean | Median | DS | Test t | |
| Age (years) | 78 | 82 | 13.3 | 68 | 68 | 14.9 | 0.001711 |
| BMI (kg/m2) | 23.9 | 23.28 | 3.58 | 24.42 | 24.77 | 2.98 | 0.498998 |
| D-dimer (ng/mL) | 1.74 | 1.1 | 1.31 | 1.95 | 1.27 | 1.82 | 0.551463 |
| Platelet count (mm3) | 241.41 | 240 | 92.24 | 252.94 | 225 | 108 | 0.60452 |
| Fibrinogen(mg/dL) | 503.4 | 470 | 198.08 | 493 | 476 | 133.78 | 0.745607 |
| LMWH Daily dose | 0.5 | 0.4 | 0.18 | 0.47 | 0.4 | 0.16 | 0.353239 |
| Creatinine(mg/dL) | 1.24 | 1 | 0.81 | 0.89 | 0.8 | 0.43 | 0.00275 |
| FiO2 (%) | 38.3 | 35 | 17.21 | 33.8 | 21 | 17.99 | 0.228329 |
| Bilirubin (mg/dL) | 0.56 | 0.53 | 0.21 | 0.58 | 0.54 | 0.26 | 0.792944 |
| GCS | 11.8 | 12.5 | 2.57 | 13.2 | 15 | 2.44 | 0.007232 |
| SBP (mmHg) | 125.3 | 127.5 | 20.77 | 121.66 | 120 | 14.59 | 0.278259 |
| NT-ProBNP(ng/L) | 4608.43 | 876.5 | 8345.56 | 581.97 | 187.5 | 1131.78 | 0.00002 |
| VTE (n = 30) | Not VTE (n = 101) | |
|---|---|---|
| Sex (female) | 15 (50%) | 48 (47%) |
| Mechanical ventilation | 8 (27%) | 32 (32%) |
| Hypertension | 19 (63%) | 17 (17%) |
| Coronary heart disease | 4 (13%) | 10 (10%) |
| Ace inhibitors | 4 (13%) | 17 (17%) |
| ARBs | 10 (33%) | 28 (28%) |
| Classifier | Train Score | Test Score | Train Time | |
|---|---|---|---|---|
| 1 | Logistic Regression | 0.862069 | 0.813953 | 0.046875 |
| 2 | Naive Bayes | 0.816092 | 0.790698 | 0.000000 |
| 3 | Random Forest | 1.000000 | 0.767442 | 2.093750 |
| 4 | Linear SVM | 0.793103 | 0.720930 | 0.000000 |
| 5 | Decision Tree | 1.000000 | 0.674419 | 0.000000 |
| Patient Proof Characteristics | |
|---|---|
| Age (Years) | 71 |
| Sex (male/female) | 1 |
| BMI (kg/m2) | 20.16 |
| D-Dimer Levels (peak) | 0.42 |
| Platelet Count (mm3) | 111 |
| Fibrinogen Levels (mg/dL) | 298 |
| Daily Dose (mg) | 99 |
| Creatinine (mg/dL) | 1.7 |
| Mechanical ventilation (yes/no) | 1 |
| FiO2 (%) | 26 |
| Bilirubin (mg/dL) | 0.59 |
| Glasgow Coma Scale | 11 |
| Systolic blood pressure | 135 |
| Hypertension (yes/no) | 1 |
| Coronary arterydisease (yes/no) | 0 |
| Ace inhibitors (yes/no) | 0 |
| ARBs (yes/no) | 0 |
| NT-proBNP (ng/L) | 24,904 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbalzano, E.; Orlando, L.; Sciacqua, A.; Nato, G.; Dentali, F.; Nassisi, V.; Russo, V.; Camporese, G.; Bagnato, G.; Cicero, A.F.G.; et al. Machine Learning to Calculate Heparin Dose in COVID-19 Patients with Active Cancer. J. Clin. Med. 2022, 11, 219. https://doi.org/10.3390/jcm11010219
Imbalzano E, Orlando L, Sciacqua A, Nato G, Dentali F, Nassisi V, Russo V, Camporese G, Bagnato G, Cicero AFG, et al. Machine Learning to Calculate Heparin Dose in COVID-19 Patients with Active Cancer. Journal of Clinical Medicine. 2022; 11(1):219. https://doi.org/10.3390/jcm11010219
Chicago/Turabian StyleImbalzano, Egidio, Luana Orlando, Angela Sciacqua, Giuseppe Nato, Francesco Dentali, Veronica Nassisi, Vincenzo Russo, Giuseppe Camporese, Gianluca Bagnato, Arrigo F. G. Cicero, and et al. 2022. "Machine Learning to Calculate Heparin Dose in COVID-19 Patients with Active Cancer" Journal of Clinical Medicine 11, no. 1: 219. https://doi.org/10.3390/jcm11010219
APA StyleImbalzano, E., Orlando, L., Sciacqua, A., Nato, G., Dentali, F., Nassisi, V., Russo, V., Camporese, G., Bagnato, G., Cicero, A. F. G., Dattilo, G., Vatrano, M., Versace, A. G., Squadrito, G., & Di Micco, P. (2022). Machine Learning to Calculate Heparin Dose in COVID-19 Patients with Active Cancer. Journal of Clinical Medicine, 11(1), 219. https://doi.org/10.3390/jcm11010219











