Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study
Abstract
:1. Introduction
- Key messages of the article:
- −
- What is already known on this topic?Prematurity is associated with altered ocular morphology and functioning in childhood and adulthood, so we investigated whether perinatal factors have long-term effects on the ocular surface and lid configuration in adulthood.
- −
- What does this study add?ROP treatment is linked to reduced tear film break-up time later in life. Furthermore, low gestational age is associated with increased bulbar redness, longer Schirmer strip measurement, and a narrower nasal lid angle.
- −
- How might this study affect research or practice?Perinatal history affects the ocular surface, tear production, and lid geometry in adults born term and preterm, which might predispose affected persons to diseases of the corneal surface and dry eye disease in later life.
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Pre-, Peri- and Postnatal Medical History
2.3. Categorization
2.4. Ophthalmological Examination
2.5. Covariates
2.6. Inclusion/Exclusion Criteria
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Descriptive Anterior Surface Parameters
3.3. Uni- and Multivariable Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GA | Gestational age |
| GCP | Good Clinical Practice |
| GEE | General estimating equations |
| GEP | Good Epidemiological Practice |
| GPES | The Gutenberg Prematurity Eye Study |
| OSDI | Ocular Surface Disease Index |
| ROP | Retinopathy of prematurity |
| UMCM | University Medical Center of the Johannes Gutenberg University Mainz in Germany |
References
- Fieß, A.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Blecha, C.; Oberacher-Velten, I.; Muether, P.S.; Bauer, J. Axial Length and Anterior Segment Alterations in Former Preterm Infants and Full-Term Neonates Analyzed With Scheimpflug Imaging. Cornea 2017, 36, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Lin, R.I.; Shih, C.P.; Wang, N.K.; Chen, Y.P.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C.; et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 2012, 119, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Christian, L.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Peripapillary Choroidal Thickness in Former Preterm and Full-Term Infants Aged From 4 to 10 Years. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6548–6553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fieß, A.; Christian, L.; Janz, J.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Functional analysis and associated factors of the peripapillary retinal nerve fibre layer in former preterm and full-term infants. Br. J. Ophthalmol. 2017, 101, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Janz, J.; Schuster, A.K.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2017, 255, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Schuster, A.K.; Nickels, S.; Urschitz, M.S.; Elflein, H.M.; Schulz, A.; Munzel, T.; Wild, P.S.; Beutel, M.E.; Schmidtmann, I.; et al. Association of Low Birth Weight With Altered Corneal Geometry and Axial Length in Adulthood in the German Gutenberg Health Study. JAMA Ophthalmol. 2019, 137, 507–514. [Google Scholar] [CrossRef]
- Darlow, B.A.; Gilbert, C. Retinopathy of prematurity—A world update. Semin. Perinatol. 2019, 43, 315–316. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Penn, J.S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef] [Green Version]
- Fieß, A.; Schuster, A.K.; Kolb-Keerl, R.; Knuf, M.; Kirchhof, B.; Muether, P.S.; Bauer, J. Corneal Aberrations in Former Preterm Infants: Results From The Wiesbaden Prematurity Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6374–6378. [Google Scholar] [CrossRef] [Green Version]
- Fieß, A.; Uschitz, M.; Nagler, M.; Mickels, S. Association of birth weight with corneal aberrations in adulthood—Results from a population-based study. J. Optom. 2021. [Google Scholar]
- Howson, C.P.; Kinney, M.V.; Lawn, J.E. Born Too Soon: The Global Action Report on Preterm Birth. March of Dimes, PMNCH, Save the Children, World Health Organization. Geneva. 2012. Available online: https://assets.publishing.service.gov.uk/media/57a08a76e5274a27b20005e3/201204_borntoosoon-report.pdf (accessed on 8 February 2019).
