Age of Red Cells for Transfusion and Outcomes in Patients with ARDS
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
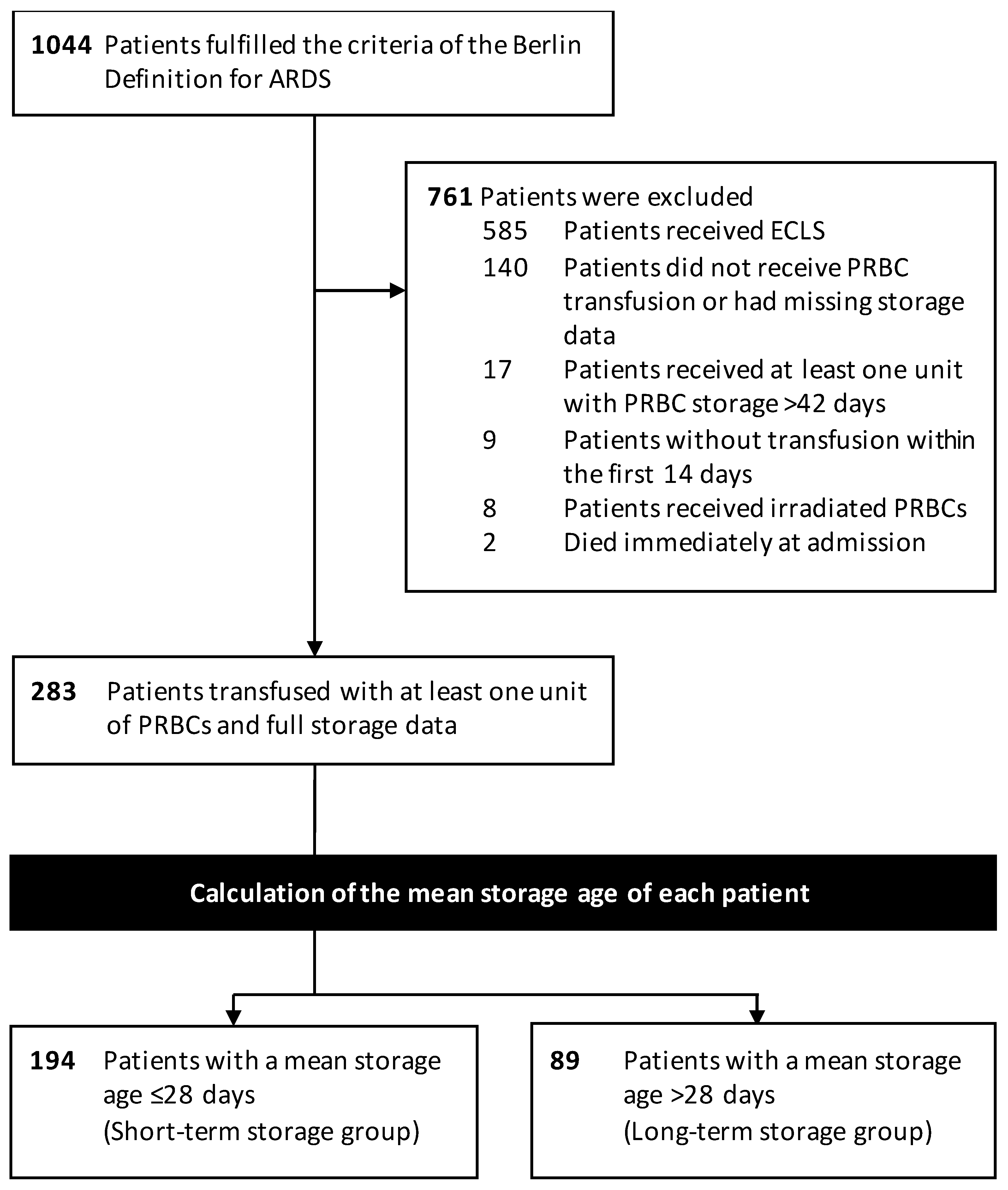
2.2. Participants
2.3. PRBC Storage Preprocessing and Grouping
2.4. Endpoints and Data Sources
2.5. Bias Handling
2.6. Statistical Analyses
3. Results
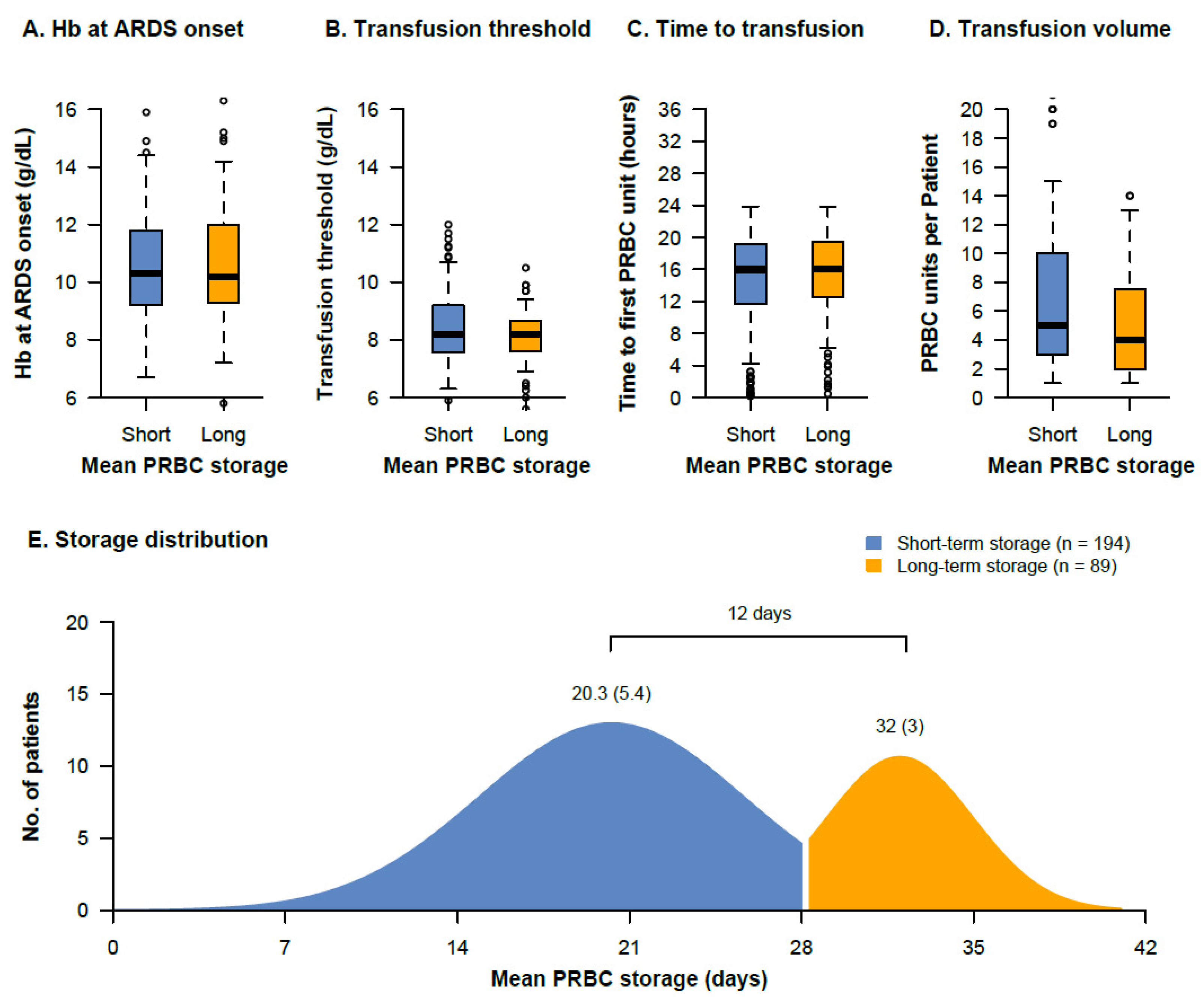
3.1. Transfusion Characteristics
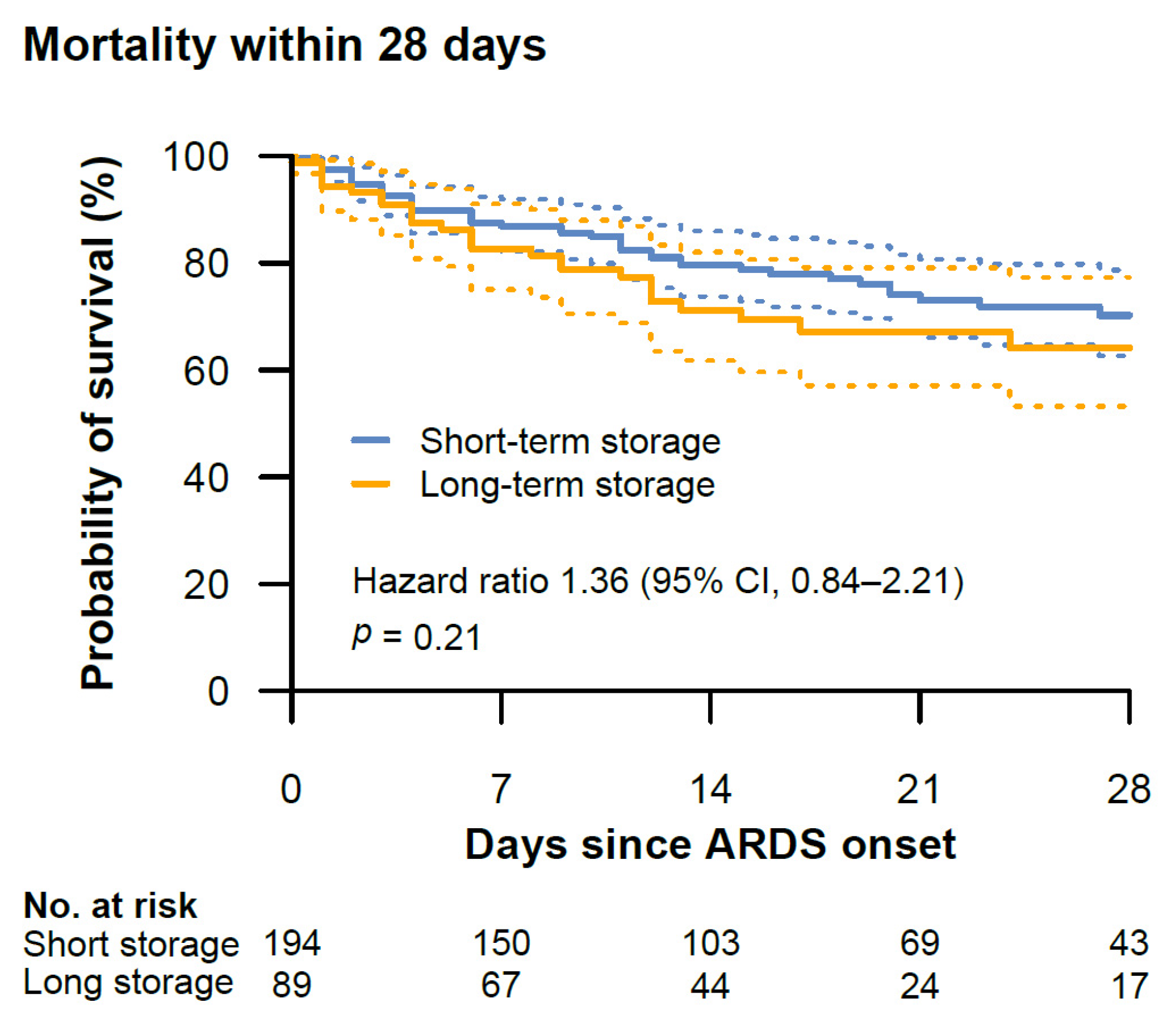
3.2. Endpoints
3.3. Secondary Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Sun, J.; Solomon, S.B.; Klein, H.G.; Natanson, C. Transfusion of older stored blood and risk of death: A meta-analysis. Transfusion 2012, 526, 1184–1195. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef] [PubMed]
- Donadee, C.; Raat, N.; Kanias, T.; Tejero, J.; Lee, J.; Kelley, E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Donadee, C.L.; Gladwin, M.T. Hemodialysis Hyperhemolysis: A Novel Mechanism of Endothelial Dysfunction and Cardiovascular Risk? J. Am. Coll. Cardiol. 2010, 55, 460–462. [Google Scholar] [CrossRef][Green Version]
- Villagra, J.; Shiva, S.; Hunter, L.A.; Machado, R.F.; Gladwin, M.T.; Kato, G.J. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood 2007, 1106, 2166–2172. [Google Scholar] [CrossRef]
- Balla, J.; Vercellotti, G.M.; Jeney, V.; Yachie, A.; Varga, Z.; Jacob, H.S.; Eaton, J.W.; Balla, G. Heme, Heme Oxygenase, and Ferritin: How the Vascular Endothelium Survives (and Dies) in an Iron-Rich Environment. Antioxid. Redox Signal. 2007, 9, 2119–2138. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 1218, 1276–1284. [Google Scholar] [CrossRef]
- Silliman, C.C.; Moore, E.E.; Kelher, M.R.; Khan, S.Y.; Gellar, L.; Elzi, D.J. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion 2011, 51, 2549–2554. [Google Scholar] [CrossRef]
- Sparrow, R.; Patton, K. Supernatant from stored red blood cell primes inflammatory cells: Influence of prestorage white cell reduction. Transfusion 2004, 44, 722–730. [Google Scholar] [CrossRef]
