Conventional Trabeculotomy versus Gonioscopy-Assisted Transluminal Trabeculotomy: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
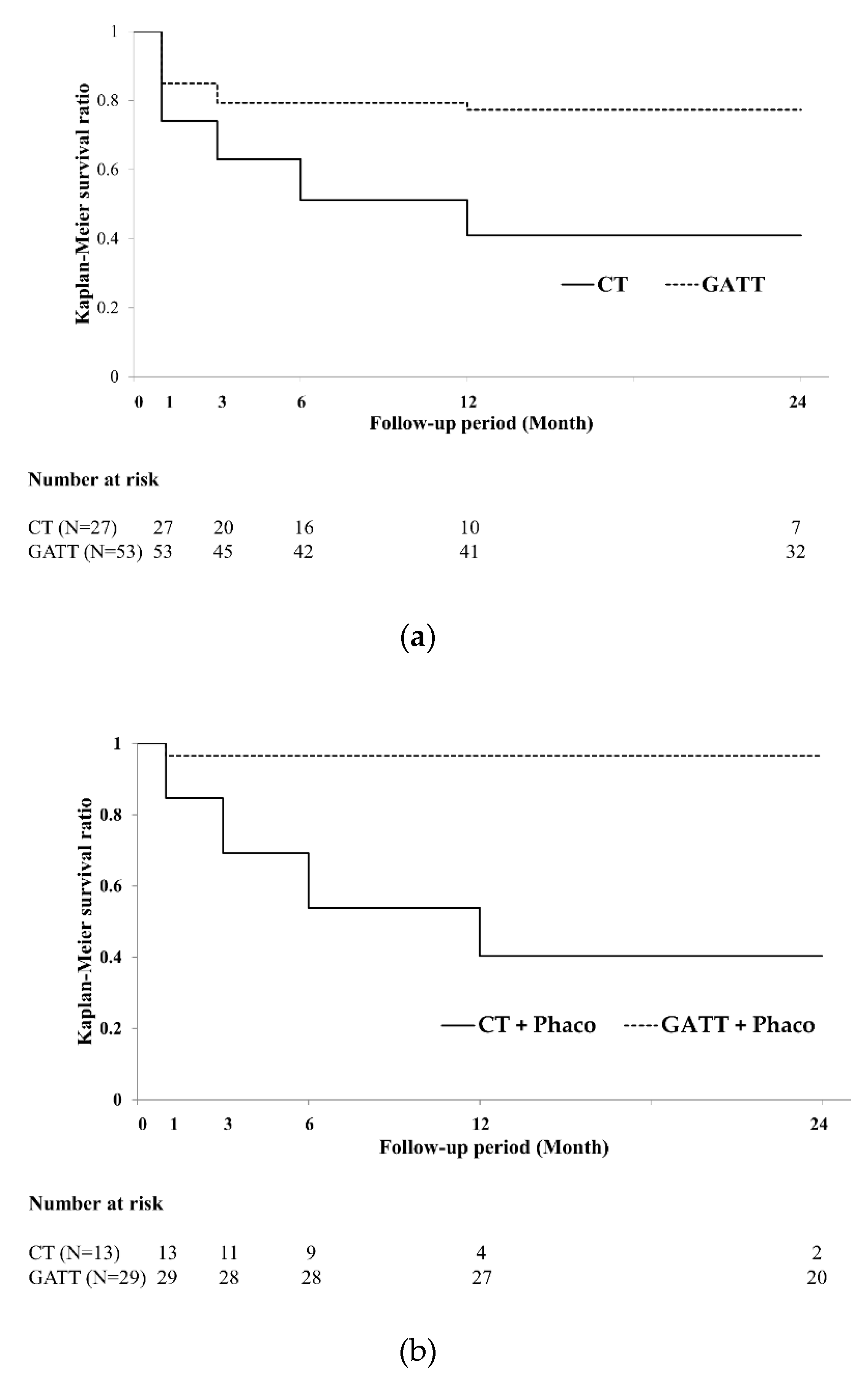
3.2. Primary Endpoint: Surgical Success Rate
3.3. Secondary Endpoints: Other Postoperative Outcomes
3.3.1. Changes in IOP
3.3.2. Changes in the Number of Glaucoma Medications
3.3.3. Medication-Free Rates among Patients
3.3.4. Incidences of Hyphema and Transient IOP Elevation
3.4. Factors Associated with the Surgical Success
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, A.; Araie, M.; Suzuki, Y.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The Tajimi Study report 2: Prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology 2005, 112, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.V.; Ervin, A.M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative effectiveness of treatments for open-angle glaucoma: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Iwao, K.; Inatani, M.; Tanihara, H.; Japanese Steroid-Induced Glaucoma Multicenter Study Group. Success rates of trabeculotomy for steroid-induced glaucoma: A comparative, multicenter, retrospective cohort study. Am. J. Ophthalmol. 2011, 151, 1047–1056.e1041. [Google Scholar] [CrossRef]
- Grover, D.S.; Godfrey, D.G.; Smith, O.; Feuer, W.J.; Montes de Oca, I.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014, 121, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, Y.; Kawase, K.; Matsushita, H.; Minobe, M. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Arch. Ophthalmol. 1991, 109, 1693–1698. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhardwaj, P. Glaucoma drainage implants. Int. J. Ophthalmol. 2020, 13, 1318–1328. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sawada, A.; Mayama, C.; Araie, M.; Ohkubo, S.; Sugiyama, K.; Kuwayama, Y.; Treatment Study Group. The 5-year incidence of bleb-related infection and its risk factors after filtering surgeries with adjunctive mitomycin C: Collaborative bleb-related infection incidence and treatment study 2. Ophthalmology 2014, 121, 1001–1006. [Google Scholar] [CrossRef]
- Grigorian, A.P.; Spaeth, G. An explanation of transient visual loss associated with leaking filtering bleb. Am. J. Ophthalmol. 2004, 138, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Parija, S.; Nayak, B. Hypotony maculopathy: A surgeon’s nightmare. BMJ Case Rep. 2019, 12, e231272. [Google Scholar] [CrossRef]
- Mäepea, O.; Bill, A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp. Eye Res. 1992, 54, 879–883. [Google Scholar] [CrossRef]