- Fielder, A.R.; Levene, M.I.; Russell-Eggitt, I.M.; Weale, R.A. Temperature—A factor in ocular development? Dev. Med. Child Neurol. 1986, 28, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Urschitz, M.S.; Marx-Groß, S.; Nagler, M.; Wild, P.S.; Münzel, T.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K. Association of Birth Weight with Central and Peripheral Corneal Thickness in Adulthood-Results from the Population-Based German Gutenberg Health Study. Children 2021, 8, 1006. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588s–595s. [Google Scholar] [CrossRef] [PubMed]
- Pellanda, L.C.; Duncan, B.B.; Vigo, A.; Rose, K.; Folsom, A.R.; Erlinger, T.P. Low birth weight and markers of inflammation and endothelial activation in adulthood: The ARIC study. Int. J. Cardiol. 2009, 134, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Chia, E.M.; Mitchell, P.; Rochtchina, E.; Lee, A.J.; Maroun, R.; Wang, J.J. Prevalence and associations of dry eye syndrome in an older population: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2003, 31, 229–232. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Hoffmann, E.M.; Brockmann, M.A.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Optic nerve head morphology in adults born extreme, very and moderate preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Am. J. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Fieß, A.; Gißler, S.; Mildenberger, E.; Urschitz, M.S.; Fauer, A.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Anterior Chamber Angle in Adults Born Extremely, Very, and Moderately Preterm with and without Retinopathy of Prematurity-Results of the Gutenberg Prematurity Eye Study. Children 2022, 9, 281. [Google Scholar] [CrossRef]
- Fieß, A.; Nauen, H.; Mildenberger, E.; Zepp, F.; Urschitz, M.S.; Pfeiffer, N.; Schuster, A.K.-G. Ocular geometry in adults born extremely, very and moderately preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Fauer, A.; Mildenberger, E.; Urschitz, M.S.; Elflein, H.M.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Refractive error, accommodation and lens opacification in adults born preterm and full-term: Results from the Gutenberg Prematurity Eye Study (GPES). Acta Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fieß, A.; Gißler, S.; Fauer, A.; Riedl, J.C.