- Baek, J.H.; D’Agnillo, F.; Vallelian, F.; Pereira, C.P.; Williams, M.C.; Jia, Y.; Schaer, D.J.; Buehler, P.W. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J. Clin. Investig. 2012, 1224, 1444–1458. [Google Scholar] [CrossRef]
- Cortés-Puch, I.; Wang, D.; Sun, J.; Solomon, S.B.; Remy, K.; Fernandez, M.; Feng, J.; Kanias, T.; Bellavia, L.; Sinchar, D.; et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood 2014, 123, 1403–1411. [Google Scholar] [CrossRef]
- Graw, J.A.; Mayeur, C.; Rosales, I.; Liu, Y.; Sabbisetti, V.S.; Riley, F.E.; Rechester, O.; Malhotra, R.; Warren, H.S.; Colvin, R.B.; et al. Haptoglobin or Hemopexin Therapy Prevents Acute Adverse Effects of Resuscitation After Prolonged Storage of Red Cells. Circulation 2016, 134, 945–960. [Google Scholar] [CrossRef]
- Koch, C.G.; Li, L.; Sessler, D.I.; Figueroa, P.; Hoeltge, G.A.; Mihaljevic, T.; Blackstone, E.H. Duration of Red-Cell Storage and Complications after Cardiac Surgery. N. Engl. J. Med. 2008, 358, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, J.; Hébert, P.C.; Fergusson, D.; Tinmouth, A.; Cook, D.J.; Marshall, J.C.; Clayton, L.; McIntyre, L.; Callum, J.; Turgeon, A.F.; et al. Age of Transfused Blood in Critically Ill Adults. N. Engl. J. Med. 2015, 372, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.E.; Ness, P.M.; Assmann, S.F.; Triulzi, D.J.; Sloan, S.R.; Delaney, M.; Granger, S.; Bennett-Guerrero, E.; Blajchman, M.A.; Scavo, V.; et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med. 2015, 37215, 1419–1429. [Google Scholar] [CrossRef]
- Heddle, N.M.; Cook, R.J.; Arnold, D.M.; Liu, Y.; Barty, R.; Crowther, M.A.; Devereaux, P.J.; Hirsh, J.; Warkentin, T.E.; Webert, K.E.; et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N. Engl. J. Med. 2016, 375, 1937–1945. [Google Scholar] [CrossRef]
- Cooper, D.J.; McQuilten, Z.K.; Nichol, A.; Ady, B.; Aubron, C.; Bailey, M.; Bellomo, R.; Gantner, D.; Irving, D.O.; Kaukonen, K.-M.; et al. Age of Red Cells for Transfusion and Outcomes in Critically Ill Adults. N. Engl. J. Med. 2017, 377, 1858–1867. [Google Scholar] [CrossRef]
