Comparison of Acquired Activated Protein C Resistance, Using the CAT and ST-Genesia® Analysers and Three Thrombin Generation Methods, in APS and SLE Patients
Abstract
:1. Introduction
2. Methods
2.1. Patients and Samples
2.2. Statistical Analysis
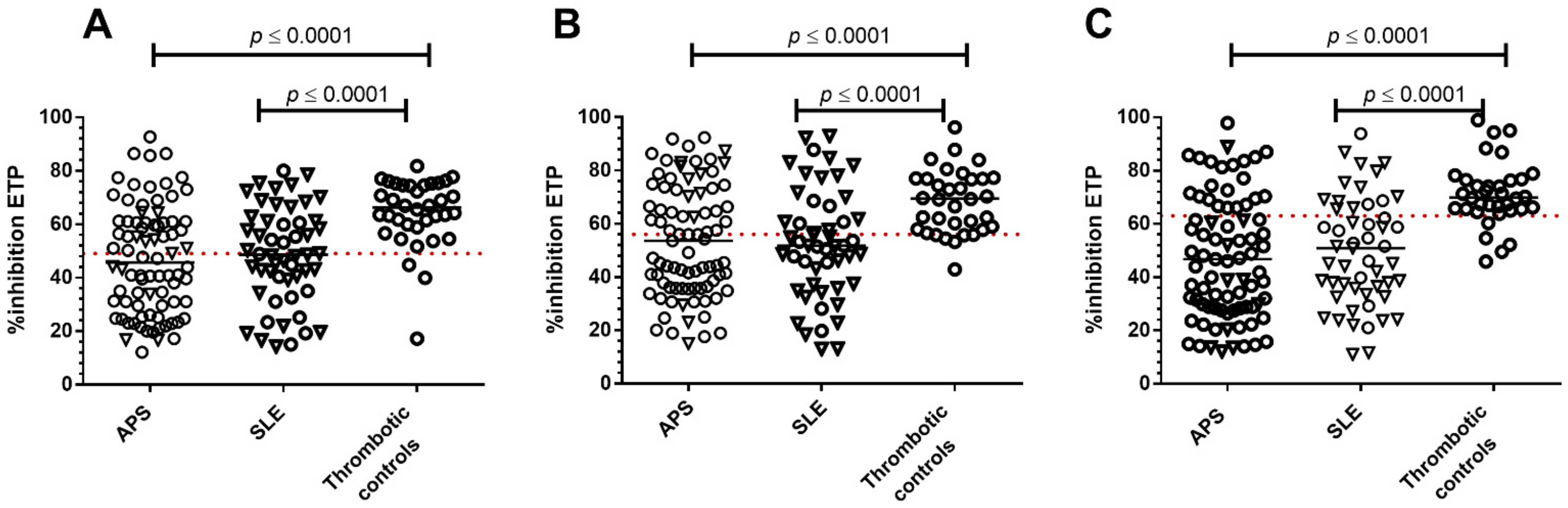
3. Results
3.1. Patients
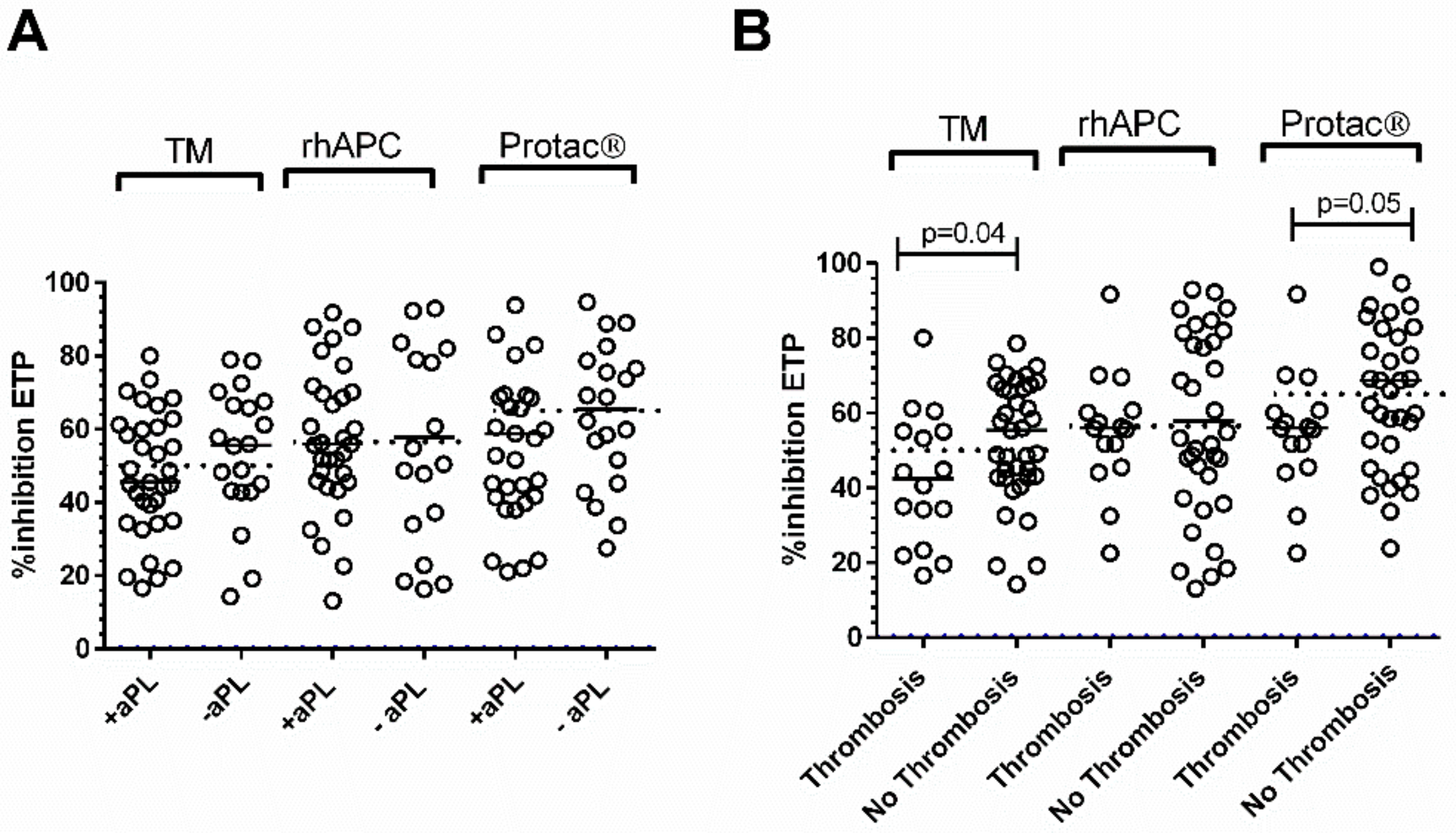
3.2. Patients with APS
3.3. Patients with SLE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouwens, E.A.; Stavenuiter, F.; Mosnier, L.O. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, K.; Kohli, S.; Al-Dabet, M.M.; Isermann, B. Cell biology of activated protein C. Curr. Opin. Hematol. 2019, 26, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bertina, R.M.; Koeleman, B.P.; Koster, T.; Rosendaal, F.R.; Dirven, R.; De Ronde, H.; Van Der Velden, P.A.; Reitsma, P.H. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994, 369, 64–67. [Google Scholar] [CrossRef]
- Dahlback, B.; Carlsson, M.; Svensson, P.J. Familial Thrombophilia Due to a Previously Unrecognized Mechanism Characterized by Poor Anticoagulant Response to Activated Protein-C—Prediction of a Cofactor to Activated Protein-C. Proc. Natl. Acad. Sci. USA 1993, 90, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.H.; Evatt, B.; Zimmerman, T.S.; Kleiss, A.J.; Wideman, C. Deficiency of protein C in congenital thrombotic disease. J. Clin. Investig. 1981, 68, 1370–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, D.A.; Mannucci, P.M.; Bauer, K.A.; Bertina, R.M.; Bochkov, N.P.; Boulyjnkov, V.; Chandy, M.; Dahlback, B.; Ginter, E.K.; Miletich, J.P.; et al. Inherited thrombophilia: Part 1. Thromb. Haemost. 1996, 76, 651–662. [Google Scholar]
- Lane, D.A.; Mannucci, P.M.; Bauer, K.A.; Bertina, R.M.; Bochkov, N.P.; Boulyjenkov, V.; Chandy, M.; Dahlbäck, B.; Ginter, E.K.; Miletich, J.P.; et al. Inherited thrombophilia: Part 2. Thromb. Haemost. 1996, 76, 824–834. [Google Scholar]
- Liestol, S.; Sandset, P.M.; Jacobsen, E.M.; Mowinckel, M.C.; Wisloff, F. Decreased anticoagulant response to tissue factor pathway inhibitor type 1 in plasmas from patients with lupus anticoagulants. Br. J. Haematol. 2007, 136, 131–137. [Google Scholar] [CrossRef]
- Arachchillage, D.R.; Efthymiou, M.; Mackie, I.J.; Lawrie, A.S.; Machin, S.J.; Cohen, H. Anti-protein C antibodies are associated with resistance to endogenous protein C activation and a severe thrombotic phenotype in antiphospholipid syndrome. J. Thromb. Haemost. 2014, 12, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Mackie, I.; Nallamilli, S.; Pires, T.; Moll, R.; Pericleous, C.; Isenberg, D.A.; Cohen, H.; Efthymiou, M. Anti-protein C antibodies and acquired protein C resistance in SLE: Novel markers for thromboembolic events and disease activity? Rheumatology 2021, 60, 1376–1386. [Google Scholar] [CrossRef]
