Concomitant Assessment of Monocyte HLA-DR Expression and Ex Vivo TNF-α Release as Markers of Adverse Outcome after Various Injuries—Insights from the REALISM Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Design
2.2. Patients
2.3. TNF Release and mHLA-DR Measurements
2.4. Definition of Endpoint
2.5. Statistical Analysis
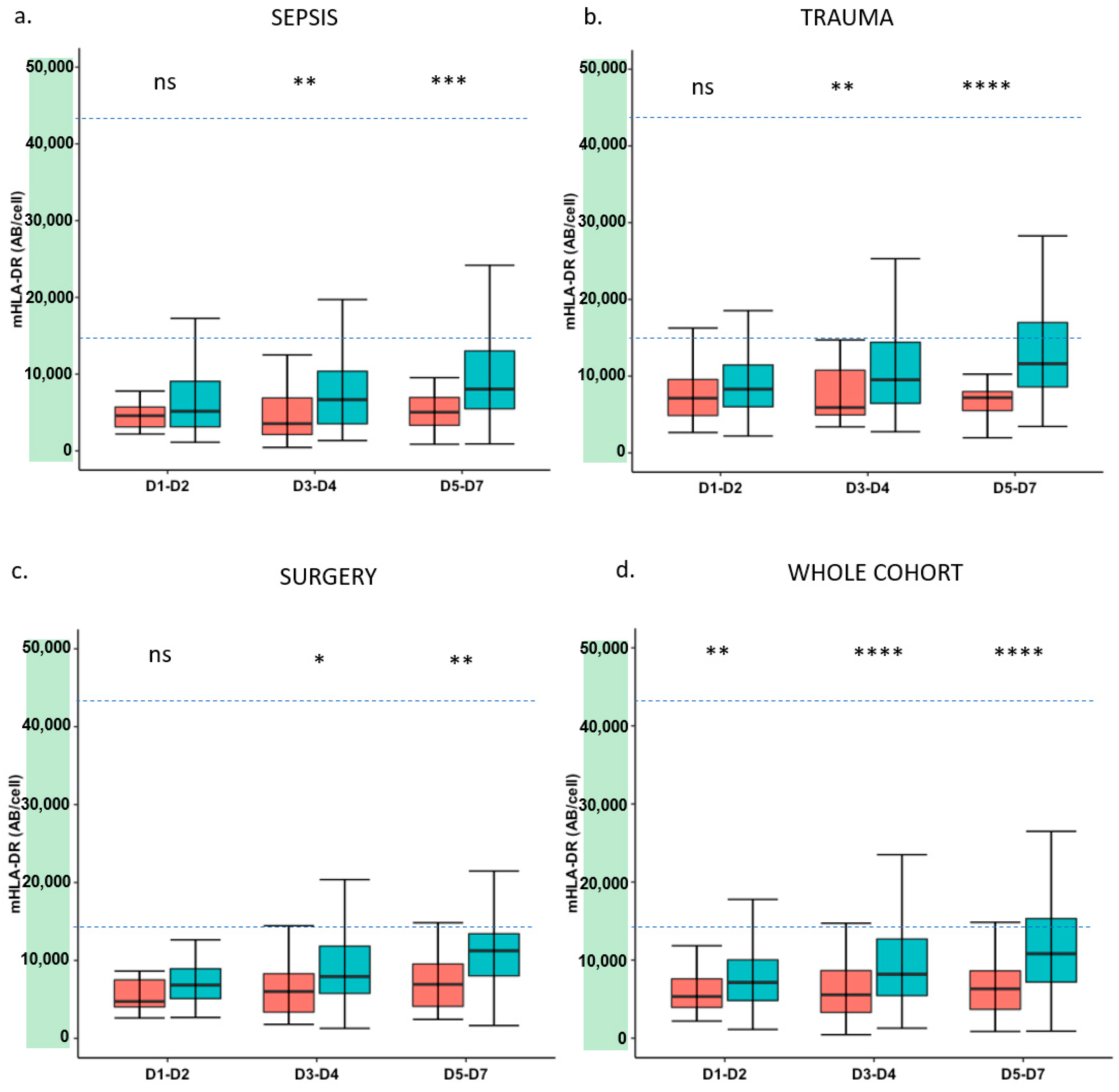
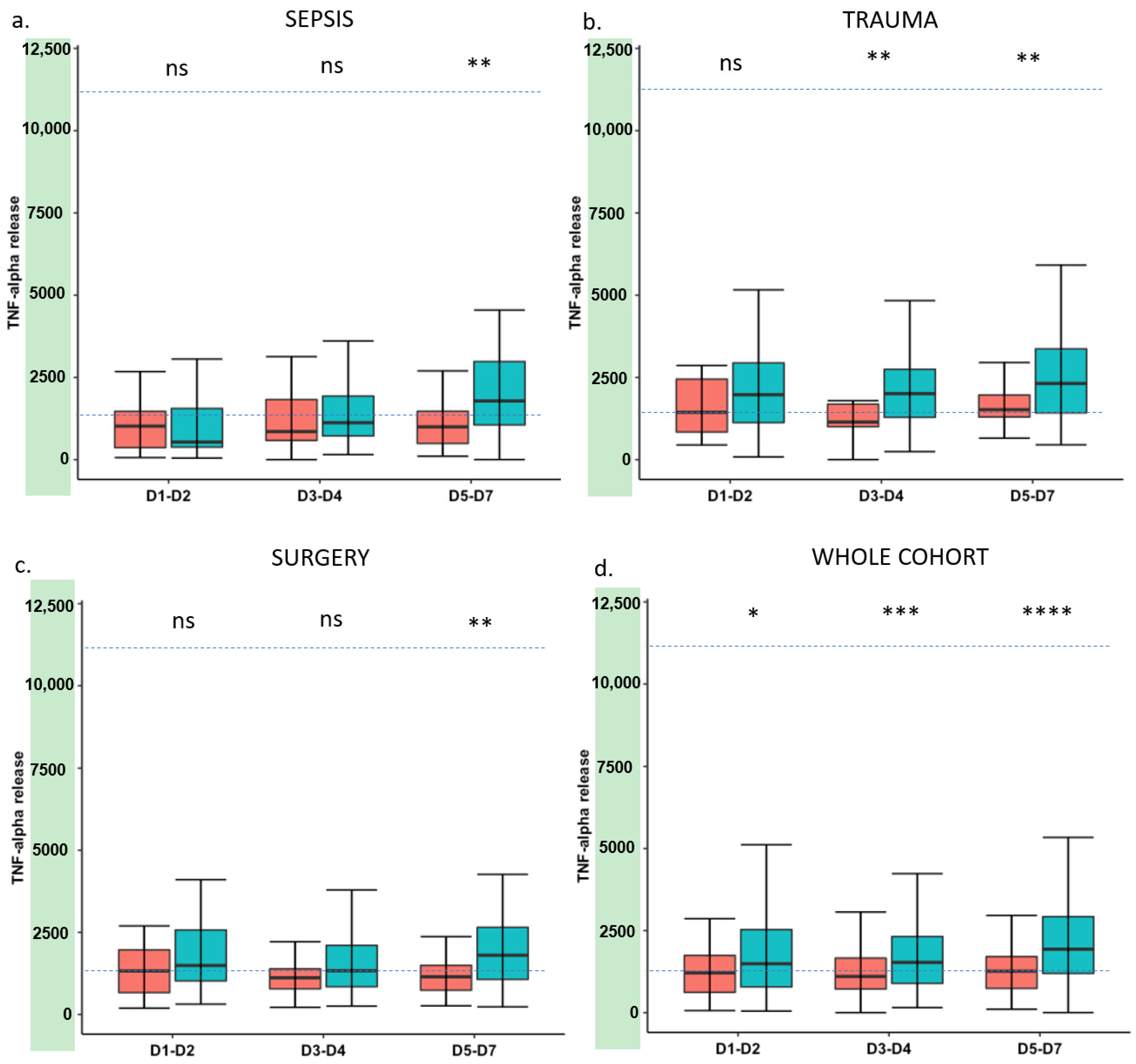
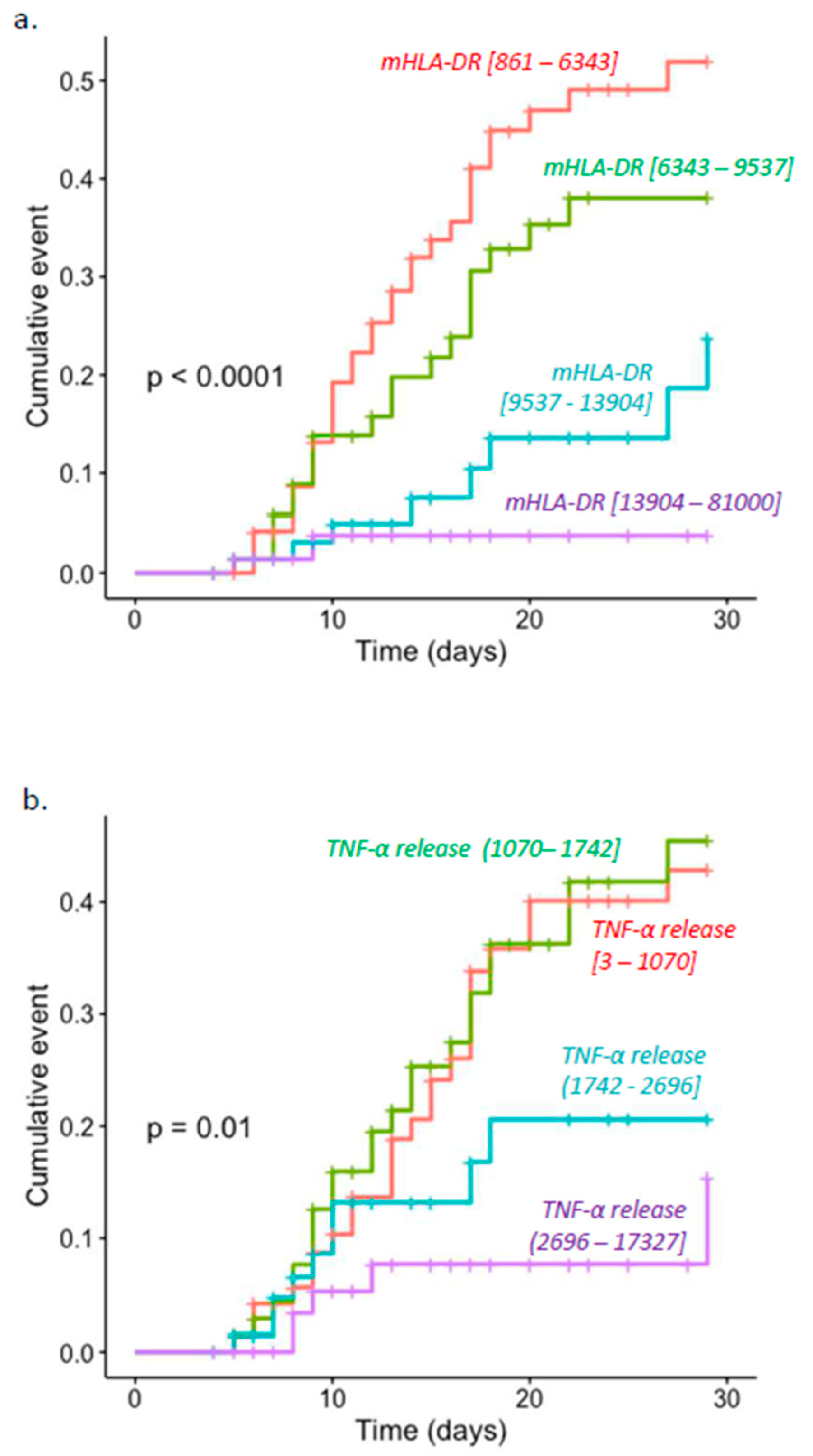
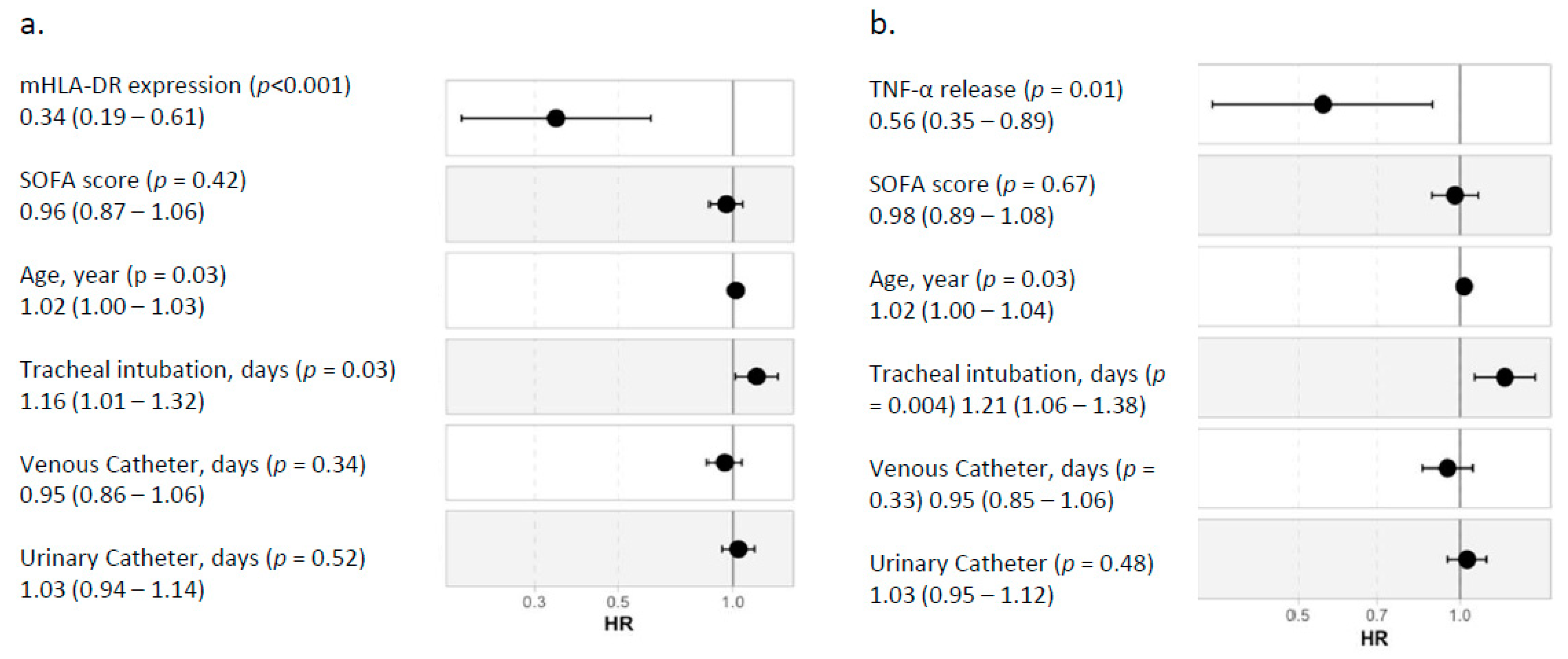
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Realism Study Group
Abbreviations
| ICU | intensive care unit |
| LPS | lipopolysaccharide |
| mHLA-DR | human leukocyte antigen DR on circulating monocytes |
| SOFA | Sequential Organ Failure Assessment |
| TNF-α | Tumor necrosis factor α |
| WB | Whole blood |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Tschaikowsky, K.; Hedwig-Geissing, M.; Schiele, A.; Bremer, F.; Schywalsky, M.; Schüttler, J. Coincidence of Pro- and Anti-Inflammatory Responses in the Early Phase of Severe Sepsis: Longitudinal Study of Mononuclear Histocompatibility Leukocyte Antigen-Dr Expression, Procalcitonin, C-Reactive Protein, and Changes in T-Cell Subsets in Septic and Postoperative Patients. Crit. Care Med. 2002, 30, 1015–1023. [Google Scholar] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the Understanding and Treatment of Sepsis-Induced Immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Ward, P.A. Sepsis-Induced Immune Dysfunction: Can Immune Therapies Reduce Mortality? J. Clin. Investig. 2016, 126, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Miyazaki, M. Immunosuppression Following Surgical and Traumatic Injury. Surg. Today 2010, 40, 793–808. [Google Scholar] [CrossRef]
