Long-Term Survival and Causes of Death in Patients below the Age of 60 with Traumatic Spinal Cord Injury in Germany
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Age at Injury and Etiology of Traumatic SCI
3.2. Life Expectancy
3.3. Causes of Death
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thietje, R. Epidemiologie, Ätiologie und Mortalität bei Querschnittlähmung. Neuroreha 2016, 8, 105–109. [Google Scholar] [CrossRef]
- Savic, G.; DeVivo, M.J.; Frankel, H.L.; Jamous, M.A.; Soni, B.M.; Charlifue, S. Long-term survival after traumatic spinal cord injury: A 70-year British study. Spinal Cord 2017, 55, 651–658. [Google Scholar] [CrossRef]
- Böthig, R.; Tiburtius, C.; Fiebag, K.; Kowald, B.; Hirschfeld, S.; Thietje, R.; Kurze, I.; Schöps, W.; Böhme, H.; Kaufmann, A.; et al. Traumatic spinal cord injury confers bladder cancer risk to patients managed without permanent urinary catheterization: Lessons from a comparison of clinical data with the national database. World J. Urol. 2020, 38, 2827–2834. [Google Scholar] [CrossRef]
- Chamberlain, J.D.; Meier, S.; Mader, L.; von Groote, P.M.; Brinkhof, M.W.G. Mortality and longevity after a spinal cord injury: Systematic review and meta-analysis. Neuroepidemiology 2015, 44, 182–198. [Google Scholar] [CrossRef]
- Hartkopp, A.; Brønnum-Hansen, H.; Seidenschnur, A.M.; Biering-Sørensen, F. Survival and cause of death after traumatic spinal cord injury. A long-term epidemiological survey from Denmark. Spinal Cord 1997, 35, 76–85. [Google Scholar] [CrossRef]
- Hartkopp, A.; Brønnum-Hansen, H.; Seidenschnur, A.M.; Biering-Sørensen, F. Suicide in a spinal cord injured population: Its relation to functional status. Arch. Phys. Med. Rehabil. 1998, 79, 1356–1361. [Google Scholar] [CrossRef]
- Soden, R.J.; Walsh, J.; Middleton, J.W.; Craven, M.L.; Rutkowski, S.B.; Yeo, J.D. Causes of death after spinal cord injury. Spinal Cord 2020, 38, 604–610. [Google Scholar] [CrossRef]
- Lidal, I.B.; Snekkevik, H.; Aamodt, G.; Hjeltnes, N.; Biering-Sørensen, F.; Stanghelle, J.K. Mortality after spinal cord injury in Norway. J. Rehabil. Med. 2007, 39, 145–151. [Google Scholar] [CrossRef]
- Jurisić, B.; Marusic, A. Suicidal ideation and behavior and some psychological correlates in physically disabled motor-vehicle accident survivors. Crisis 2009, 30, 34–38. [Google Scholar] [CrossRef]
- van den Berg, M.E.L.; Castellote, J.M.; de Pedro-Cuesta, J.; Mahillo-Fernandez, I. Survival after spinal cord injury: A systematic review. J. Neurotrauma. 2010, 27, 1517–1528. [Google Scholar] [CrossRef]
- Divanoglou, A.; Westgren, N.; Seiger, A.; Hulting, C.; Levi, R. Late mortality during the first year after acute traumatic spinal cord injury: A prospective, population-based study. J. Spinal. Cord Med. 2010, 33, 117–127. [Google Scholar] [CrossRef][Green Version]
- Böthig, R.; Golka, K.; Tiburtius, C.; Balzer, O.; Kowald, B.; Hirschfeld, S.; Kurze, I.; Schöps, W.; Kadhum, T.; Thietje, R. Incidental bladder cancer at initial urological workup of spinal cord injury patients. Spinal Cord Ser. Cases 2020, 6, 55. [Google Scholar] [CrossRef]
- Böthig, R.; Schöps, W.; Zellner, M.; Fiebag, K.; Kowald, B.; Hirschfeld, S.; Thietje, R.; Kurze, I.; Böhme, H.; Kaufmann, A.; et al. Urinary bladder cancer as a late sequela of spinal cord injury: Decision-making aids for assessment of this causal association. Urol. A 2020, 59, 700–709. [Google Scholar]
- Kriz, J.; Sediva, K.; Maly, M. Causes of death after spinal cord injury in the Czech Republic. Spinal Cord 2021, 59, 814–820. [Google Scholar]