- Dargaud, Y.; Luddington, R.; Gray, E.; Negrier, C.; Lecompte, T.; Petros, S.; Hogwood, J.; Bordet, J.-C.; Regnault, V.; Siegemund, A.; et al. Effect of standardization and normalization on imprecision of calibrated automated thrombography: An international multicentre study. Br. J. Haematol. 2007, 139, 303–309. [Google Scholar] [CrossRef]
- Dargaud, Y.; Luddington, R.; Gray, E.; Lecompte, T.; Siegemund, T.; Baglin, T.; Hogwood, J.; Regnault, V.; Negrier, C. Standardisation of thrombin generation test—Which reference plasma for TGT? An international multicentre study. Thromb. Res. 2010, 125, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, R.; Kleinegris, M.C.; Loubele, S.T.; Pluijmen, P.H.M.; Fens, D.; Van Oerle, R.; Cate, H.T.; Spronk, H.M.H. Preanalytic variables of thrombin generation: Towards a standard procedure and validation of the method. J. Thromb. Haemost. 2012, 10, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.E.; Wong, A.; Gopal, R.D.; Dale, B.J.; Duncan, E.M.; McRae, S.J. Evaluation of pre-analytical variables in a commercial thrombin generation assay. Thromb. Res. 2014, 134, 160–164. [Google Scholar] [CrossRef]
- Dargaud, Y.; Wolberg, A.S.; Gray, E.; Negrier, C.; Hemker, H.C. Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2017, 15, 1704–1707. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.; Dielis, A.W.J.H.; De Smedt, E.; van Oerle, R.; Fens, D.; Prins, M.H.; Hamulyák, K.; ten Cate, H. Assessment of thrombin generation II: Validation of the Calibrated Automated Thrombogram in platelet-poor plasma in a clinical laboratory. Thromb. Haemost. 2008, 100, 362–364. [Google Scholar] [PubMed]
- Douxfils, J.; Morimont, L.; Delvigne, A.S.; Devel, P.; Masereel, B.; Haguet, H.; Bouvy, C.; Dogné, J.-M. Validation and standardization of the ETP-based activated protein C resistance test for the clinical investigation of steroid contraceptives in women: An unmet clinical and regulatory need. Clin. Chem. Lab. Med. 2020, 58, 294–305. [Google Scholar] [CrossRef]
- Calzavarini, S.; Brodard, J.; Quarroz, C.; Maire, L.; Nützi, R.; Jankovic, J.; Rotondo, L.C.; Giabbani, E.; Fiedler, G.M.; Nagler, M.; et al. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: Normal values and variability. Res. Pract. Thromb. Haemost. 2019, 18, 758–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douxfils, J.; Morimont, L.; Bouvy, C.; De Saint-Hubert, M.; Devalet, B.; Devroye, C.; Dincq, A.; Dogné, J.; Guldenpfennig, M.; Baudar, J.; et al. Assessment of the analytical performances and sample stability on ST Genesia system using the STG-DrugScreen application. J. Thromb. Haemost. 2019, 17, 1273–1287. [Google Scholar] [CrossRef]