- Cheron, A.; Floccard, B.; Allaouchiche, B.; Guignant, C.; Poitevin, F.; Malcus, C.; Crozon, J.; Faure, A.; Guillaume, C.; Marcotte, G.; et al. Lack of Recovery in Monocyte Human Leukocyte Antigen-Dr Expression Is Independently Associated with the Development of Sepsis after Major Trauma. Crit. Care 2010, 14, R208. [Google Scholar] [CrossRef] [Green Version]
- Venet, F.; Lukaszewicz, A.C.; Payen, D.; Hotchkiss, R.; Monneret, G. Monitoring the Immune Response in Sepsis: A Rational Approach to Administration of Immunoadjuvant Therapies. Curr. Opin. Immunol. 2013, 25, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, N.A.; Unsinger, J.; Hotchkiss, R.S.; Ayala, A. The New Normal: Immunomodulatory Agents against Sepsis Immune Suppression. Trends Mol. Med. 2014, 20, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, L.K.; Pickkers, P.; van der Poll, T. Sepsis-Induced Immunosuppression. Annu. Rev. Physiol. 2021, 84. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Venet, F.; Pachot, A.; Lepape, A. Monitoring Immune Dysfunctions in the Septic Patient: A New Skin for the Old Ceremony. Mol. Med. 2008, 14, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Piani, A.; Hossle, J.P.; Birchler, T.; Siegrist, C.A.; Heumann, D.; Davies, G.; Loeliger, S.; Seger, R.; Lauener, R.P. Expression of Mhc Class Ii Molecules Contributes to Lipopolysaccharide Responsiveness. Eur. J. Immunol. 2000, 30, 3140–3146. [Google Scholar] [CrossRef]