- Rapido, F.; Brittenham, G.M.; Bandyopadhyay, S.; La Carpia, F.; L’Acqua, C.; McMahon, D.J.; Rebbaa, A.; Wojczyk, B.S.; Netterwald, J.; Wang, H.; et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Investig. 2017, 1271, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef]
- Hod, E.A.; Brittenham, G.M.; Billote, G.B.; Francis, R.O.; Ginzburg, Y.Z.; Hendrickson, J.E.; Jhang, J.; Schwartz, J.; Sharma, S.; Sheth, S.; et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non–transferrin-bound iron. Blood 2011, 118, 6675–6682. [Google Scholar] [CrossRef]
- Berra, L.; Pinciroli, R.; Stowell, C.P.; Wang, L.; Yu, B.; Fernandez, B.O.; Feelisch, M.; Mietto, C.; Hod, E.A.; Chipman, D.; et al. Autologous Transfusion of Stored Red Blood Cells Increases Pulmonary Artery Pressure. Am. J. Respir. Crit. Care Med. 2014, 190, 800–807. [Google Scholar] [CrossRef]
- Zallen, G.; Offner, P.J.; Moore, E.E.; Blackwell, J.; Ciesla, D.J.; Gabriel, J.; Denny, C.; Silliman, C.C. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am. J. Surg. 1999, 1786, 570–572. [Google Scholar] [CrossRef]
- Remy, K.E.; Sun, J.; Wang, D.; Welsh, J.; Solomon, S.B.; Klein, H.G.; Natanson, C.; Cortés-Puch, I. Transfusion of recently donated (fresh) red blood cells (RBCs) does not improve survival in comparison with current practice, while safety of the oldest stored units is yet to be established: A meta-analysis. Vox Sang. 2016, 111, 43–54. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Hunsicker, O.; Materne, L.; Bünger, V.; Krannich, A.; Balzer, F.; Spies, C.; Francis, R.C.; Weber-Carstens, S.; Menk, M.; Graw, J.A. Lower versus higher hemoglobin threshold for transfusion in ARDS patients with and without ECMO. Crit. Care 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 30723, 2526–2533. [Google Scholar]