- Roullet, S.; Labrouche, S.; Freyburger, G. Comparison of Two Thrombin Generation Methods, CAT and ST-Genesia, in Liver Transplant Patients. Thromb. Haemost. 2019, 119, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Siguret, V.; Abdoul, J.; Delavenne, X.; Curis, E.; Carlo, A.; Blanchard, A.; Salem, J.-E.; Gaussem, P.; Funck-Brentano, C.; Azizi, M.; et al. Rivaroxaban pharmacodynamics in healthy volunteers evaluated with thrombin generation and the active protein C system: Modeling and assessing interindividual variability. J. Thromb. Haemost. 2019, 17, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Isenberg, D.A.; Rahman, A.; Allen, E.; Farewell, V.; Akil, M.; Bruce, I.N.; D’Cruz, D.D.; Griffiths, B.; Khamashta, M.; Maddinson, P.; et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005, 44, 902–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Yee, C.S.; Cresswell, L.; Farewell, V.; Rahman, A.; Teh, L.-S.; Griffiths, B.; Bruce, I.N.; Ahmad, Y.; Prabu, A.; Akil, M.; et al. Numerical scoring for the BILAG-2004 index. Rheumatology 2010, 49, 1665–1669. [Google Scholar] [CrossRef] [Green Version]
- Pengo, V.; Tripodi, A.; Reber, G.; Rand, J.H.; Ortel, T.L.; Galli, M.; De Groot, P.G. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2009, 7, 1737–1740. [Google Scholar] [CrossRef]
- Keeling, D.; Mackie, I.; Moore, G.W.; Greer, I.A.; Greaves, M.; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br. J. Haematol. 2012, 157, 47–58. [Google Scholar] [CrossRef]
- Efthymiou, M.; Lawrie, A.S.; Mackie, I.; Arachchillage, D.; Lane, P.J.; Machin, S.; Cohen, H. Thrombin generation and factor X assays for the assessment of warfarin anticoagulation in thrombotic antiphospholipid syndrome. Thromb. Res. 2015, 135, 1191–1197. [Google Scholar] [CrossRef]
- Liestol, S.; Sandset, P.M.; Mowinckel, M.C.; Wisloff, F. Activated protein C resistance determined with a thrombin generation-based test is associated with thrombotic events in patients with lupus anticoagulants. J. Thromb. Haemost. 2007, 5, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Lebreton, A.; Sinegre, T.; Pereira, B.; Lamblin, G.; Duron, C.; Abergel, A. Plasma hypercoagulability in the presence of thrombomodulin but not of activated protein C in patients with cirrhosis. J. Gastroenterol. Hepatol. 