Gastric Xanthelasma, Microsatellite Instability and Methylation of Tumor Suppressor Genes in the Gastric Mucosa: Correlation and Comparison as a Predictive Marker for the Development of Synchronous/Metachronous Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analyses of MSI and Gene Methylation
2.3. Statistical Analysis
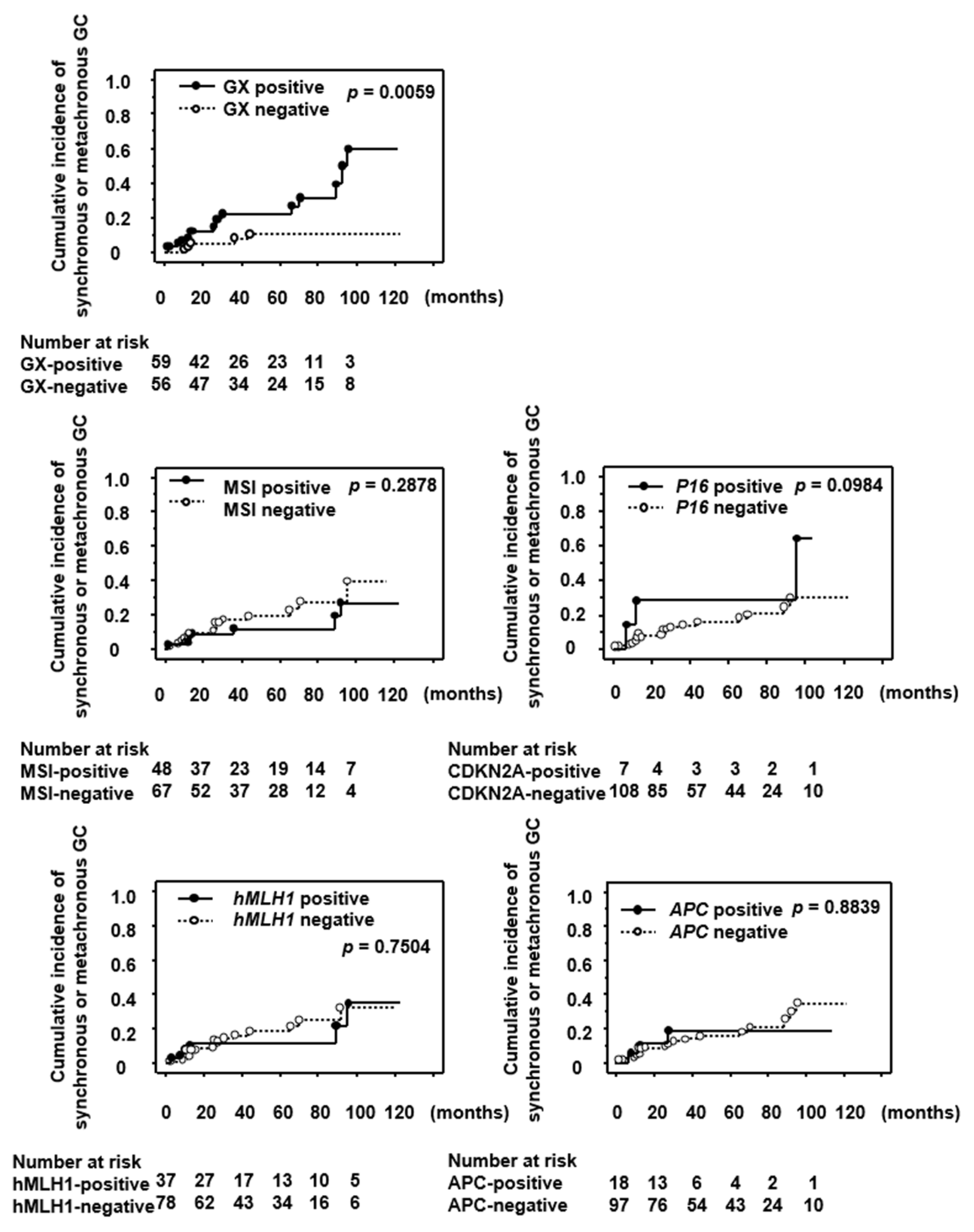
3. Results
3.1. Relationship of GX to Clinical/Endoscopic Features in Patients with Early GC Treated by ESD
3.2. Relationship of MSI or Methylation of hMLH1, CDKN2A or APC to Clinical/Endoscopic Features in Patients with Early GC Treated by ESD
3.3. Relationship between GX and MSI or Methylation of hMLH1, CDKN2A or APC in Patients with Early GC Treated by ESD
3.4. Significance of GX, MSI and Methylation of Tumor Suppressor Genes as a Predictive Marker for the Development of Synchronous/Metachronous GC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Ohnita, K.; Isomoto, H.; Shikuwa, S.; Yajima, H.; Minami, H.; Matsushima, K.; Akazawa, Y.; Yamaguchi, N.; Fukuda, E.; Nishiyama, H.; et al. Early and long-term outcomes of endoscopic submucosal dissection for early gastric cancer in a large patient series. Exp. Med. 2014, 7, 594–598. [Google Scholar] [CrossRef][Green Version]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Mori, G.; Nonaka, S.; Yoshinaga, S.; Saito, Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016, 19, 198–205. [Google Scholar] [CrossRef]
- Abe, S.; Oda, I.; Suzuki, H.; Nonaka, S.; Yoshinaga, S.; Nakajima, T.; Sekiguchi, M.; Mori, G.; Taniguchi, H.; Sekine, S.; et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015, 47, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; Nakajima, T.; Asada, K.; Shimazu, T.; Yamamichi, N.; Maekita, T.; Yokoi, C.; Fujishiro, M.; Gotoda, T.; Ichinose, M.; et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: Results of a large-scale, multicenter cohort study in Japan. Gastric Cancer 2016, 19, 911–918. [Google Scholar] [CrossRef]
- Watari, J.; Chen, N.; Amenta, P.S.; Fukui, H.; Oshima, T.; Tomita, T.; Miwa, H.; Lim, K.J.; Das, K.M. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014, 20, 5461–5473. [Google Scholar] [CrossRef]
- Kawanaka, M.; Watari, J.; Kamiya, N.; Yamasaki, T.; Kondo, T.; Toyoshima, F.; Ikehara, H.; Tomita, T.; Oshima, T.; Fukui, H.; et al. Effects of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic treatment: Analysis of molecular alterations by a randomised controlled trial. Br. J. Cancer 2016, 114, 21–29. [Google Scholar] [CrossRef]