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Short report on retinal vessel metrics and arterial blood pressure in adult individuals born preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Acta Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Fusch, C.; Olbertz, D. Analyse des Neugeborenenkollektivs der Bundesrepublik Deutschland 12. Mitteilung: Vorstellung engmaschiger Perzentilwerte (-kurven) für die Körpermaße Neugeborener. Geburtsh Frauenheilk 2006, 66, 956–970. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Fieß, A.; Schuster, A.K.; Pfeiffer, N.; Nickels, S. Association of birth weight with corneal power in early adolescence: Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2008. PLoS ONE 2017, 12, e0186723. [Google Scholar] [CrossRef]
- Fieß, A.; Nickels, S.; Urschitz, M.S.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Hoffmann, E.M.; Pfeiffer, N.; Schuster, A.K. Association of Birth Weight with Peripapillary Retinal Nerve Fiber Layer Thickness in Adulthood—Results from a Population-Based Study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Fieß, A.; Ponto, K.A.; Urschitz, M.S.; Nickels, S.; Schulz, A.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; et al. Birthweight and its association with retinal vessel equivalents—Results from the population-based German Gutenberg Health Study. Acta Ophthalmol. 2020, 99, e773–e774. [Google Scholar] [CrossRef]
- Fieß, A.; Stingl, J.; Urschitz, M.S.; Hoffmann, E.M.; Münzel, T.; Wild, P.S.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; Schuster, A.K. Birth weight and its association with optic nerve head morphology—Results from the population-based German Gutenberg Health Study. Acta Ophthalmol. 2021. [Google Scholar]
- Fieß, A.; Wagner, F.M.; Urschitz, M.S.; Nagler, M.; Stoffelns, B.; Wild, P.S.; Münzel, T.; Beutel, M.E.; Lackner, K.J.; Pfeiffer, N.; et al. Association of Birth Weight With Foveolar Thickness in Adulthood: Results From a Population-Based Study. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef]
- Raffa, L.H.; Hellstrom, A.; Aring, E.; Andersson, S.; Gronlund, M.A. Ocular dimensions in relation to auxological data in a sample of Swedish children aged 4–15 years. Acta Ophthalmol. 2014, 92, 682–688. [Google Scholar] [CrossRef]