2017, 32, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuily, S.; Regnault, V.; Guillemin, F.; Kaminsky, P.; Rat, A.-C.; Lecompte, T.; Wahl, D. Superficial vein thrombosis, thrombin generation and activated protein C resistance as predictors of thromboembolic events in lupus and antiphospholipid patients. A prospective cohort study. Thromb. Res. 2013, 132, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Meesters, E.W.; Hansen, H.; Spronk, H.M.; Hamulyak, K.; Rosing, J.; Rowshani, A.; ten Berge, I.J.M.; ten Cate, H. The inflammation and coagulation cross-talk in patients with systemic lupus erythematosus. Blood Coagul Fibrinolysis 2007, 18, 21–28. [Google Scholar] [CrossRef]
- Nojima, J.; Kuratsune, H.; Suehisa, E.; Iwatani, Y.; Kanakura, Y. Acquired activated protein C resistance associated with IgG antibodies against beta2-glycoprotein I and prothrombin as a strong risk factor for venous thromboembolism. Clin. Chem. 2005, 51, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Rodríguez, F.J.; Reverter, J.C.; Font, J.; Tassies, D.; Espinosa, G.; Cervera, R.; Carmona, F.; Balasch, J.; Ingelmo, M.; Ordinas, A. Clinical significance of acquired activated protein C resistance in patients with systemic lupus erythematosus. Lupus 2002, 11, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Perrin, J.; Depasse, F.; Lecompte, T.; De Raucourt, E.; Planche, V.; Ajzenberg, N.; Ollivier, V.; Helley, D.; Dignat-Georges, F.; Morange, P.; et al. Large external quality assessment survey on thrombin generation with CAT: Further evidence for the usefulness of normalisation with an external reference plasma. Thromb. Res. 2015, 136, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tans, G.; van Hylckama Vlieg, A.; Thomassen, M.C.; Curvers, J.; Bertina, R.M.; Rosing, J.; Rosendaal, F.R. Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br. J. Haematol. 2003, 122, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Dargaud, Y.; Wolberg, A.S.; Luddington, R.; Regnault, V.; Spronk, H.; Baglin, T.; Lecompte, T.; Cate, H.T.; Negrier, C. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: An international multicentre study. Thromb. Res. 2012, 130, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Morimont, L.; Bouvy, C.; Delvigne, A.S.; Dogné, J.M.; Douxfils, J. Proof of concept of a new scale for the harmonization and the standardization of the ETP-based APC resistance. J. Thromb. Haemost. 2020, 18, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ninivaggi, M.; de Laat-Kremers, R.; Tripodi, A.; Wahl, D.; Zuily, S.; Dargaud, Y.; ten Cate, H.; Ignjatovic, V.; Devreese, K.M.J.; de Laat, B. Recommendations for the measurement of thrombin generation: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2021, 19, 1372–1378. [Google Scholar] [CrossRef]