- Venet, F.; Demaret, J.; Gossez, M.; Monneret, G. Myeloid Cells in Sepsis-Acquired Immunodeficiency. Ann. N. Y. Acad Sci. 2020, 1499, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.W.; Knatz, N.L.; Vetterly, C.; Tomarello, S.; Wewers, M.D.; Volk, H.D.; Carcillo, J.A. Immunoparalysis and Nosocomial Infection in Children with Multiple Organ Dysfunction Syndrome. Intensive Care Med. 2011, 37, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Muszynski, J.A.; Nofziger, R.; Greathouse, K.; Nateri, J.; Hanson-Huber, L.; Steele, L.; Nicol, K.; Groner, J.I.; Besner, G.E.; Raffel, C.; et al. Innate Immune Function Predicts the Development of Nosocomial Infection in Critically Injured Children. Shock 2014, 42, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Van Vught, L.A.; Wiewel, M.A.; Hoogendijk, A.J.; Scicluna, B.P.; Belkasim, H.; Horn, J.; Schultz, M.J.; Van der Poll, T. Reduced Responsiveness of Blood Leukocytes to Lipopolysaccharide Does Not Predict Nosocomial Infections in Critically Ill Patients. Shock 2015, 44, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segre, E.; Fullerton, J.N. Stimulated Whole Blood Cytokine Release as a Biomarker of Immunosuppression in the Critically Ill: The Need for a Standardized Methodology. Shock 2016, 45, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Drewry, A.M.; Ablordeppey, E.A.; Murray, E.T.; Beiter, E.R.; Walton, A.H.; Hall, M.W.; Hotchkiss, R.S. Comparison of Monocyte Human Leukocyte Antigen-Dr Expression and Stimulated Tumor Necrosis Factor Alpha Production as Outcome Predictors in Severe Sepsis: A Prospective Observational Study. Crit. Care 2016, 20, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, M.S.; Rissiek, A.; Priefler, M.; Schwedhelm, E.; Robbe, L.; Bauer, A.; Zahrte, C.; Zoellner, C.; Kluge, S.; Nierhaus, A. Human Leucocyte Antigen (Hla-Dr) Gene Expression Is Reduced in Sepsis and Correlates with Impaired Tnfalpha Response: A Diagnostic Tool for Immunosuppression? PLoS ONE 2017, 12, e0182427. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Textoris, J.; Blein, S.; Rol, M.L.; Bodinier, M.; Canard, B.; Cortez, P.; Meunier, B.; Tan, L.; Tipple, C.; et al. Immune Profiling Demonstrates a Common Immune Signature of Delayed Acquired Immunodeficiency in Patients with Various Etiologies of Severe Injury. Crit. Care Med. 2021. [Google Scholar] [CrossRef]
- Rol, M.L.; Venet, F.; Rimmele, T.; Moucadel, V.; Cortez, P.; Quemeneur, L.; Gardiner, D.; Griffiths, A.; Pachot, A.; Textoris, J.; et al. The Reanimation Low Immune Status Markers (Realism) Project: A Protocol for Broad Characterisation and Follow-up of Injury-Induced Immunosuppression in Intensive Care Unit (Icu) Critically Ill Patients. BMJ Open 2017, 7, e015734. [Google Scholar] [CrossRef] [Green Version]
- Demaret, J.; Walencik, A.; Jacob, M.C.; Timsit, J.F.; Venet, F.; Lepape, A.; Monneret, G. Inter-Laboratory Assessment of Flow Cytometric Monocyte Hla-Dr Expression in Clinical Samples. Cytometry B Clin. Cytom 2013, 84, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohé, J.; Venet, F.; Debard, A.L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting Low Monocyte Human Leukocyte Antigen-Dr Expression Predicts Mortality in Septic Shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Leijte, G.P.; Rimmelé, T.; Kox, M.; Bruse, N.; Monard, C.; Gossez, M.; Monneret, G.; Pickkers, P.; Venet, F. Monocytic Hla-Dr Expression Kinetics in Septic Shock Patients with Different Pathogens, Sites of Infection and Adverse Outcomes. Crit. Care 2020, 24, 110. [Google Scholar] [CrossRef] [Green Version]