- Grey, C.; Yap, M. Influence of lid position on astigmatism. Am. J. Optom. Physiol. Opt. 1986, 63, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J.; Carney, L.G. The influence of eyelid morphology on normal corneal shape. Investig. Ophthalmol. Vis. Sci. 2007, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, K.; Abetz, L.; Mertzanis, P.; Espindle, D.; Begley, C.; Chalmers, R.; Caffery, B.; Snyder, C.; Nelson, J.D.; Simpson, T.; et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health J. Int. Soc. Pharm. Outcomes Res. 2005, 8, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, L.; Chapin, W.J.; Zhu, M. Assessment of vision-related quality of life in dry eye patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5722–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.J.; Liu, Y.; Zou, H.D.; Jiang, Y.J.; Liang, X.Q.; Sheng, M.J.; Li, B.; Xu, X. Epidemiologic study of dry eye in populations equal or over 20 years old in Jiangning District of Shanghai. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2009, 45, 486–491. [Google Scholar]
- Bielory, L.; Syed, B.A. Pharmacoeconomics of anterior ocular inflammatory disease. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 537–542. [Google Scholar] [CrossRef]
- Upadhyaya, S.; Sourander, A.; Luntamo, T.; Matinolli, H.M.; Chudal, R.; Hinkka-Yli-Salomäki, S.; Filatova, S.; Cheslack-Postava, K.; Sucksdorff, M.; Gissler, M.; et al. Preterm Birth Is Associated With Depression From Childhood to Early Adulthood. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 1127–1136. [Google Scholar] [CrossRef]
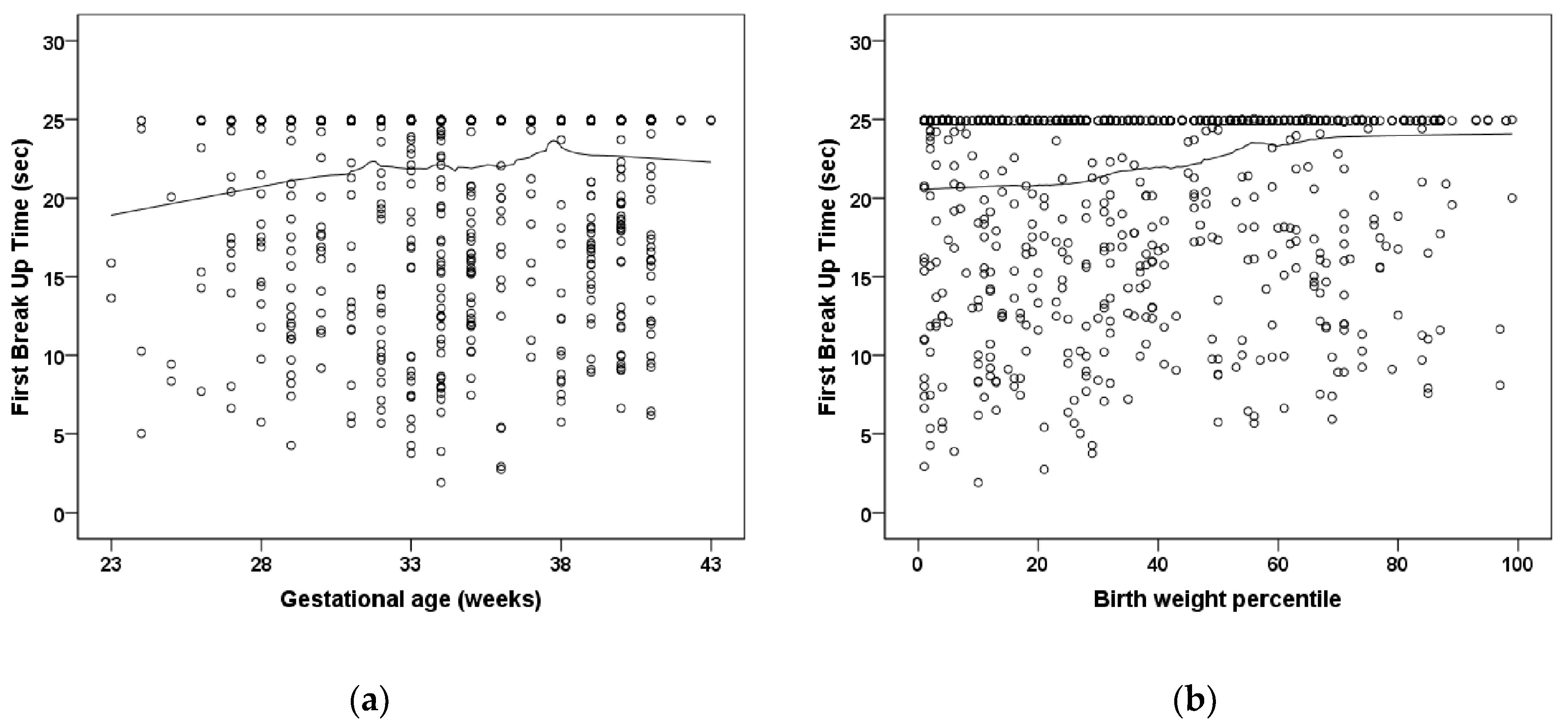
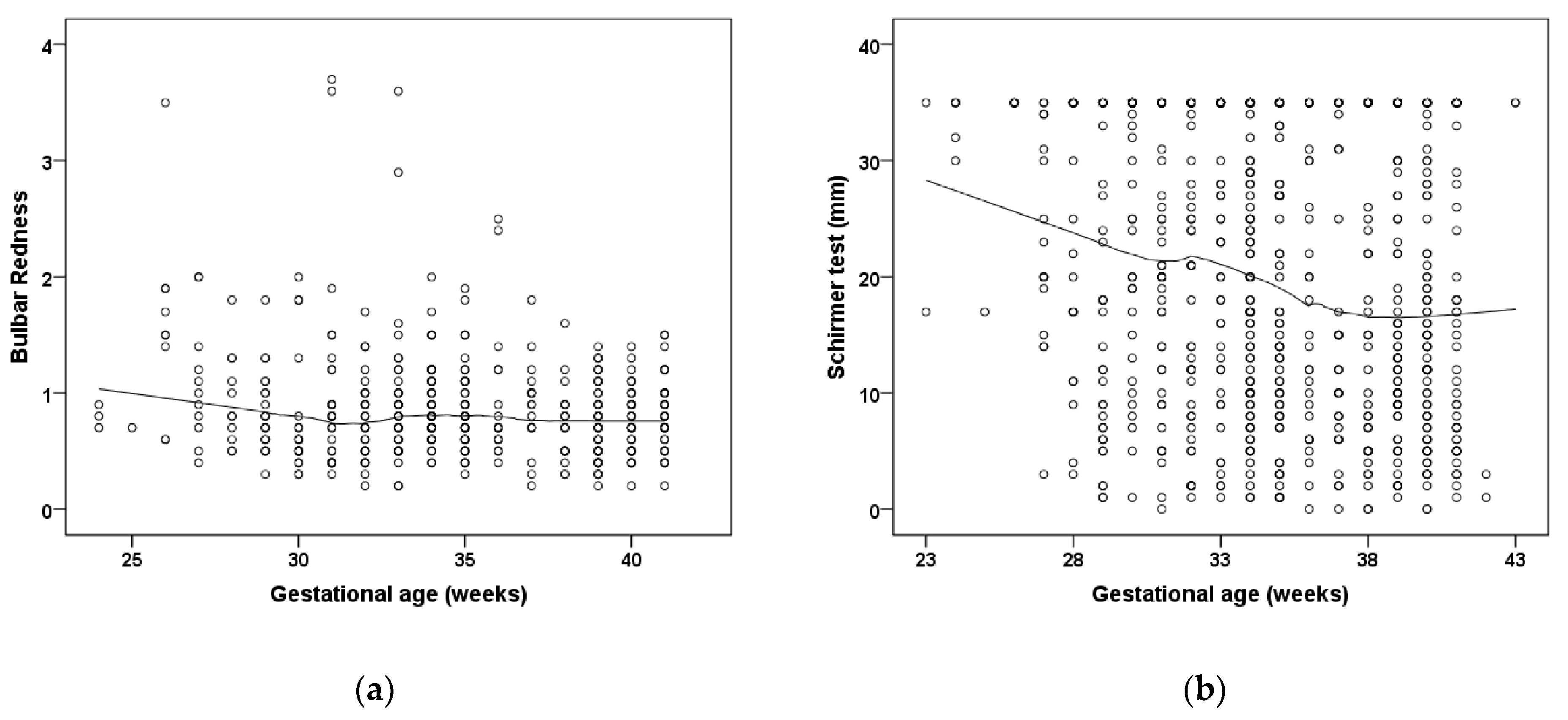
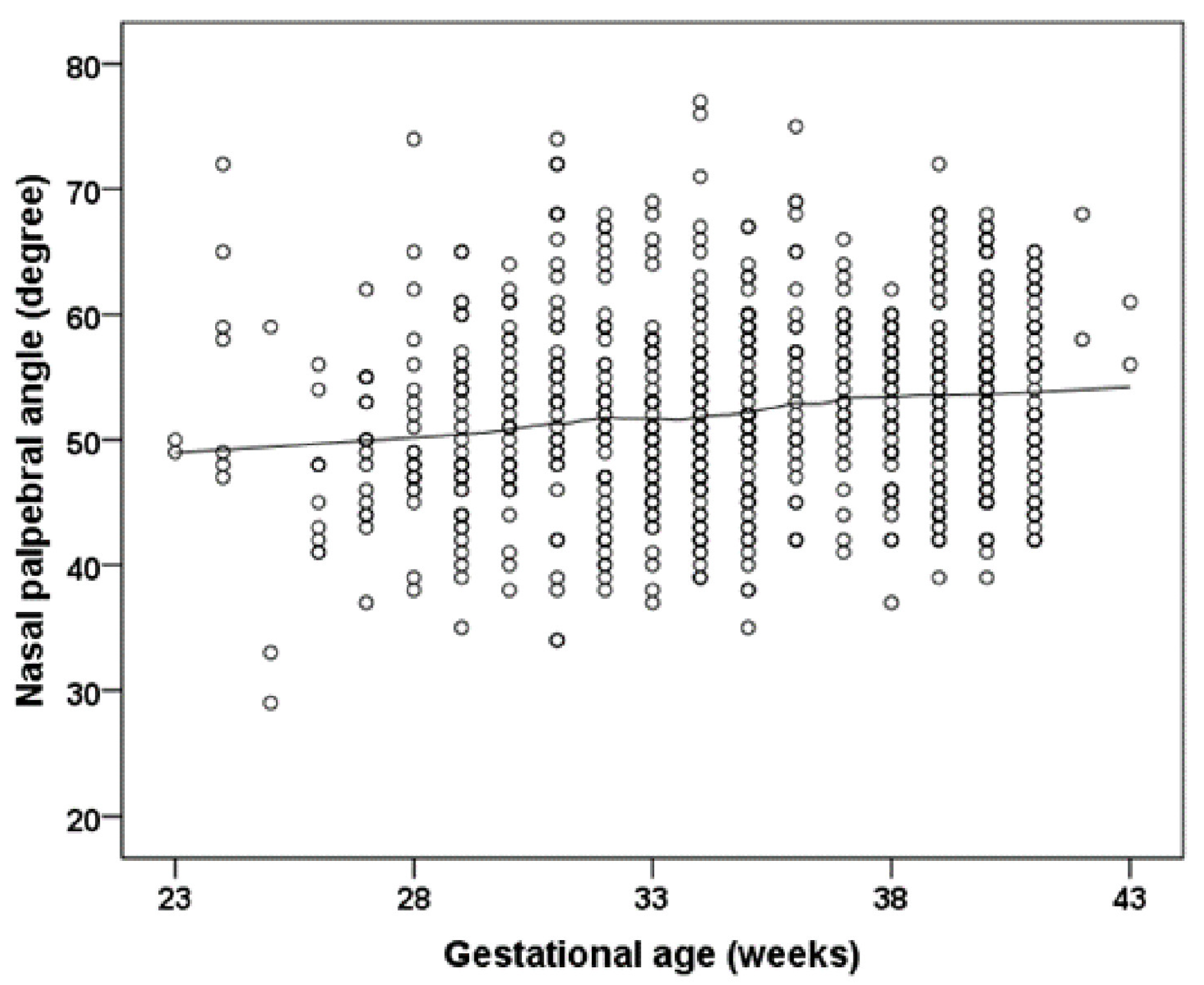
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational Age [Weeks] | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| no ROP | no ROP | no ROP | ROP | ROP with Treatment | ||
| Participants/eyes (n) | 139/277 | 129/256 | 70/133 | 14/28 | 33/56 | 9/16 |
| Sex (Women) (%) | 81 (58.3%) | 77 (59.7%) | 37 (52.9%) | 8 (57.1%) | 15 (45.5%) | 2 (22.2%) |
| Age (y) | 29.9 ± 9.2 | 29.3 ± 9.2 | 27.8 ± 8.1 | 23.9 ± 8.2 | 24.5 ± 5.3 | 27.9 ± 6.3 |
| Birth weight (g) | 3420 ± 393 | 2064 ± 473 | 1587 ± 347 | 930 ± 218 | 1120 ± 392 | 862 ± 304 |
| Birth weight < 1500 g (yes) | 0 (0%) | 13 (10.1%) | 28 (40%) | 14 (100%) | 28 (84.8%) | 9 (100%) |
| Birth weight < 1000 g (yes) | 0 (0%) | 0 (0%) | 4 (5.7%) | 7 (50%) | 12 (36.4%) | 6 (66.7%) |
| Birth weight percentile | 48.7 ± 21.4 | 25.2 ± 24.5 | 45.2 ± 23.8 | 41.1 ± 24.6 | 37.7 ± 29.4 | 24.2 ± 27.1 |
| Gestational age (wks) | 39.3 ± 1.3 | 34.3 ± 1.0 | 30.7 ± 1.1 | 26.8 ± 1.6 | 28.4 ± 1.9 | 27.4 ± 2.7 |