- Maekita, T.; Nakazawa, K.; Mihara, M.; Nakajima, T.; Yanaoka, K.; Iguchi, M.; Arii, K.; Kaneda, A.; Tsukamoto, T.; Tatematsu, M.; et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin. Cancer Res. 2006, 12, 989–995. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Watanabe, M.; Ezaki, T.; Kanai, T.; Ishii, H.; Mukai, M.; Hibi, T. Clinical usefulness of microsatellite instability for the prediction of gastric adenoma or adenocarcinoma in patients with chronic gastritis. Br. J. Cancer 2000, 82, 1814–1818. [Google Scholar] [CrossRef]
- Nanjo, S.; Asada, K.; Yamashita, S.; Nakajima, T.; Nakazawa, K.; Maekita, T.; Ichinose, M.; Sugiyama, T.; Ushijima, T. Identification of gastric cancer risk markers that are informative in individuals with past H. pylori. Infect. Gastr. Cancer 2012, 15, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Yoshida, T.; Enomoto, S.; Asada, K.; Tatematsu, M.; Ichinose, M.; Sugiyama, T.; Ushijima, T. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: Its possible involvement in the formation of epigenetic field defect. Int. J. Cancer 2009, 124, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T.; Hattori, N. Molecular pathways: Involvement of Helicobacter pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin. Cancer Res. 2012, 18, 923–929. [Google Scholar] [CrossRef]
- Michigami, Y.; Watari, J.; Ito, C.; Nakai, K.; Yamasaki, T.; Kondo, T.; Kono, T.; Tozawa, K.; Tomita, T.; Oshima, T.; et al. Long-term effects of H. pylori eradication on epigenetic alterations related to gastric carcinogenesis. Sci. Rep. 2018, 8, 14369. [Google Scholar] [CrossRef]
- Kaiserling, E.; Heine, H.; Itabe, H.; Takano, T.; Remmele, W. Lipid islands in human gastric mucosa: Morphological and immunohistochemical findings. Gastroenterology 1996, 110, 369–374. [Google Scholar] [CrossRef]
- Sekikawa, A.; Fukui, H.; Maruo, T.; Tsumura, T.; Kanesaka, T.; Okabe, Y.; Osaki, Y. Gastric xanthelasma may be a warning sign for the presence of early gastric cancer. J. Gastroenterol. Hepatol. 2014, 29, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Sada, R.; Fukuhara, M.; Marui, S.; Tanke, G.; Endo, M.; Ohara, Y.; Matsuda, F.; Nakajima, J.; et al. Gastric atrophy and xanthelasma are markers for predicting the development of early gastric cancer. J. Gastroenterol. 2016, 51, 35–42. [Google Scholar] [CrossRef]
- Kimura, K.; Takemoto, T. An endoscopic recognization of the atrophic border and its significance in chronic gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Kitahara, F.; Kobayashi, K.; Sato, T.; Kojima, Y.; Araki, T.; Fujino, M.A. Accuracy of screening for gastric cancer using serum pepsinogen concentration. Gut 1999, 44, 693–697. [Google Scholar] [CrossRef]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Balic, M.; Pichler, M.; Strutz, J.; Heitzer, E.; Ausch, C.; Samonigg, H.; Cote, R.J.; Dandachi, N. High quality assessment of DNA methylation in archival tissues from colorectal cancer patients using quantitative high-resolution melting analysis. J. Mol. Diagn. 2009, 11, 102–108. [Google Scholar] [CrossRef]