- Tanihara, H.; Negi, A.; Akimoto, M.; Terauchi, H.; Okudaira, A.; Kozaki, J.; Takeuchi, A.; Nagata, M. Surgical effects of trabeculotomy ab externo on adult eyes with primary open angle glaucoma and pseudoexfoliation syndrome. Arch. Ophthalmol. 1993, 111, 1653–1661. [Google Scholar] [CrossRef]
- McPherson, S.D. Results of external trabeculotomy. Am. J. Ophthalmol. 1973, 76, 918–920. [Google Scholar] [CrossRef]
- Grover, D.S.; Smith, O.; Fellman, R.L.; Godfrey, D.G.; Gupta, A.; Montes de Oca, I.; Feuer, W.J. Gonioscopy-assisted Transluminal Trabeculotomy: An Ab Interno Circumferential Trabeculotomy: 24 Months Follow-up. J. Glaucoma 2018, 27, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Rahmatnejad, K.; Pruzan, N.L.; Amanullah, S.; Shaukat, B.A.; Resende, A.F.; Waisbourd, M.; Zhan, T.; Moster, M.R. Surgical Outcomes of Gonioscopy-assisted Transluminal Trabeculotomy (GATT) in Patients with Open-angle Glaucoma. J. Glaucoma 2017, 26, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Dannheim, R. Epicritical consideration of 300 cases of trabeculotomy ‘ab externo’. Trans. Ophthalmol. Soc. UK 1970, 89, 491–499. [Google Scholar]
- Sato, T.; Hirata, A.; Mizoguchi, T. Prospective, noncomparative, nonrandomized case study of short-term outcomes of 360° suture trabeculotomy ab interno in patients with open-angle glaucoma. Clin. Ophthalmol. 2015, 9, 63–68. [Google Scholar] [CrossRef] [PubMed]
- GRANT, W.M. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 1963, 69, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Nitta, T.; Shinmei, Y.; Aoyagi, M.; Nitta, A.; Ohno, S.; Ishida, S.; Yoshida, K. Reduction of intraocular pressure using a modified 360-degree suture trabeculotomy technique in primary and secondary open-angle glaucoma: A pilot study. J. Glaucoma 2012, 21, 401–407. [Google Scholar] [CrossRef]
- Manabe, S.I.; Sawaguchi, S.; Hayashi, K. The effect of the extent of the incision in the Schlemm canal on the surgical outcomes of suture trabeculotomy for open-angle glaucoma. JPN J. Ophthalmol. 2017, 61, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Otori, Y.; Matsuoka, T.; Kumoi, M.; Tachibana, E.; Tsujino, C.; Matsuda, S. Comparison of Surgical Outcomes Between Ab Interno Suture Trabeculotomy and Ab Externo Metal Trabeculotomy in Adult Patients with Glaucoma. Clin. Ophthalmol. 2021, 15, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Buller, C.; Johnson, D. Segmental variability of the trabecular meshwork in normal and glaucomatous eyes. Investig. Ophthalmol. Vis. Sci 1994, 35, 3841–3851. [Google Scholar]