- D’Alessandro, A.; Kriebardis, A.; Rinalducci, S.; Antonelou, M.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015, 55, 205–219. [Google Scholar] [CrossRef]
- Yehya, N.; Harhay, M.O.; Curley, M.A.Q.; Schoenfeld, D.A.; Reeder, R.W. Reappraisal of Ventilator-free Days in Critical Care Research. Am. J. Respir. Crit. Care Med. 2019, 200, 828–836. [Google Scholar] [CrossRef]
- Hoenig, J.M.; Heisey, D.M. The Abuse of Power: The Pervasive Fallacy of Power Calculations for Data Analysis. Am. Stat. 2001, 551, 19–24. [Google Scholar] [CrossRef]
- Spinella, P.C.; Tucci, M.; Fergusson, D.A.; Lacroix, J.; Hebert, P.C.; Leteutre, S.; Schechtman, K.B.; Doctor, A.; Berg, R.A.; Bockelmann, T.; et al. Effect of Fresh vs. Standard-issue Red Blood Cell Transfusions on Multiple Organ Dysfunction Syndrome in Critically Ill Pediatric Patients: A Randomized Clinical Trial. JAMA 2019, 322, 2179–2190. [Google Scholar] [CrossRef]
- Shah, A.; Brunskill, S.J.; Desborough, M.; Doree, C.; Trivella, M.; Stanworth, S.J. Transfusion of red blood cells stored for shorter versus longer duration for all conditions. Cochrane Database Syst. Rev. 2018, 2018, CD010801. [Google Scholar] [CrossRef]
- Hod, E.A.; Spitalnik, S. Harmful effects of transfusion of older stored red blood cells: Iron and inflammation. Transfusion 2011, 51, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Puch, I.; Remy, K.E.; Solomon, S.B.; Sun, J.; Wang, D.; Al-Hamad, M.; Kelly, S.M.; Sinchar, D.; Bellavia, L.; Kanias, T.; et al. In a canine pneumonia model of exchange transfusion, altering the age but not the volume of older red blood cells markedly alters outcome. Transfusion 2015, 55, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen Windsant, I.C.; de Wit, N.C.; Sertorio, J.T.; van Bijnen, A.A.; Ganushchak, Y.M.; Heijmans, J.H.; Tanus-Santos, J.E.; Jacobs, M.J.; Maessen, J.G.; Buurman, W.A. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 2014, 5, 340. [Google Scholar] [CrossRef]