| (min–max) | (37–43) | (33–36) | (29–32) | (23–28) | (24–32) | (24–32) |
| ROP stage (1/2/3/4/5) | 0/0/0/0/0 | 0/0/0/0/0 | 0/0/0/0/0 | 0/0/0/0/0 | 25/25/5/0/1 | 0/2/14/0/0 |
| Preeclampsia (yes) | 11 (7.9%) | 23 (17.8%) | 8 (11.4%) | 2 (14.3%) | 7 (21.2%) | 2 (22.2%) |
| Placental insufficiency (yes) | 2 (1.4%) | 16 (12.4%) | 1 (1.4%) | 1 (7.1%) | 2 (6.1%) | 0 (0%) |
| HELLP-syndrome | 0 (0%) | 6 (4.7%) | 0 (0%) | 0 (0%) | 4 (12.1%) | 0 (0%) |
| Maternal smoking (yes) | 7 (5%) | 7 (5.4%) | 5 (7.1%) | 1 (7.1%) | 3 (9.1%) | 1 (11.1%) |
| Gestational diabetes (yes) | 1 (0.7%) | 6 (4.7%) | 1 (1.4%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Breastfeeding (yes) | 78 (56.1%) | 69 (53.5%) | 37 (52.9%) | 7 (50%) | 16 (48.5%) | 5 (55.6%) |
| Ocular parameters | ||||||
| Visual acuity (logMAR) OD | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.1) | 0.0 (0.0; 0.3) |
| Visual acuity (logMAR) OS | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.1 (0.0; 0.7) |
| Spherical equivalent (diopter) OD | −0.98 ± 2.2 | −1.10 ± 2.20 | −0.59 ± 2.19 | −0.9 ± 2.53 | −1.52 ± 3.39 | −5.12 ± 7.51 |
| Spherical equivalent (diopter) OS | −0.97 ± 2.09 | −1.16 ± 2.18 | −0.63 ± 2.19 | −0.45 ± 2.19 | −1.75 ± 3.41 | −4.26 ± 10.98 |
| Intraocular pressure (mmHg) OD | 15.3 ± 2.8 | 14.7 ± 2.9 | 14.6 ± 3.3 | 16.1 ± 3.0 | 15.8 ± 3.5 | 16.0 ± 4.1 |
| Intraocular pressure (mmHg) OS | 15.2 ± 2.8 | 14.5 ± 3.0 | 14.5 ± 3.1 | 14.7 ± 3.0 | 16.3 ± 3.7 | 17.0 ± 4.4 |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational Age [Weeks] | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| no ROP | no ROP | no ROP | ROP | ROP with Treatment | ||
| Participants/eyes (n) | 139/277 | 129/256 | 70/133 | 14/28 | 33/56 | 9/16 |
| Break-up time | ||||||
| First BUT OD | 20.7 ± 5.8 | 20.7 ± 6.2 | 20.2 ± 6.8 | 21.4 ± 5.4 | 19.6 ± 6.2 | 14.9 ± 7.8 # |
| First BUT OS | 21.6 ± 5.2 | 19.6 ± 6.8 # | 20.9 ± 5.9 | 20.3 ± 5.1 | 20.3 ± 5.1 | 12.2 ± 7.6 # |
| BUT ≤ 20 s OD + OS | 89 (33.0%) | 90 (36.0%) | 41 (32.3%) | 10 (37.0%) | 21 (42.0%) | 10 (71.4%) # |
| BUT ≤ 10 s OD + OS | 21 (7.8%) | 29 (11.6%) | 14 (11.0%) | 1 (3.7%) | 3 (6.0%) | 6 (42.9%) # |
| BUT ≤ 5 s OD + OS | 0 (0%) | 6 (2.4%) # | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7.1%) # |
| BUT grade OD | 13.9 ± 6.1 | 14.7 ± 5.9 | 13.9 ± 6.6 | 16.0 ± 5.0 | 14.0 ± 6.4 | 10.4 ± 5.3 |
| BUT grade OS | 15.4 ± 5.3 | 13.6 ± 5.9 # | 15.6 ± 6.0 | 14.4 ± 4.8 | 14.6 ± 4.6 | 8.5 ± 5.6 # |