- Saatian, M.; Nayereh, K.; Mohamadi, Y.; Sangestani, S.; Abdoli, A.; Mazloumi, E. Epidemiological Study of Traumatic Spinal Injuries in Iranian Patients from 2007 to 2017. Hormozgan Med. J. 2021, 25, e103203. [Google Scholar]
- Savic, G.; DeVivo, M.J.; Frankel, H.L.; Jamous, M.A.; Soni, B.M.; Charlifue, S. Causes of death after traumatic spinal cord injury-a 70-year British study. Spinal Cord 2017, 55, 891–897. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications In Brief. American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar]
- Kirshblum, S.; Botticello, A.; Benedetto, J.; Donovan, J.; Marino, R.; Hsieh, S.; Wagaman, N. A Comparison of Diagnostic Stability of the ASIA Impairment Scale Versus Frankel Classification Systems for Traumatic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2020, 101, 1556–1562. [Google Scholar] [CrossRef]
- Villaveces, A.; Peck, M.; Faraklas, I.; Hsu-Chang, N.; Joe, V.; Wibbenmeyer, L. Process evaluation of software using the International classification of external causes of injuries for collecting burn injury data at burn centers in the United States. J. Burn Care Res. 2014, 35, 28–40. [Google Scholar]
- DeVivo, M.J.; Biering-Sørensen, F.; New, P.; Chen, Y. International Spinal Cord Injury Data Set. Standardization of data analysis and reporting of results from the International Spinal Cord Injury Core Data Set. Spinal Cord 2011, 49, 596–599. [Google Scholar]
- DeVivo, M.; Executive Committee for the International SCI Data Sets Committees; Biering-Sørensen, F.; Charlifue, S.; Noonan, V.; Post, M.; Stripling, T.; Wing, P. International Spinal Cord Injury Core Data Set. Spinal Cord 2006, 44, 535–540. [Google Scholar] [CrossRef]
- Biering-Sørensen, F.; Bickenbach, J.E.; El Masry, W.S.; Officer, A.; von Groote, P.M. ISCoS-WHO collaboration. International Perspectives of Spinal Cord Injury (IPSCI) report. Spinal Cord 2011, 49, 679–683. [Google Scholar] [CrossRef]
- Dahlberg, A.; Kotila, M.; Leppänen, P.; Kautiainen, H.; Alaranta, H. Prevalence of spinal cord injury in Helsinki. Spinal Cord 2005, 43, 47–50. [Google Scholar] [CrossRef]
- Hagen, E.M.; Eide, G.E.; Rekand, T.; Gilhus, N.E.; Gronning, M. A 50-year follow-up of the incidence of traumatic spinal cord injuries in Western Norway. Spinal Cord 2010, 48, 313–318. [Google Scholar] [CrossRef]
- Noonan, V.K.; Fingas, M.; Farry, A.; Baxter, D.; Singh, A.; Fehlings, M.G. Incidence and prevalence of spinal cord injury in Canada: A national perspective. Neuroepidemiology 2012, 38, 219–226. [Google Scholar] [CrossRef]
- Alshahri, S.S.; Cripps, R.A.; Lee, B.B.; Al-Jadid, M.S. Traumatic spinal cord injury in Saudi Arabia: An epidemiological estimate from Riyadh. Spinal Cord 2012, 50, 882–884. [Google Scholar] [CrossRef]
- Ahoniemi, E.; Pohjolainen, T.; Kautiainen, H. Survival after spinal cord injury in Finland. J. Rehabil. Med. 2011, 43, 481–485. [Google Scholar] [CrossRef][Green Version]
- O’Connor, P.J. Survival after spinal cord injury in Australia. Arch. Phys. Med. Rehabil. 2005, 86, 37–47. [Google Scholar] [CrossRef]
- Garshick, E.; Kelley, A.; Cohen, S.A.; Garrison, A.; Tun, C.G.; Gagnon, D. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005, 43, 408–416. [Google Scholar] [CrossRef]
- Middleton, J.W.; Dayton, A.; Walsh, J.; Rutkowski, S.B.; Leong, G.; Duong, S. Life expectancy after spinal cord injury: A 50-year study. Spinal Cord 2012, 50, 803–811. [Google Scholar] [CrossRef]
- National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance 2016. University of Alabama at Birmingham Webpage. Available online: https://www.nscisc.uab.edu/public_pages/FactsFigures_archive (accessed on 25 October 2021).