- Siguret, V.; Foulon, G.; Abdoul, J. Thrombin generation analysis with a new automated system (ST-Genesia): Inter-series performances during DRIVING study and comparison with CAT system PB584. Res. Pract. Thromb. Haemost. 2018, 2, 1–368. [Google Scholar] [CrossRef]
- Billoir, P.; Miranda, S.; Levesque, H.; Benhamou, Y.; Le Cam Duchez, V. Hypercoagulability Evaluation in Antiphospholipid Syndrome without Anticoagulation Treatment with Thrombin Generation Assay: A Preliminary Study. J. Clin. Med. 2021, 10, 2728. [Google Scholar] [CrossRef] [PubMed]
- Arus, M.; Vilalta, N.; Romero, L. Comparison between the standard thrombin generation method and the automated thrombin generation techinque. In Proceedings of the ECTH 2018, Marseille, France, 24–26 October 2018; p. 238. [Google Scholar]
- Talon, L.; Sinegre, T.; Lecompte, T.; Pereira, B.; Massoulié, S.; Abergel, A.; Lebreton, A. Hypercoagulability (thrombin generation) in patients with cirrhosis is detected with ST-Genesia. J. Thromb. Haemost. 2020, 18, 2177–2190. [Google Scholar] [CrossRef]
- Devreese, K.; Peerlinck, K.; Arnout, J.; Hoylaerts, M.F. Laboratory detection of the antiphospholipid syndrome via calibrated automated thrombography. Thromb. Haemost. 2009, 101, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| APCr Prevalence n (%) (Median, 95% Confidence Intervals) | Agreemen | ||||||
|---|---|---|---|---|---|---|---|
| Patients | ST-Genesia® ™ | CAT (rhAPC) | CAT (Protac®) | n(%) ST-Genesia®/CAT (rhAPC) | K-Coefficient | n(%) ST-Genesia®/CAT (Protac®) | K-Coefficient |
| APS (n = 106) | 57(53.8%) (45.7%, 39.6–55.5%) | 61 (57.5%) (53.6%, 48.9–58.0%) | 67 (63.2%) (46.8%, 43.3–53.9%) | 35 (56.8%) | 0.16 Poor | 44 (65.9%) | 0.20 Poor |
| SLE (n = 53) | 27 (50%) (49.1%, 45.2–58.5%) | 32 (59.3%) (51.8%, 48.2–60.6%) | 38 (70.4%) (50.9%, 44.6–56.6%) | 21 (68.5%) | 0.37 Fair | 21 (64.8%) | 0.29 Fair |
| Thrombotic controls (n = 36) | 3 (8.3%) (66.3%, 61.7–72.3%) | 6 (16.7%) (69.5%, 65.4–74.7%) | 5 (13.9%) (69.9%, 67.5–76.6%) | 0 | −0.12 No | 0 | −0.12 No |
| APCr Prevalence n (%) (Median, 95% Confidence Intervals) | Agreement | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients | ST-Genesia® (TM) | CAT (rhAPC) | CAT (Protac®) | n (%) ST-Genesia®/CAT (rhAPC) | K-Coefficient | n (%) ST-Genesia®/CAT (Protac®) | K-Coefficient | |