| OSDI score | 4.73 ± 7.79 | 4.73 ± 7.47 | 3.58 ± 5.69 | 2.79 ± 6.09 | 4.14 ± 5.64 | 5.13 ± 10.16 |
| Available measurements OD/OS | 130/129 | 117/116 | 61/61 | 13/13 | 22/23 | 6/6 |
| Schirmer test OD (mm/5 min) | 17.6 ± 12.1 | 20.8 ± 11.8 # | 21.2 ± 12 | 26.9 ± 9.5 # | 19.3 ± 13 | 24.5 ± 12.1 |
| Schirmer test OS (mm/5 min) | 16.2 ± 11.3 | 20.1 ± 11.7 # | 20.7 ± 11.7 # | 28.5 ± 9.6 # | 21.1 ± 12.5 | 23.5 ± 12.1 |
| Schirmer test ≤ 10 mm/5 min OD + OS | 102 (39.2%) | 64 (27.2%) # | 31 (24.8%) # | 1 (3.8%) # | 12 (26.7%) # | 2 (18.2%) # |
| Schirmer test ≤ 5 mm/5 min OD + OS | 52 (20%) | 29 (12.3%) # | 15 (12%) # | 0 (0%) ** | 7 (15.6%) | 2 (18.2%) |
| Bulbar redness | ||||||
| Valid measurements participants OD/OS | 81/76 | 71/69 | 42/39 | 9/6 | 16/13 | 6/3 |
| Global bulbar redness OD | 0.82 ± 0.32 | 0.87 ± 0.4 | 0.87 ± 0.61 | 0.92 ± 0.32 | 1.15 ± 0.53 # | 1.08 ± 0.43 |
| Global bulbar redness OS | 0.72 ± 0.31 | 0.96 ± 0.56 # | 0.81 ± 0.59 | 0.97 ± 0.56 | 1.08 ± 0.89 | 0.9 ± 0.2 |
| Bulbar temporal OD | 0.7 ± 0.33 | 0.78 ± 0.35 | 0.75 ± 0.43 | 0.86 ± 0.32 | 0.92 ± 0.38 # | 1.0 ± 0.4 |
| Bulbar temporal OS | 0.66 ± 0.28 | 0.79 ± 0.38 # | 0.69 ± 0.34 | 0.78 ± 0.38 | 0.78 ± 0.34 | 1.03 ± 0.15 # |
| Bulbar nasal OD | 1.01 ± 0.4 | 0.96 ± 0.4 | 0.88 ± 0.48 | 1.12 ± 0.39 | 1.52 ± 0.78 # | 1.17 ± 0.52 |
| Bulbar nasal OS | 0.91 ± 0.45 | 1.05 ± 0.52 | 0.92 ± 0.49 | 1.42 ± 0.7 | 1.27 ± 0.82 | 1.13 ± 0.32 |
| Limbal temporal OD | 0.37 ± 0.28 | 0.43 ± 0.23 | 0.39 ± 0.28 | 0.62 ± 0.45 | 0.53 ± 0.3 # | 0.52 ± 0.34 |
| Limbal temporal OS | 0.39 ± 0.22 | 0.5 ± 0.31 # | 0.44 ± 0.29 | 0.5 ± 0.35 | 0.47 ± 0.29 | 0.53 ± 0.23 |
| Limbal nasal OD | 0.53 ± 0.3 | 0.5 ± 0.27 | 0.5 ± 0.35 | 0.61 ± 0.28 | 0.79 ± 0.48 | 0.67 ± 0.33 |
| Limbal nasal OS | 0.47 ± 0.31 | 0.58 ± 0.38 | 0.49 ± 0.39 | 0.9 ± 0.81 | 0.68 ± 0.53 | 0.87 ± 0.55 |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational Age [Weeks] | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| no ROP | no ROP | no ROP | ROP | Treated ROP | ||
| Available lid measurements Participants/eyes (n) | 134/268 | 125/245 | 64/125 | 14/28 | 29/50 | 8/15 |
| Palpebral fissure (mm) OD | 9.52 ± 1.11 | 9.41 ± 1.43 | 9.39 ± 1.19 | 9.25 ± 1.03 | 9.5 ± 1.17 | 8.85 ± 1.97 |
| Palpebral fissure (mm) OS | 9.17 ± 1.19 | 8.98 ± 1.56 | 9.11 ± 1.27 | 8.61 ± 1.41 | 9.36 ± 2.35 | 8.39 ± 2.34 |
| Nasal palpebral angle (degree) OD | 53.86 ± 6.83 | 52.46 ± 7.62 | 52.92 ± 8.78 | 51.43 ± 8.48 | 51.8 ± 6.41 | 47.88 ± 10.52 |