- Vermeulen Windsant, I.C.; Snoeijs, M.G.; Hanssen, S.J.; Altintas, S.; Heijmans, J.H.; Koeppel, T.A.; Schurink, G.W.; Buurman, W.A.; Jacobs, M.J. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010, 7710, 913–920. [Google Scholar] [CrossRef] [PubMed]
- McVey, M.; Kuebler, W.; Orbach, A.; Arbell, D.; Zelig, O.; Barshtein, G.; Yedgar, S. Reduced deformability of stored red blood cells is associated with generation of extracellular vesicles. Transfus. Apher. Sci. 2020, 59, 102851. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Ma, T.; Liu, X.; Wang, B.; Wu, Z.; Dang, S.; Lv, Y.; Wu, R. Transfusion of Older Red Blood Cells Increases the Risk of Acute Kidney Injury After Orthotopic Liver Transplantation: A Propensity Score Analysis. Anesth. Analg. 2018, 1271, 202–209. [Google Scholar] [CrossRef]
- Materne, L.A.; Hunsicker, O.; Menk, M.; Graw, J.A. Hemolysis in patients with Extracorporeal Membrane Oxygenation therapy for severe Acute Respiratory Distress Syndrome—A systematic review of the literature. Int. J. Med Sci. 2021, 18, 1730–1738. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Muller, A.; Weis, B.; Spies, C.D.; Ralf, B.; Andreas, B.; Rolf, B.; Stephan, B.; Hartmut, B.; Peter, D.; Süha, D.; et al. Analgesie, Sedierung und Delirmanagement—Die neue S3-Leitlinie „Analgesie, Sedierung und Delirmanagement in der Intensivmedizin“(DAS-Leitlinie 2015). Anästhesiol. Intensivmed. Notf. Schmerzther. 2015, 50, 698–703. [Google Scholar] [CrossRef]

| Characteristic | Short-Term Storage Group (n = 194) | Long-Term Storage Group (n = 89) | p-Value |
|---|---|---|---|
| Age (years) | 55.50 (42.25, 66.00) | 57.00 (47.00, 69.00) | 0.248 |
| Male sex, n (%) | 120 (61.9) | 56 (62.9) | 0.968 |
| Body mass index (kg/cm) | 27.78 (24.39, 32.65) | 26.73 (23.67, 31.37) | 0.212 |
| Charlson comorbidity index | 3.00 (1.00, 5.00) | 3.00 (1.00, 6.00) | 0.430 |
| Chronic kidney disease, n (%) | 19 (9.8) | 16 (18.0) | 0.081 |
| Immunocompromised, n (%) | 44 (22.7) | 22 (24.7) | 0.822 |