- Wolk, K.; Döcke, W.D.; von Baehr, V.; Volk, H.D.; Sabat, R. Impaired Antigen Presentation by Human Monocytes During Endotoxin Tolerance. Blood 2000, 96, 218–223. [Google Scholar] [CrossRef]
- Allen, M.L.; Peters, M.J.; Goldman, A.; Elliott, M.; James, I.; Callard, R.; Klein, N.J. Early Postoperative Monocyte Deactivation Predicts Systemic Inflammation and Prolonged Stay in Pediatric Cardiac Intensive Care. Crit. Care Med. 2002, 30, 1140–1145. [Google Scholar] [CrossRef]
- Satoh, A.; Miura, T.; Satoh, K.; Masamune, A.; Yamagiwa, T.; Sakai, Y.; Shibuya, K.; Takeda, K.; Kaku, M.; Shimosegawa, T. Human Leukocyte Antigen-Dr Expression on Peripheral Monocytes as a Predictive Marker of Sepsis During Acute Pancreatitis. Pancreas 2002, 25, 245–250. [Google Scholar] [CrossRef]
- Levin, G.; Boyd, J.G.; Day, A.; Hunt, M.; Maslove, D.M.; Norman, P.; O’Callaghan, N.; Sibley, S.; Muscedere, J. The Relationship between Immune Status as Measured by Stimulated Ex-Vivo Tumour Necrosis Factor Alpha Levels and the Acquisition of Nosocomial Infections in Critically Ill Mechanically Ventilated Patients. Intensive Care Med. Exp. 2020, 8, 55. [Google Scholar] [CrossRef]
- Quadrini, K.J.; Patti-Diaz, L.; Maghsoudlou, J.; Cuomo, J.; Hedrick, M.N.; McCloskey, T.W. A Flow Cytometric Assay for Hla-Dr Expression on Monocytes Validated as a Biomarker for Enrollment in Sepsis Clinical Trials. Cytom. B Clin. Cytom. 2021, 100, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Elmenkouri, N.; Bohe, J.; Debard, A.L.; Gutowski, M.C.; Bienvenu, J.; Lepape, A. Analytical Requirements for Measuring Monocytic Human Lymphocyte Antigen Dr by Flow Cytometry: Application to the Monitoring of Patients with Septic Shock. Clin. Chem. 2002, 48, 1589–1592. [Google Scholar] [CrossRef] [Green Version]