| APS | Thrombotic APS (n = 83) | 42 (50.6%) (45.7%, 39.6–55.5%) | 48 (57.8%) (54.3%, 42.057.5%) | 49 (59.0%) (48.8%, 36.8–59.0%) | 34 (59.0%) | 0.22 Fair | 33 (45.2%) | 0.13 Poor |
| Pregnancy Morbidity APS (n = 23) | 15 (65.2%) (36.9%, 21.9–49.0%) | 13 (56.5%) (46.3%, 36.177.0%) | 18 (78.3%) (41.5%, 21.2–55.9%) | 11 (48.2%) | 0.17 Poor | 14 (55.6%) | 0.16 Poor | |
| Triple aPL positive (n = 15) | 10 (66.6%) (37.1%, 24.7–60.5%) | 12 (80.0%) (52.3%, 40.8–61.0%) | 14 (93.3%) (38.4%, 23.0–52.1%) | 9 (80.0%) | 0.53 Moderate | 11 (80.0%) | 0.41 Moderate | |
| Double aPL positive (n = 36) | 16 (44.4%) (52.3%, 43.2–59.4%) | 21 (58.3%) (51.0%, 37–65.4%) | 21 (58.3%) (45.7%, 35.6–66.2%) | 12 (64.0%) | 0.22 Fair | 15 (70.0%) | 0.37 Fair | |
| Single aPL positive (n = 32) | 16 (50.0%) (52.1%, 40.7–60.6%) | 18 (56.2%) (49.0%, 34.6–68.6%) | 22 (68.8%) (47.5%, 32.7–70.3%) | 9 (48.5%) | 0.13 Poor | 12 (47.2%) | 0.19 Poor | |
| SLE | aPL positive SLE (n = 34) | 17 (50%) (45.6%, 40.4–58.5%) | 18 (52.9%) (56.1%, 48.669.9%) | 18 (52.9%) (58.8%, 44.7–68.7%) | 11 (47.5%) | 0.09 Poor | 12 (54.5%) | 0.08 Poor |
| aPL negative SLE (n = 20) | 9 (45.0%) (56.0%, 43.2–66.5%) | 10 (50.0%) (57.8%, 34.8–90.1%) | 9 (45.0%) (65.5%, 51.6–76.5%) | 3 (35.0%) | 0.20 Poor | 3 (35.0%) | 0.20 Poor | |
| Thrombotic SLE (n = 16) | 10 (62.5%) (42.7%, 23.4–55.1%) | 9 (56.2%) (56.1%, 45.6–69.7%) | 10 (62.5%) (56.1%, 45.6–69.7%) | 8 (64.8%) | 0.27 Fair | 8 (68.8%) | 0.31 Fair | |
| Non thrombotic SLE (n = 37) | 17 (45.9%) (57.8.4%, 48.0–79.1% | 19 (51.4%) (57.8%, 47.9–79.1%) | 17 (45.9%) (68.7%, 58.4–76.5%) | 8 (47.4%) | 0.06 Poor | 8 (47.4%) | 0.06 Poor | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efthymiou, M.; Lane, P.J.; Isenberg, D.; Cohen, H.; Mackie, I.J. Comparison of Acquired Activated Protein C Resistance, Using the CAT and ST-Genesia® Analysers and Three Thrombin Generation Methods, in APS and SLE Patients. J. Clin. Med. 2022, 11, 69. https://doi.org/10.3390/jcm11010069
Efthymiou M, Lane PJ, Isenberg D, Cohen H, Mackie IJ. Comparison of Acquired Activated Protein C Resistance, Using the CAT and ST-Genesia® Analysers and Three Thrombin Generation Methods, in APS and SLE Patients. Journal of Clinical Medicine. 2022; 11(1):69. https://doi.org/10.3390/jcm11010069
Chicago/Turabian StyleEfthymiou, Maria, Philip J. Lane, David Isenberg, Hannah Cohen, and Ian J. Mackie. 2022. "Comparison of Acquired Activated Protein C Resistance, Using the CAT and ST-Genesia® Analysers and Three Thrombin Generation Methods, in APS and SLE Patients" Journal of Clinical Medicine 11, no. 1: 69. https://doi.org/10.3390/jcm11010069