| Nasal palpebral angle (degree) OS | 53.47 ± 7.42 | 51.72 ± 7.6 | 52.49 ± 8.6 | 50.79 ± 6.93 | 47.68 ± 5.39 | 46.86 ± 13.23 |
| Bulbar area OD | 11.3 ± 4.3 | 10.9 ± 5.0 | 11.1 ± 4.9 | 10.4 ± 3.1 | 9.6 ± 2.5 # | 8.8 ± 3.6 |
| Bulbar area OS | 11.2 ± 4.6 | 9.8 ± 5.2 | 10.3 ± 5.2 | 9.7 ± 4.1 | 9.4 ± 4.4 | 8.1 ± 2.3 # |
| First Break-Up Time < 20 s (yes) | Univariate | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR [95% CI] | p | B [95% CI] | p | B [95% CI] | p | |
| Gestational age (weeks) | 0.981 (0.947; 1.017) | 0.29 | - | - | - | - |
| Birth weight (kg) | 0.878 (0.750; 1.028) | 0.88 | * | * | * | * |
| Birth weight percentile | 0.994 (0.988; 1.000) | 0.057 | - | - | - | - |
| ROP (yes) | 1.813 (1.083; 3.036) | 0.024 | - | - | 1.425 (0.783; 2.591) | 0.25 |
| ROP treatment (yes) | 4.711 (1.463; 15.17) | 0.009 | - | - | 4.421 (1.200; 16.28) | 0.025 |
| Placental insufficiency (yes) | 1.204 (0.617; 2.349) | 0.59 | - | - | - | - |
| Preeclampsia (yes) | 1.389 (0.896; 2.153) | 0.14 | - | - | - | - |
| Breastfeeding (yes) | 0.962 (0.711; 1.301) | 0.80 | - | - | - | - |
| Smoking pregnancy (yes) | 1.144 (0.623; 2.103) | 0.66 | - | - | - | - |
| Bulbar redness | B [95% CI] | p | B [95% CI] | p | B [95% CI] | p |
| Gestational age (weeks) | −0.018 (−0.03; −0.006) | 0.004 | −0.019 (−0.030; −0.008) | <0.001 | −0.015 (−0.027; −0.003) | 0.011 |
| Birth weight (kg) | −0.076 (−0.127; −0.026) | 0.003 | * | * | * | * |
| Birth weight percentile | −0.002 (−0.003; 0) | 0.098 | - | - | - | - |
| ROP (yes) | 0.202 (−0.001; 0.405) | 0.05 | - | - | 0.108 (−0.102; 0.319) | 0.31 |
| ROP treatment (yes) | 0.147 (−0.097; 0.390) | 0.24 | - | - | - | - |
| Placental insufficiency (yes) | −0.067 (−0.255; 0.121) | 0.49 | - | - | - | - |
| Preeclampsia (yes) | 0.055 (−0.098; 0.207) | 0.48 | - | - | - | - |
| Breastfeeding (yes) | −0.087 (−0.193; 0.018) | 0.10 | - | - | - | - |
| Smoking pregnancy (yes) | −0.135 (−0.328; 0.058) | 0.17 | - | - | - | - |
| Wetting Schirmer test (mm) | B [95% CI] | p | B [95% CI] | p | B [95% CI] | p |
| Gestational age (weeks) | −1.067 (−1.638; −0.496) | <0.001 | −0.692 (−1.158; −0.226) | 0.003 | ||
| Birth weight (kg) | −4.918 (−7.330; −2.506) | 0.001 | * | * | ||
| Birth weight percentile | 0.048 (−0.068; 0.163) | 0.41 | - | - | ||
| ROP (yes) | 3.0 (−3.137; 9.137) | 0.34 | - | - | ||
| ROP treatment (yes) | 3.0 (−13.203; 19.203) | 0.22 | - | - | ||