- Hamada, S.; Jeannet, R.; Gossez, M.; Cour, M.; Argaud, L.; Francois, B.; Daix, T.; Venet, F.; Monneret, G. Evaluation of Stabilizing Sampling Tubes for Assessment of Monocyte Hla-Dr Expression in Clinical Samples. Cytom. B Clin. Cytom. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bourgoin, P.; Taspinar, R.; Gossez, M.; Venet, F.; Delwarde, B.; Rimmelé, T.; Morange, P.E.; Malergue, F.; Monneret, G. Toward Monocyte Hla-Dr Bedside Monitoring: A Proof-Of-Concept Study. Shock 2021, 55, 782–789. [Google Scholar] [CrossRef] [PubMed]


| Adverse Outcome (n = 90) | Favorable Outcome (n = 263) | p-Value | |
|---|---|---|---|
| Gender (n, %) | 57 (63.3) | 174 (66.2) | 0.720 |
| Age (years) | 67.5 (55.3–75.0) | 57.0 (44.0–70.0) | <0.001 |
| BMI (kg/m2) | 26.1 (22.2–29.9) | 24.7 (22.3–27.6) | 0.093 |
| Severity scores | |||
| SAPSII | 35.0 (26.3–49.8) | 26.0 (18.0–40.0) | <0.001 |
| Charlson | 2.0 (0.0–3.0) | 1.0 (0.0–2.0) | <0.001 |
| SOFA | 7.0 (2.0–10.0) | 4.0 (1.0–8.0) | <0.001 |
| Biological values | |||
| ALT (UI/L) | 84.0 (41.8–150.0) | 63.0 (32.0–160.0) | 0.419 |
| AST (UI/L) | 126.0 (50.5–235.0) | 83.0 (48.0–177.0) | 0.189 |
| Bilirubin (µmol/L) | 17.5 (11.8–34.0) | 15.0 (9.0–35.0) | 0.335 |
| Creatinine (µmol/L) | 108.5 (71.8–183.8) | 87.0 (66.3–125.8) | 0.016 |
| Leucocytes (×109/L) | 13.9 (10.1–18.4) | 12.7 (10.32–16.4) | 0.389 |
| Lymphocytes (×109/L) | 1.1 (0.8–1.6) | 1.3 (0.82–1.8) | 0.059 |
| Monocytes (×109/L) | 0.9 (0.6–1.4) | 0.9 ( 0.67–1.3) | 0.709 |
| Neutrophils (×109/L) | 11.6 (8.3–15.9) | 10.7 (7.88–14.1) | 0.271 |