- Vranka, J.A.; Bradley, J.M.; Yang, Y.F.; Keller, K.E.; Acott, T.S. Mapping molecular differences and extracellular matrix gene expression in segmental outflow pathways of the human ocular trabecular meshwork. PLoS ONE 2015, 10, e0122483. [Google Scholar]
- Cha, E.D.; Xu, J.; Gong, L.; Gong, H. Variations in active outflow along the trabecular outflow pathway. Exp. Eye Res. 2016, 146, 354–360. [Google Scholar] [CrossRef]
- Dvorak-Theobald, G. Further studies on the canal of Schlemm; its anastomoses and anatomic relations. Am. J. Ophthalmol. 1955, 39, 65–89. [Google Scholar] [CrossRef]
- Hann, C.R.; Bentley, M.D.; Vercnocke, A.; Ritman, E.L.; Fautsch, M.P. Imaging the aqueous humor outflow pathway in human eyes by three-dimensional micro-computed tomography (3D micro-CT). Exp. Eye Res. 2011, 92, 104–111. [Google Scholar] [CrossRef]
- Kim, D.D.; Doyle, J.W.; Smith, M.F. Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients. Ophthalmic Surg. Lasers 1999, 30, 37–40. [Google Scholar] [CrossRef]
- Tanito, M.; Ohira, A.; Chihara, E. Surgical outcome of combined trabeculotomy and cataract surgery. J. Glaucoma 2001, 10, 302–308. [Google Scholar] [CrossRef]
- Albanis, C.V.; Dwyer, M.A.; Ernest, J.T. Outcomes of extracapsular cataract extraction and phacoemulsification performed in a university training program. Ophthalmic Surg. Lasers 1998, 29, 643–648. [Google Scholar] [CrossRef]
- Bektas, C.; Aktas, Z.; Ucgul, A.Y.; Karamert, S.S. Prognostic factors affecting the surgical success of gonioscopy-assisted transluminal trabeculotomy. Indian J. Ophthalmol. 2021, 69, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Shinmei, Y.; Kijima, R.; Nitta, T.; Ishijima, K.; Ohguchi, T.; Chin, S.; Ishida, S. Modified 360-degree suture trabeculotomy combined with phacoemulsification and intraocular lens implantation for glaucoma and coexisting cataract. J. Cataract Refract. Surg. 2016, 42, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Alnahrawy, O.; Blumenstock, G.; Ziemssen, F.; Szurman, P.; Leitritz, M.A.; Dimopoulos, S.; Voykov, B. Exit strategies in canaloplasty: Intraoperative conversion into 180-degree trabeculotomy or 360-degree trabeculotomy in cases of unsuccessful catheterisation of Schlemm’s canal: Influence of degree of canal cleavage. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 779–784. [Google Scholar] [CrossRef] [PubMed]



| Total | CT | GATT | p-Value | |
|---|---|---|---|---|
| 80 Eyes | 27 Eyes | 53 Eyes | ||
| Age (mean ± SD) years | 68.6 ± 13.6 | 65.8 ± 12.8 | 70.0 ± 13.7 | 0.06 |
| Sex (male), n (%) | 37 (46.3) | 11 (40.7) | 26 (49.1) | 0.48 |