| Placental insufficiency (yes) | 12.0 (1.259; 22.74) | 0.029 | 8.596 (4.643; 12.55) | <0.001 | ||
| Preeclampsia (yes) | 0.0 (−10.73; 10.73) | 1.0 | - | - | ||
| Breastfeeding (yes) | 3.0 (−2.514; 8.515) | 0.29 | - | - | ||
| Smoking pregnancy (yes) | 8.0 (−1.875; 17.87) | 0.11 | - | - | ||
| Nasal palpebral angle (degree) | B [95% CI] | p | B [95% CI] | p | B [95% CI] | p |
| Gestational age (weeks) | 0.266 (0.098; 0.435) | 0.002 | 0.220 (0.050; 0.390) | 0.011 | 0.142 (0.039; 0.323) | 0.125 |
| Birth weight (kg) | 1.159 (0.418; 1.9) | 0.002 | * | * | * | * |
| Birth weight percentile | 0.019 (−0.009; 0.048) | 0.19 | - | - | - | - |
| ROP (yes) | −3.643 (−5.998; −1.288) | 0.002 | - | - | −2.384 (−4.986; 0.219) | 0.073 |
| ROP treatment (yes) | −5.175 (−11.934; 1.584) | 0.13 | - | - | - | - |
| Placental insufficiency (yes) | −0.555 (−4.721; 3.611) | 0.79 | - | - | - | - |
| Preeclampsia (yes) | 2.31 (0.107; 4.513) | 0.040 | 2.360 (0.177; 4.544) | 0.034 | 2.492 (0.320; 4.664) | 0.025 |
| Breastfeeding (yes) | −0.668 (−2.098; 0.762) | 0.36 | - | - | - | - |
| Smoking pregnancy (yes) | 0.622 (−2.422; 3.667) | 0.69 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fieß, A.; Hufschmidt-Merizian, C.; Gißler, S.; Hampel, U.; Mildenberger, E.; Urschitz, M.S.; Zepp, F.; Stoffelns, B.; Pfeiffer, N.; Schuster, A.K. Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study. J. Clin. Med. 2022, 11, 2702. https://doi.org/10.3390/jcm11102702
Fieß A, Hufschmidt-Merizian C, Gißler S, Hampel U, Mildenberger E, Urschitz MS, Zepp F, Stoffelns B, Pfeiffer N, Schuster AK. Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study. Journal of Clinical Medicine. 2022; 11(10):2702. https://doi.org/10.3390/jcm11102702
Chicago/Turabian StyleFieß, Achim, Clara Hufschmidt-Merizian, Sandra Gißler, Ulrike Hampel, Eva Mildenberger, Michael S. Urschitz, Fred Zepp, Bernhard Stoffelns, Norbert Pfeiffer, and Alexander K. Schuster. 2022. "Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study" Journal of Clinical Medicine 11, no. 10: 2702. https://doi.org/10.3390/jcm11102702
APA StyleFieß, A., Hufschmidt-Merizian, C., Gißler, S., Hampel, U., Mildenberger, E., Urschitz, M. S., Zepp, F., Stoffelns, B., Pfeiffer, N., & Schuster, A. K. (2022). Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study. Journal of Clinical Medicine, 11(10), 2702. https://doi.org/10.3390/jcm11102702