- Shibukawa, N.; Ouchi, S.; Wakamatsu, S.; Wakahara, Y.; Kaneko, A. Gastric xanthoma is a predictive marker for metachronous and synchronous gastric cancer. World J. Gastrointest. Oncol. 2017, 9, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Tsutsumi, Y. Helicobacter pylori infection in gastric xanthomas: Lmmunohistochemical analysis of 145 lesions. Pathol. Int. 1996, 46, 589–593. [Google Scholar] [CrossRef]
- Farinati, F.; Cardin, R.; Dagan, P.; Rugge, M.; Mario, F.D.; Bonvicini, P.; Naccarato, R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut 1998, 42, 351–356. [Google Scholar] [CrossRef]
- Kountouras, J.; Chatzopoulos, D.; Zavos, C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology 2001, 48, 743–751. [Google Scholar] [PubMed]
- Mizoshita, T.; Tsukamoto, T.; Cao, X.; Otsuka, T.; Ito, S.; Takahashi, E.; Nakamura, S.; Nakamura, T.; Yamamura, Y.; Tatematsu, M. Microsatellite instability is linked to loss of hMLH1 expression in advanced gastric cancers: Lack of a relationship with the histological type and phenotype. Gastric Cancer 2005, 8, 164–172. [Google Scholar] [CrossRef]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dang, S.; Hou, P. Gene methylation in gastric cancer. Clin. Chim. Acta 2013, 424, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Tamura, G.; Sato, K.; Endoh, Y.; Sakata, K.; Jin, Z.; Motoyama, T.; Usuba, O.; Kimura, W.; Nishizuka, S.; et al. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene 2000, 19, 3642–3646. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zheng, T.; Hu, K.; Zhu, C.; Guo, L.; Ye, G. Promoter methylation of tumor-related genes as a potential biomarker using blood samples for gastric cancer detection. Oncotarget 2017, 8, 77783–77793. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, A.; Takai, A.; Shimizu, T.; Matsumoto, T.; Kumagai, K.; Miyamoto, S.; Seno, H.; Marusawa, H. Genetic features of multicentric/multifocal intramucosal gastric carcinoma. Int. J. Cancer 2018, 143, 1923–1934. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Yuan, Y.; Dong, C.; Yang, M. APC promoter methylation in gastrointestinal cancer. Front. Oncol. 2021, 11, 653222. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.H.; Watari, J.; Tanabe, H.; Sato, R.; Moriichi, K.; Tanaka, A.; Maemoto, A.; Fujiya, M.; Ashida, T.; Kohgo, Y. Clinicopathologic implications of genetic instability in intestinal-type gastric cancer and intestinal metaplasia as a precancerous lesion: Proof of field cancerization in the stomach. Am. J. Clin. Pathol. 2008, 129, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Businello, G.; Angerilli, V.; Parente, P.; Realdon, S.; Savarino, E.; Farinati, F.; Grillo, F.; Vanoli, A.; Galuppini, F.; Paccagnella, S.; et al. Molecular landscapes of gastric pre-neoplastic and pre-invasive lesions. Int. J. Mol. Sci. 2021, 22, 9950. [Google Scholar] [CrossRef]