| Preoperative IOP (mean ± SD) (mmHg) | 30.7 ± 8.63 | 30.3 ± 8.38 | 30.96 ± 8.74 | 0.76 |
| Preoperative number of glaucoma medications | 4.04 ± 1.02 | 4.11 ± 0.99 | 4.00 ± 1.03 | 0.59 |
| Preoperative MD of HFA 30-2 (dB) (mean ± SD) | −7.36 ± 5.45 | −5.33 ± 3.43 | −8.34 ± 5.95 | 0.03 |
| Type of glaucoma | 0.55 | |||
| POAG, n (%) | 31 (38.75) | 10 (37.04) | 21 (39.62) | |
| Exfoliative, n (%) | 28 (35.0) | 8 (29.63) | 20 (37.74) | |
| Steroid-induced, n (%) | 14 (17.5) | 7 (25.93) | 7 (13.21) | |
| Others, n (%) | 7 (8.75) | 2 (7.40) | 5 (9.43) | |
| Lens status | ||||
| Phakic, n (%) | 53 (66.25) | 17 (63.0) | 35 (66.0) | 0.79 |
| Combined cataract extraction, n (%) | 43 (53.8) | 13 (48.1) | 30 (56.6) | 0.47 |
| Extent of incision in SC, mean ± SD (degrees) | 257.3 ± 111.3 | 117.8 ± 11.3 | 328.3 ± 60.7 | * <0.001 |
| Follow-up period (mean ± SD, range) (months) | 19.5 ± 6.5 (3–24) | 18.4 ± 7.5 (3–24) | 20.0 ± 5.8 (6–24) | 0.41 |
| Preoperative | 1 Day | 1 Week | 1 Month | 3 Months | 6 Months | 12 Months | 24 Months | |
|---|---|---|---|---|---|---|---|---|
| CT, mean (median) ± SD (mmHg) | 30.04 (27) ± 8.38 (n = 27) | 26.78 (26) ± 9.14 (n = 27) | 18.00 (18) ± 4.19 (n = 27) | 16.07 (16) ± 3.54 (n = 27) | 16.33 (17) ± 3.47 (n = 27) | 17.38 (17.5) ± 2.97 (n = 24) | 18.15 (18) ± 4.33 (n = 20) | 17.75 (18) ± 3.42 (n = 16) |
| GATT, mean (median) ± SD (mmHg) | 30.96 (28) ± 8.73 (n = 53) | 18.26 (16) ± 9.01 (n = 53) | 17.51 (16) ± 7.94 (n = 53) | 14.85 (14) ± 6.30 (n = 52) | 14.76 (14) ± 5.27 (n = 50) | 14.31 (14) ± 3.65 (n = 49) | 14.93 (14) ± 6.57 (n = 45) | 15.37 (16) ± 3.54 (n = 35) |
| p-Value | NA | * <0.001 | 0.717 | 0.376 | 0.179 | * <0.001 | * 0.024 | * 0.020 |
| CT with phacoemulsification, mean (median) ± SD (mmHg) | 27.69 (26) ± 5.03 (n = 13) | 28.00 (26) ± 8.38 (n = 13) | 18.31 (16) ± 3.93 (n = 13) | 16.31 (16) ± 2.52 (n = 13) | 16.08 (17) ± 1.90 (n = 13) | 17.17 (17.5) ± 2.82 (n = 12) | 17.25 (16.5) ± 3.27 (n = 8) | 19.14 (20) ± 2.42 (n = 7) |
| GATT with phacoemulsification, mean (median) ± SD (mmHg) | 28.60 (26.5) ± 7.14 (n = 30) | 19.33 (16) ± 9.28 (n = 30) | 17.30 (15.5) ± 7.40 (n = 30) | 13.67 (14) ± 3.06 (n = 30) | 13.30 (13) ± 2.97 (n = 30) | 13.80 (13) ± 3.39 (n = 30) | 13.25 (13.5) ± 2.06 (n = 28) | 15.05 (16) ± 3.39 (n = 21) |
| p-Value | NA | * 0.010 | 0.615 | * 0.013 | * 0.004 | * 0.008 | * <0.001 | * 0.004 |
| CT without phacoemulsification, mean (median) ± SD (mmHg) | 32.21 (30) ± 10.11 (n = 14) | 25.64 (26) ± 9.66 (n = 14) | 17.71 (18) ± 4.40 (n = 14) | 15.86 (16.5) ± 4.26 (n = 14) | 16.57 (17) ± 4.45 (n = 14) | 17.58 (17.5) ± 3.09 (n = 12) | 18.75 (19) ± 5.37 (n = 12) | 16.67 (16) ± 3.68 (n = 9) |