| Platelets (×109/L) | 193 (144–241) | 206 (162.00–274) | 0.112 |
| PaO2/FiO2 (mm Hg) | 242 (160–316) | 246 (181–358) | 0.146 |
| Hemoglobin (g/dL) | 113(93–127) | 117 (103–132) | 0.075 |
| pH | 7.34 (7.28, 7.39) | 7.36 (7.30–7.41) | 0.116 |
| Lactate (mmol/L) | 2.50 (1.70, 3.50) | 2.20 (1.60–3.20) | 0.205 |
| Organ failure | |||
| Coma (n, %) | 7 (7.8) | 19 (7.2) | 1.000 |
| Vasopressors (n, %) | 57 (63) | 121 (46) | 0.007 |
| Renal Replacement Therapy (n, %) | 30 (33) | 31 (12) | <0.001 |
| Exposure to invasive devices | |||
| Urinary Catheter (n, %) | 81 (90) | 213 (81) | 0.070 |
| Venous Catheter (n, %) | 74 (82) | 145 (55) | <0.001 |
| Tracheal intubation (n, %) | 59 (66) | 105 (40) | <0.001 |
| Invasive Ventilation D30 Free Days | 17.00 (0.00, 28.75) | 29.00 (27.00, 29.00) | <0.001 |
| Urinary Catheter D30 Free Days | 7(0–22) | 27 (22–28) | <0.001 |
| Venous Catheter D30 Free Days | 0 (0–15) | 23.00 (17–27) | <0.001 |
| Follow-up | |||
| Length of ICU stay | 9.50 (4.00, 15.00) | 5.00 (3.00, 8.00) | <0.001 |
| Time to adverse outcome (days) | 9.00 (5.00, 14.00) | NA |
| Population | ρ | p-Value |
|---|---|---|
| All cohort (all time points) | 0.41 | <0.001 |
| D1–D2 | 0.36 | <0.001 |
| D3–D4 | 0.39 | <0.001 |
| D5–D7 | 0.49 | <0.001 |
| Sepsis (all time points) | 0.45 | <0.001 |
| Sepsis D1–D2 | 0.32 | 0.003 |
| Sepsis D3–D4 | 0.40 | <0.001 |
| Sepsis D5–D7 | 0.52 | <0.001 |
| Trauma (all time points) | 0.34 | <0.001 |
| Trauma D1–D2 | 0.17 | 0.04 |
| Trauma D3–D4 | 0.36 | <0.001 |
| Trauma D5–D7 | 0.46 | <0.001 |
| Surgery (all time points) | 0.38 | <0.001 |
| Surgery Day 1–2 | 0.39 | <0.001 |
| Surgery D3–D4 | 0.24 | 0.02 |