- Kato, M.; Nishida, T.; Yamamoto, K.; Hayashi, S.; Kitamura, S.; Yabuta, T.; Yoshio, T.; Nakamura, T.; Komori, M.; Kawai, N.; et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: A multicentre retrospective cohort study by Osaka University ESD study group. Gut 2013, 62, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Shibukawa, N.; Ouchi, S.; Wakamatsu, S.; Wakahara, Y.; Kaneko, A. Gastric xanthoma is a predictive marker for early gastric cancer detected after Helicobacter pylori eradication. Intern. Med. 2019, 58, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Tan, P.; Yeoh, K.-G. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology 2015, 149, 1153–1162.e3. [Google Scholar] [CrossRef] [PubMed]
- Jacome, A.A.; Coutinho, A.K.; Lima, E.M.; Andrade, A.C.; Dos Santos, J.S. Personalized medicine in gastric cancer: Where are we and where are we going? World J. Gastroenterol. 2016, 22, 1160–1171. [Google Scholar] [CrossRef]
- Enomoto, S.; Maekita, T.; Tsukamoto, T.; Nakajima, T.; Nakazawa, K.; Tatematsu, M.; Ichinose, M.; Ushijima, T. Lack of association between CpG island methylator phenotype in human gastric cancers and methylation in their background noncancerous gastric mucosae. Cancer Sci. 2007, 98, 1853–1861. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamamoto, E.; Nojima, M.; Maruyama, R.; Yamano, H.O.; Yoshikawa, K.; Kimura, T.; Harada, T.; Ashida, M.; Niinuma, T.; et al. Aberrant methylation of microRNA-34b/c is a predictive marker of metachronous gastric cancer risk. J. Gastroenterol. 2014, 49, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Nakajima, T.; Shimazu, T.; Yamamichi, N.; Maekita, T.; Yokoi, C.; Oda, I.; Ando, T.; Yoshida, T.; Nanjo, S.; et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut 2015, 64, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, K.; Sasaki, Y.; Abe, Y.; Sato, H.; Shoji, M.; Yaoita, T.; Yagi, M.; Mizumoto, N.; Onozato, Y.; Kon, T.; et al. Somatic alterations and mutational burden are potential predictive factors for metachronous development of early gastric cancer. Sci. Rep. 2020, 10, 22071. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Patients (n = 115) | Patients with GX (n = 59) | Patients without GX (n = 56) | p Value |
|---|---|---|---|---|
| Age | ||||