| GATT without phacoemulsification, mean (median) ± SD (mmHg) | 34.04 (30) ± 9.62 (n = 23) | 16.87 (14) ± 8.46 (n = 23) | 17.78 (16) ± 8.58 (n = 23) | 16.45 (13.5) ± 8.75 (n = 22) | 16.95 (16) ± 6.94 (n = 20) | 15.11 (14) ± 3.91 (n = 19) | 17.71 (16) ± 9.74 (n = 17) | 15.86 (16) ± 3.70 (n = 14) |
| p-Value | NA | * 0.011 | 0.932 | 0.792 | 0.831 | 0.089 | 0.535 | 0.584 |
| Preoperative | 1 Week | 1 Month | 3 Months | 6 Months | 12 Months | 24 Months | |
|---|---|---|---|---|---|---|---|
| CT, mean (median) ± SD | 4.11 (4) ± 0.99 (n = 27) | 1.93 (2) ± 1.27 (n = 27) | 2.11 (2) ± 1.37 (n = 27) | 2.37 (2) ± 1.22 (n = 27) | 2.50 (2) ± 1.32 (n = 24) | 2.80 (3) ± 1.57 (n = 20) | 2.88 (3) ± 1.54 (n = 16) |
| GATT, mean (median) ± SD | 4.00 (4) ± 1.03 (n = 53) | 1.42 (1) ± 1.51 (n = 53) | 1.68 (2) ± 1.55 (n = 53) | 1.72 (2) ± 1.58 (n = 50) | 1.82 (2) ± 1.61 (n = 49) | 1.87 (2) ± 1.61 (n = 45) | 1.97 (2) ± 1.73 (n = 35) |
| p-Value | NA | 0.139 | 0.222 | 0.070 | 0.081 | * 0.018 | 0.095 |
| CT with phacoemulsification, mean (median) ± SD | 3.69 (4) ± 1.07 (n = 13) | 1.85 (2) ± 1.35 (n = 13) | 2.23 (2) ± 1.19 (n = 13) | 2.31 (2) ± 1.26 (n = 13) | 2.42 (2) ± 1.38 (n = 12) | 2.63 (3.5) ± 1.65 (n = 8) | 3.14 (4) ± 1.55 (n = 7) |
| GATT with phacoemulsification, mean (median) ± SD | 4.03 (4) ± 0.75 (n = 30) | 1.60 (1.5) ± 1.52 (n = 30) | 1.57 (1) ± 1.56 (n = 30) | 1.57 (1) ± 1.50 (n = 30) | 1.73 (1.5) ± 1.63 (n = 30) | 1.79 (1.5) ± 1.61 (n = 28) | 2.00 (2) ± 1.80 (n = 21) |
| p-Value | NA | 0.766 | 0.225 | 0.164 | 0.249 | 0.227 | 0.206 |
| CT without phacoemulsification, mean (median) ± SD | 4.50 (4.5) ± 0.73 (n = 14) | 2.00 (2) ± 1.20 (n = 14) | 2.00 (2) ± 1.51 (n = 14) | 2.43 (2) ± 1.18 (n = 14) | 2.58 (2) ± 1.26 (n = 12) | 2.92 (3) ± 1.50 (n = 12) | 2.67 (2) ± 1.49 (n = 9) |
| GATT without phacoemulsification, mean (median) ± SD | 3.96 (4) ± 1.30 (n = 23) | 1.17 (0) ± 1.46 (n = 23) | 1.77 (2) ± 1.54 (n = 22) | 1.91 (2) ± 1.65 (n = 20) | 1.95 (2) ± 1.57 (n = 19) | 2.00 (2) ± 1.61 (n = 17) | 1.93 (2) ± 1.62 (n = 14) |
| p-Value | NA | 0.165 | 0.688 | 0.356 | 0.202 | 0.09 | 0.306 |
| Time Point | Total | With Phacoemulsification | Without Phacoemulsification | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CT (%) | GATT (%) | p-Value | CT (%) | GATT (%) | p-Value | CT (%) | GATT (%) | p-Value | |
| 1 month | 22.20 (6/27) | 37.70 (20/53) | 0.161 | 15.38 (2/13) | 40.00 (12/30) | 0.108 | 28.57 (4/14) | 34.78 (8/23) | 0.493 |
| 3 months | 11.10 (3/27) | 36.00 (18/50) | 0.016 | 15.38 (2/13) | 36.67 (11/30) | 0.151 | 7.14 (1/14) | 35.00 (7/20) | 0.067 |