| Surgery D5–D7 | 0.43 | <0.001 |
| Clinical Worsening | No Clinical Worsening | |
|---|---|---|
| Group 1 (mHLA-DR expression and TNF-α release lower than medians) | 40 (41%) | 57 (59%) |
| Group 2 (only mHLA-DR expression lower than median) | 11 (24%) | 35 (76%) |
| Group 3 (only TNF-α release lower than median) | 7 (16%) | 38 (84%) |
| Group 4 (mHLA-DR expression and TNF- α release higher than medians) | 3 (3%) | 94 (97%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidar, F.; Bodinier, M.; Venet, F.; Lukaszewicz, A.-C.; Brengel-Pesce, K.; Conti, F.; Quemeneur, L.; Leissner, P.; Tan, L.K.; Textoris, J.; et al. Concomitant Assessment of Monocyte HLA-DR Expression and Ex Vivo TNF-α Release as Markers of Adverse Outcome after Various Injuries—Insights from the REALISM Study. J. Clin. Med. 2022, 11, 96. https://doi.org/10.3390/jcm11010096
Bidar F, Bodinier M, Venet F, Lukaszewicz A-C, Brengel-Pesce K, Conti F, Quemeneur L, Leissner P, Tan LK, Textoris J, et al. Concomitant Assessment of Monocyte HLA-DR Expression and Ex Vivo TNF-α Release as Markers of Adverse Outcome after Various Injuries—Insights from the REALISM Study. Journal of Clinical Medicine. 2022; 11(1):96. https://doi.org/10.3390/jcm11010096
Chicago/Turabian StyleBidar, Frank, Maxime Bodinier, Fabienne Venet, Anne-Claire Lukaszewicz, Karen Brengel-Pesce, Filippo Conti, Laurence Quemeneur, Philippe Leissner, Lionel K. Tan, Julien Textoris, and et al. 2022. "Concomitant Assessment of Monocyte HLA-DR Expression and Ex Vivo TNF-α Release as Markers of Adverse Outcome after Various Injuries—Insights from the REALISM Study" Journal of Clinical Medicine 11, no. 1: 96. https://doi.org/10.3390/jcm11010096