| SOFA at ARDS onset | 11.00 (8.25, 14.00) | 12.00 (8.00, 14.00) | 0.669 |
| SAPS II at ARDS onset | 50.00 (38.00, 64.00) | 55.00 (38.00, 70.00) | 0.320 |
| RASS at ARDS onset | −5.00 (−5.00, −4.00) | −5.00 (−5.00, −4.00) | 0.719 |
| Chronic lung disease, n (%) | 39 (20.1) | 24 (27.0) | 0.256 |
| Mechanical ventilation before admission (days) | 1.00 (0.00, 4.00) | 1.00 (0.00, 4.00) | 0.523 |
| ARDS severity, n (%) | 0.618 | ||
| Mild | 10 (5.2) | 7 (7.9) | |
| Moderate | 47 (24.2) | 19 (21.3) | |
| Severe | 137 (70.6) | 63 (70.8) | |
| ARDS etiology, n (%) | 0.495 | ||
| Pneumonia | 102 (52.6) | 51 (57.3) | |
| Aspiration | 44 (22.7) | 17 (19.1) | |
| Sepsis | 15 (7.7) | 11 (12.4) | |
| Pancreatitis | 6 (3.1) | 2 (2.2) | |
| Other | 27 (13.9) | 8 (9.0) | |
| Ventilation parameters after initial optimization | |||
| PaO2:FiO2 (mmHg) | 157.37 (109.17, 214.44) | 164.47 (111.25, 228.05) | 0.401 |
| Oxygenation index | 15.19 (9.56, 22.57) | 14.35 (8.88, 21.36) | 0.244 |
| PEEP (cm H2O) | 16.60 (14.00, 20.00) | 16.00 (13.25, 18.02) | 0.075 |
| Driving pressure (cm H2O) | 15.52 (13.00, 18.00) | 15.29 (11.85, 18.84) | 0.771 |
| Tidal volume (mL/kg PBW) | 6.86 (5.74, 7.76) | 6.07 (5.48, 7.05) | 0.016 |
| Respiratory rate (breaths/min) | 20.00 (18.00, 24.00) | 21.00 (20.00, 25.00) | 0.306 |
| Compliance (mL/cm H2O) | 36.40 (27.45, 47.15) | 33.85 (26.98, 49.30) | 0.735 |
| Rescue therapy | |||
| Inhaled nitric oxide, n (%) | 131 (67.5) | 57 (64.0) | 0.660 |
| Prone positioning, n (%) | 132 (68.0) | 52 (58.4) | 0.150 |
| Septic shock, n (%) | 83 (43.2) | 37 (42.5) | 0.999 |
| Lactate (mg/dL) | 16.00 (10.00, 30.00) | 16.00 (10.00, 31.00) | 0.742 |
| RRT, n (%) | 120 (61.9) | 50 (56.2) | 0.439 |
| Characteristic | Short-Term Storage Group (n = 194) | Long-Term Storage Group (n = 89) | p-Value |
|---|---|---|---|
| Hemoglobin at ARDS onset (g/dL) | 10.30 (9.22, 11.80) | 10.20 (9.30, 12.00) | 0.647 |
| Transfusion threshold * (g/dL) | 8.22 (7.59, 9.20) | 8.20 (7.60, 8.65) | 0.171 |