| ≥65 | 97 (84.3) | 52 (88.1) | 45 (80.4) | 0.3088 |
| <65 | 18 (15.7) | 7 (11.9) | 11 (19.6) | |
| Sex | ||||
| Male | 82 (71.3) | 45 (76.3) | 37 (66.1) | 0.3027 |
| Female | 33 (28.7) | 14 (23.7) | 19 (33.9) | |
| BMI | 23.0 ± 3.4 | 22.9 ± 3.0 | 23.1 ± 3.9 | 0.8843 |
| Atrophy | ||||
| Open-type | 108 (93.9) | 57 (96.6) | 51 (91.1) | 0.2928 |
| Closed-type | 5 (4.4) | 2 (3.4) | 3 (5.3) | |
| None | 2 (1.7) | 0 (0.0) | 2 (3.6) | |
| H. pylori | ||||
| Negative | 15 (13.0) | 5 (8.5) | 10 (17.9) | 0.2553 |
| Era-negative | 11 (9.6) | 7 (11.9) | 4 (7.1) | |
| Positive | 89 (77.4) | 47 (79.6) | 42 (75.0) |
| Characteristics | MSI (+) (n = 48) | MSI (−) (n = 67) | p Value | hMLH1 (+) (n = 37) | hMLH1 (−) (n = 78) | p Value | CDKN2A (+) (n = 7) | CDKN2A (−) (n = 108) | p Value | APC (+) (n = 18) | APC (−) (n = 97) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||||
| ≥65 | 40 (83.3) | 57 (85.1) | 0.8008 | 28 (75.7) | 69 (88.5) | 0.1003 | 5 (71.4) | 92 (85.2) | 0.3008 | 13 (72.2) | 84 (86.6) | 0.1553 |
| <65 | 8 (16.7) | 10 (14.9) | 9 (24.3) | 9 (11.5) | 2 (28.6) | 16 (14.8) | 5 (27.8) | 13 (13.4) | ||||
| Sex | ||||||||||||
| Male | 35 (72.9) | 47 (70.1) | 0.8356 | 30 (81.1) | 52 (66.7) | 0.1272 | 4 (57.1) | 78 (72.2) | 0.4074 | 16 (88.9) | 66 (68.0) | 0.0915 |
| Female | 13 (27.1) | 20 (29.9) | 7 (18.9) | 26 (33.3) | 3 (42.9) | 30 (27.8) | 2 (11.1) | 31 (32.0) | ||||
| BMI | 23.5 ± 3.6 | 22.6 ± 3.2 | 0.3127 | 22.9 ± 3.0 | 23.0 ± 3.6 | 0.8131 | 21.4 ± 2.3 | 23.1 ± 3.5 | 0.1226 | 21.9 ± 2.8 | 23.1 ± 3.5 | 0.2124 |
| Atrophy | ||||||||||||
| Open-type | 44 (91.7) | 64 (95.5) | 0.6750 | 35 (94.6) | 73 (93.6) | 0.7285 | 6 (85.7) | 102 (94.5) | 0.0284 | 16 (88.9) | 92 (94.9) | 0.3836 |
| Closed-type | 3 (6.2) | 2 (3.0) | 1 (2.7) | 4 (5.1) | 0 (0.0) | 5 (4.6) | 1 (5.55) | 4 (4.1) | ||||
| None | 1 (2.1) | 1 (1.5) | 1 (2.7) | 1 (1.3) | 1 (14.3) | 1 (0.9) | 1 (5.55) | 1 (1.0) | ||||
| H. pylori | ||||||||||||
| Negative | 4 (8.3) | 11 (16.4) | 0.3368 | 4 (10.8) | 11 (14.1) | 0.8591 | 1 (14.3) | 14 (12.9) | 0.8973 | 1 (5.6) | 14 (14.4) | 0.5863 |
| Era-negative | 6 (12.5) | 5 (7.5) | 4 (10.8) | 7 (9.0) | 1 (14.3) | 10 (9.3) | 2 (11.1) | 9 (9.3) | ||||
| Positive | 38 (79.2) | 51 (76.1) | 29 (78.4) | 60 (76.9) | 5 (71.4) | 84 (77.8) | 15 (83.3) | 74 (76.3) |
| Characteristics | Patients with GX (n = 59) | Patients without GX (n = 56) | p Value |
|---|---|---|---|
| MSI | |||
| positive | 23 (39.0) | 25 (44.6) | 0.5744 |
| negative | 36 (61.0) | 31 (55.4) | |
| hMLH1 methylation | |||
| positive | 19 (32.2) | 18 (32.1) | 0.9945 |
| negative | 40 (67.8) | 38 (67.9) | |
| CDKN2A methylation | |||
| positive | 3 (5.1) | 4 (7.1) | 0.7121 |
| negative | 56 (94.9) | 52 (92.9) | |