| 6 months | 12.50 (3/24) | 34.70 (17/49) | 0.039 | 16.67 (2/12) | 36.67 (11/30) | 0.187 | 8.33 (1/12) | 31.58 (6/19) | 0.143 |
| 12 months | 15.00 (3/20) | 31.11 (14/45) | 0.144 | 25.00 (2/8) | 32.14 (9/28) | 0.532 | 8.33 (1/12) | 29.41 (5/17) | 0.182 |
| 24 months | 12.50 (2/16) | 29.41 (10/34) | 0.171 | 14.29 (1/7) | 30.00 (6/20) | 0.393 | 11.11 (1/9) | 28.57 (4/14) | 0.327 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| GATT (vs. CT) | 4.81 (1.66–13.99) | * 0.004 | 4.30 (1.37–13.43) | * 0.012 |
| Combined phacoemulsification | 4.43 (1.55–12.64) | * 0.005 | 5.39 (1.62–17.88) | * 0.006 |
| Occurrence of IOP spike | 0.44 (0.13–1.55) | 0.202 | 3.11 (0.76–12.78) | 0.115 |
| Age | 1.02 (0.98–1.07) | 0.297 | ||
| Preoperative IOP | 0.99 (0.93–1.04) | 0.669 | ||
| Preoperative glaucoma medications | 1.20 (0.73–1.98) | 0.466 | ||
| Occurrence of hyphema | 0.89 (0.33–2.34) | 0.810 | ||
| POAG | 1.93 (0.68–5.46) | 0.216 | ||
| PEG | 0.66 (0.24–1.83) | 0.422 | ||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| GATT (vs. CT) | 4.95 (1.59–15.38) | * 0.006 | 4.25 (1.26–14.38) | * 0.020 |
| Combined phacoemulsification | 3.70 (1.29–11.47) | * 0.014 | 4.44 (1.30–15.20) | * 0.018 |
| Occurrence of IOP spike | 0.26 (0.06–1.17) | 0.080 | 3.44 (0.71–16.64) | 0.124 |
| Age | 1.02 (0.98–1.06) | 0.292 | ||
| Preoperative IOP | 0.98 (0.93–1.04) | 0.570 | ||
| Preoperative glaucoma medications | 1.05 (0.63–1.73) | 0.858 | ||
| Occurrence of hyphema | 0.75 (0.27–2.04) | 0.569 | ||
| POAG | 2.20 (0.77–6.78) | 0.142 | ||
| PEG | 0.59 (0.20–1.70) | 0.329 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, M.; Ishikawa, H.; Ikeda, T.; Gomi, F. Conventional Trabeculotomy versus Gonioscopy-Assisted Transluminal Trabeculotomy: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 46. https://doi.org/10.3390/jcm11010046
Takata M, Ishikawa H, Ikeda T, Gomi F. Conventional Trabeculotomy versus Gonioscopy-Assisted Transluminal Trabeculotomy: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(1):46. https://doi.org/10.3390/jcm11010046
Chicago/Turabian StyleTakata, Masashi, Hiroto Ishikawa, Tomohiro Ikeda, and Fumi Gomi. 2022. "Conventional Trabeculotomy versus Gonioscopy-Assisted Transluminal Trabeculotomy: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 1: 46. https://doi.org/10.3390/jcm11010046
APA StyleTakata, M., Ishikawa, H., Ikeda, T., & Gomi, F. (2022). Conventional Trabeculotomy versus Gonioscopy-Assisted Transluminal Trabeculotomy: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(1), 46. https://doi.org/10.3390/jcm11010046