| PRBC units transfused per patient with the first 14 days (number) | 5.00 (3.00, 9.75) | 4.00 (2.00, 8.00) | 0.143 |
| PRBC units transfused per patient with the first 28 days (number) | 6.00 (2.25, 10.00) | 4.00 (2.00, 9.50) | 0.193 |
| Mean PRBC storage age per patient (days) | 20.23 (5.38) | 32.26 (3.10) | <0.001 |
| Oldest PRBC unit per patient (days) | 27.81 (8.13) | 37.49 (3.31) | <0.001 |
| Time to first PRBC transfusion (hours) | 15.90 (11.63, 19.20) | 16.08 (12.83, 19.40) | 0.657 |
| Patients receiving transfusion of other blood components, n (%) | |||
| Platelets | 137 (70.6) | 57 (64.0) | 0.333 |
| Fresh frozen plasma | 53 (27.3) | 22 (24.7) | 0.753 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graw, J.A.; Bünger, V.; Materne, L.A.; Krannich, A.; Balzer, F.; Francis, R.C.E.; Pruß, A.; Spies, C.D.; Kuebler, W.M.; Weber-Carstens, S.; et al. Age of Red Cells for Transfusion and Outcomes in Patients with ARDS. J. Clin. Med. 2022, 11, 245. https://doi.org/10.3390/jcm11010245
Graw JA, Bünger V, Materne LA, Krannich A, Balzer F, Francis RCE, Pruß A, Spies CD, Kuebler WM, Weber-Carstens S, et al. Age of Red Cells for Transfusion and Outcomes in Patients with ARDS. Journal of Clinical Medicine. 2022; 11(1):245. https://doi.org/10.3390/jcm11010245
Chicago/Turabian StyleGraw, Jan A., Victoria Bünger, Lorenz A. Materne, Alexander Krannich, Felix Balzer, Roland C. E. Francis, Axel Pruß, Claudia D. Spies, Wolfgang M. Kuebler, Steffen Weber-Carstens, and et al. 2022. "Age of Red Cells for Transfusion and Outcomes in Patients with ARDS" Journal of Clinical Medicine 11, no. 1: 245. https://doi.org/10.3390/jcm11010245
APA StyleGraw, J. A., Bünger, V., Materne, L. A., Krannich, A., Balzer, F., Francis, R. C. E., Pruß, A., Spies, C. D., Kuebler, W. M., Weber-Carstens, S., Menk, M., & Hunsicker, O. (2022). Age of Red Cells for Transfusion and Outcomes in Patients with ARDS. Journal of Clinical Medicine, 11(1), 245. https://doi.org/10.3390/jcm11010245