- Rabadi, M.H.; Mayanna, S.K.; Vincent, A.S. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord 2013, 51, 784–788. [Google Scholar] [CrossRef]


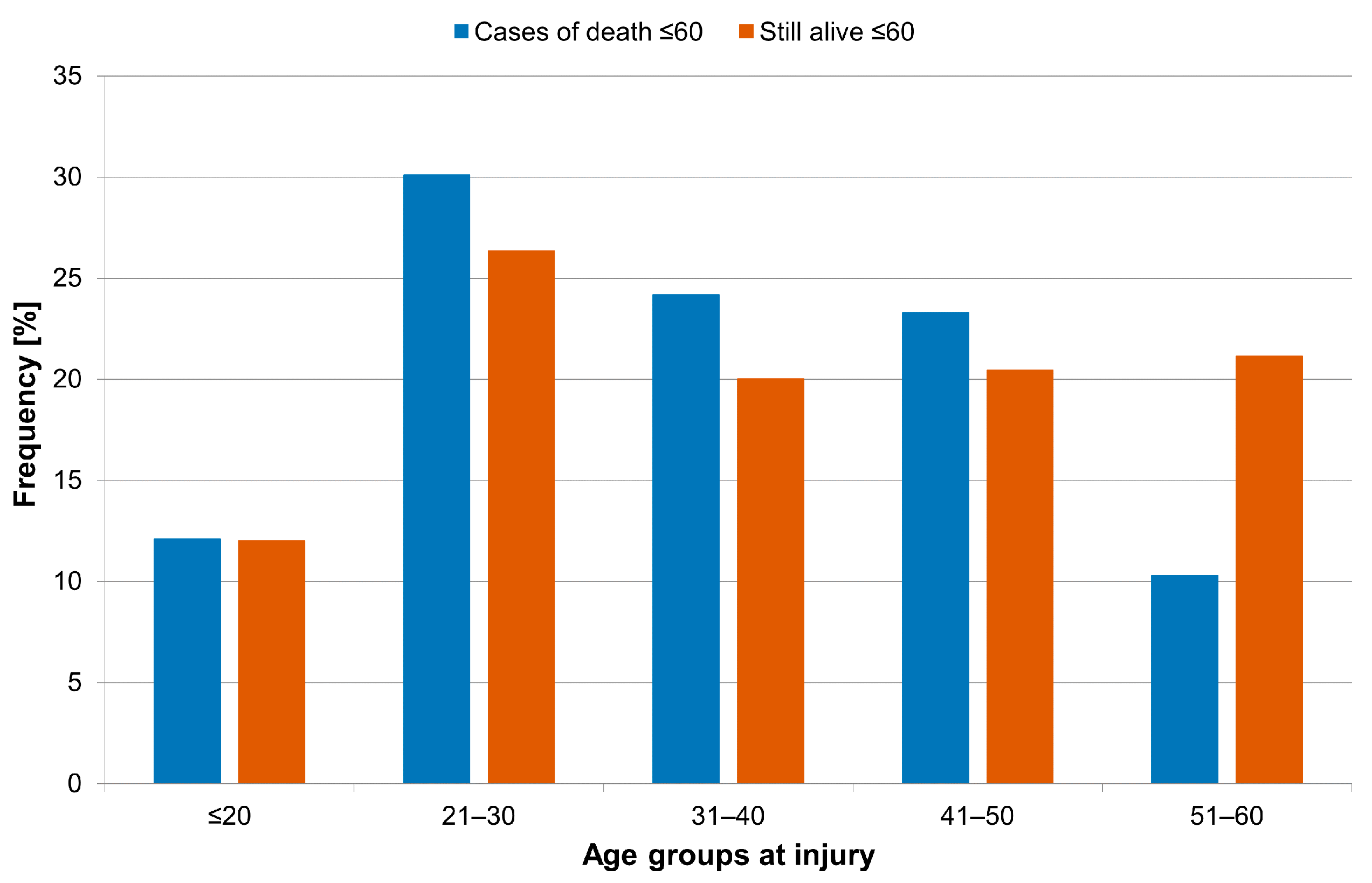
| AIS-Type | Control Group ± 60 n | Cases of Death ± 60 n |
|---|---|---|
| A | 804 | 159 |
| B | 166 | 10 |
| C | 380 | 40 |
| D | 248 | 14 |
| Sum | 1598 | 223 |
| Cause of TSCI | n | Percentage |
|---|---|---|
| Sports and leisure | 17 | 7.3 |
| Assaults | 6 | 2.7 |
| Transport | 78 | 35.0 |
| Fall | 118 | 52.9 |
| Others | 4 | 1.8 |
| Level of Injury and Severity | Average | Range | n |
|---|---|---|---|
| C1–4 AIS A, B or C | 17.3 | 1.1–43.4 | 53 |
| C5–8 AIS A, B or C | 22.3 | 4.0–51.9 | 49 |
| T1–S5 AIS A, B or C | 31.1 | 1.1–63.3 | 98 |
| AIS D at any level | 28.9 | 13.3–53.1 | 14 |
| Ventilator-dependent | 12.6 | 2.8–34.6 | 9 |
| Total | 25.0 | 1.1–63.3 | 223 |
| Cause of Death | n | Percentage |
|---|---|---|
| Cardiovascular diseases | 63 | 28.2 |
| Pneumonia | 58 | 26.0 |
| Pressure sore | 22 | 9.9 |
| Suicide | 19 | 8.5 |
| Other tumor | 18 | 8.1 |
| Bladder cancer | 15 | 6.7 |
| Urosepsis | 14 | 6.3 |
| Other sepsis | 6 | 2.7 |
| Others | 8 | 3.6 |
| Cause of Death | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|
| Pneumonia | 50.9% | 21.3% | 15.3% | 14.3% | 22.2% | 26.0% |
| Cardiovascular Diseases | 13.2% | 19.1% | 39.8% | 57.2% | 0.0% | 28.2% |
| Pressure sore | 5.7% | 12.8% | 13.3% | 0.0% | 0.00% | 9.9% |
| Urosepsis | 11.3% | 6.4% | 5.1% | 0.0% | 0.0% | 6.3% |
| Other sepsis | 0.0% | 4.3% | 2.0% | 0.0% | 22.2% | 2.7% |
| Bladder cancer | 1.9% | 12.8% | 7.1% | 7.1% | 0.0% | 6.7% |
| Other tumor | 1.9% | 8.5% | 10.2% | 14.3% | 11.1% | 8.1% |