| APC methylation | |||
| positive | 7 (11.9) | 11 (19.6) | 0.3088 |
| negative | 52 (88.1) | 45 (80.4) |
| Characteristics | Total with Synch or Metach GC/Total Patients | Univariate | Multivariate | |
|---|---|---|---|---|
| p Value | 95% CI | p Value | ||
| Age | ||||
| ≥65 | 18/97 | 0.847 | 1.096 (0.295–4.074) | 0.892 |
| <65 | 3/18 | 1.0 | ||
| Sex | ||||
| Male | 16/82 | 0.790 | 1.828 (0.507–6.579) | 0.357 |
| Female | 5/33 | 1.0 | ||
| GX | ||||
| Present | 16/59 | 0.015 | 3.257 (1.134–9.346) | 0.028 |
| Absent | 5/56 | 1.0 | ||
| MSI | ||||
| positive | 7/48 | 0.468 | 0.711 (0.273–1.855) | 0.486 |
| Negative | 14/67 | 1.0 | ||
| hMLH1 methylation | ||||
| Positive | 6/37 | 0.800 | 0.512 (0.142–1.842) | 0.305 |
| Negative | 15/78 | 1.0 | ||
| CDKN2A methylation | ||||
| Positive | 3/7 | 0.113 | 4.673 (0.671–32.258) | 0.120 |
| Negative | 18/108 | 1.0 | ||
| APC methylation | ||||
| Positive | 3/18 | 0.849 | 1.300 (0.335–5.051) | 0.705 |
| Negative | 18/97 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, M.; Fukui, H.; Watari, J.; Ito, C.; Hara, K.; Eda, H.; Tomita, T.; Oshima, T.; Miwa, H. Gastric Xanthelasma, Microsatellite Instability and Methylation of Tumor Suppressor Genes in the Gastric Mucosa: Correlation and Comparison as a Predictive Marker for the Development of Synchronous/Metachronous Gastric Cancer. J. Clin. Med. 2022, 11, 9. https://doi.org/10.3390/jcm11010009
Fukushima M, Fukui H, Watari J, Ito C, Hara K, Eda H, Tomita T, Oshima T, Miwa H. Gastric Xanthelasma, Microsatellite Instability and Methylation of Tumor Suppressor Genes in the Gastric Mucosa: Correlation and Comparison as a Predictive Marker for the Development of Synchronous/Metachronous Gastric Cancer. Journal of Clinical Medicine. 2022; 11(1):9. https://doi.org/10.3390/jcm11010009
Chicago/Turabian StyleFukushima, Masashi, Hirokazu Fukui, Jiro Watari, Chiyomi Ito, Ken Hara, Hirotsugu Eda, Toshihiko Tomita, Tadayuki Oshima, and Hiroto Miwa. 2022. "Gastric Xanthelasma, Microsatellite Instability and Methylation of Tumor Suppressor Genes in the Gastric Mucosa: Correlation and Comparison as a Predictive Marker for the Development of Synchronous/Metachronous Gastric Cancer" Journal of Clinical Medicine 11, no. 1: 9. https://doi.org/10.3390/jcm11010009
APA StyleFukushima, M., Fukui, H., Watari, J., Ito, C., Hara, K., Eda, H., Tomita, T., Oshima, T., & Miwa, H. (2022). Gastric Xanthelasma, Microsatellite Instability and Methylation of Tumor Suppressor Genes in the Gastric Mucosa: Correlation and Comparison as a Predictive Marker for the Development of Synchronous/Metachronous Gastric Cancer. Journal of Clinical Medicine, 11(1), 9. https://doi.org/10.3390/jcm11010009