| Suicide | 9.4% | 10.5% | 5.2% | 7.1% | 33.3% | 8.5% |
| Others | 5.7% | 4.3% | 2.0% | 0.0% | 11.1% | 3.6% |
| Total | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| Causes of Death | Statutory Health Insurance (n = 99) | German Statutory Accident Insurance (n = 124) |
|---|---|---|
| SCI-related | 54.5% | 40.3% |
| Non-SCI-related | 45.5% | 59.7% |
| Total | 100.0% | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thietje, R.; Kowald, B.; Böthig, R.; Schulz, A.P.; Northmann, M.; Rau, Y.; Hirschfeld, S. Long-Term Survival and Causes of Death in Patients below the Age of 60 with Traumatic Spinal Cord Injury in Germany. J. Clin. Med. 2022, 11, 26. https://doi.org/10.3390/jcm11010026
Thietje R, Kowald B, Böthig R, Schulz AP, Northmann M, Rau Y, Hirschfeld S. Long-Term Survival and Causes of Death in Patients below the Age of 60 with Traumatic Spinal Cord Injury in Germany. Journal of Clinical Medicine. 2022; 11(1):26. https://doi.org/10.3390/jcm11010026
Chicago/Turabian StyleThietje, Roland, Birgitt Kowald, Ralf Böthig, Arndt P. Schulz, Markus Northmann, Yannick Rau, and Sven Hirschfeld. 2022. "Long-Term Survival and Causes of Death in Patients below the Age of 60 with Traumatic Spinal Cord Injury in Germany" Journal of Clinical Medicine 11, no. 1: 26. https://doi.org/10.3390/jcm11010026
APA StyleThietje, R., Kowald, B., Böthig, R., Schulz, A. P., Northmann, M., Rau, Y., & Hirschfeld, S. (2022). Long-Term Survival and Causes of Death in Patients below the Age of 60 with Traumatic Spinal Cord Injury in Germany. Journal of Clinical Medicine, 11(1), 26. https://doi.org/10.3390/jcm11010026






