Artificial Intelligence in Laryngeal Endoscopy: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods, Types of Studies, and Participants
2.2. Index Tests and Target Conditions
2.3. Data Collection and Analysis/Selection Process
2.4. Risk of Bias Assessment
2.5. Statistical Analysis and Data Synthesis
- (1)
- Analysis of the overall accuracy of AI in assessing laryngeal lesions;
- (2)
- The ability of AI to identify healthy tissue;
- (3)
- The ability of AI to differentiate benign lesions from potentially malignant and malignant ones;
- (4)
- Analysis of diagnostic performance of AI using NBI and WLE images.
3. Results
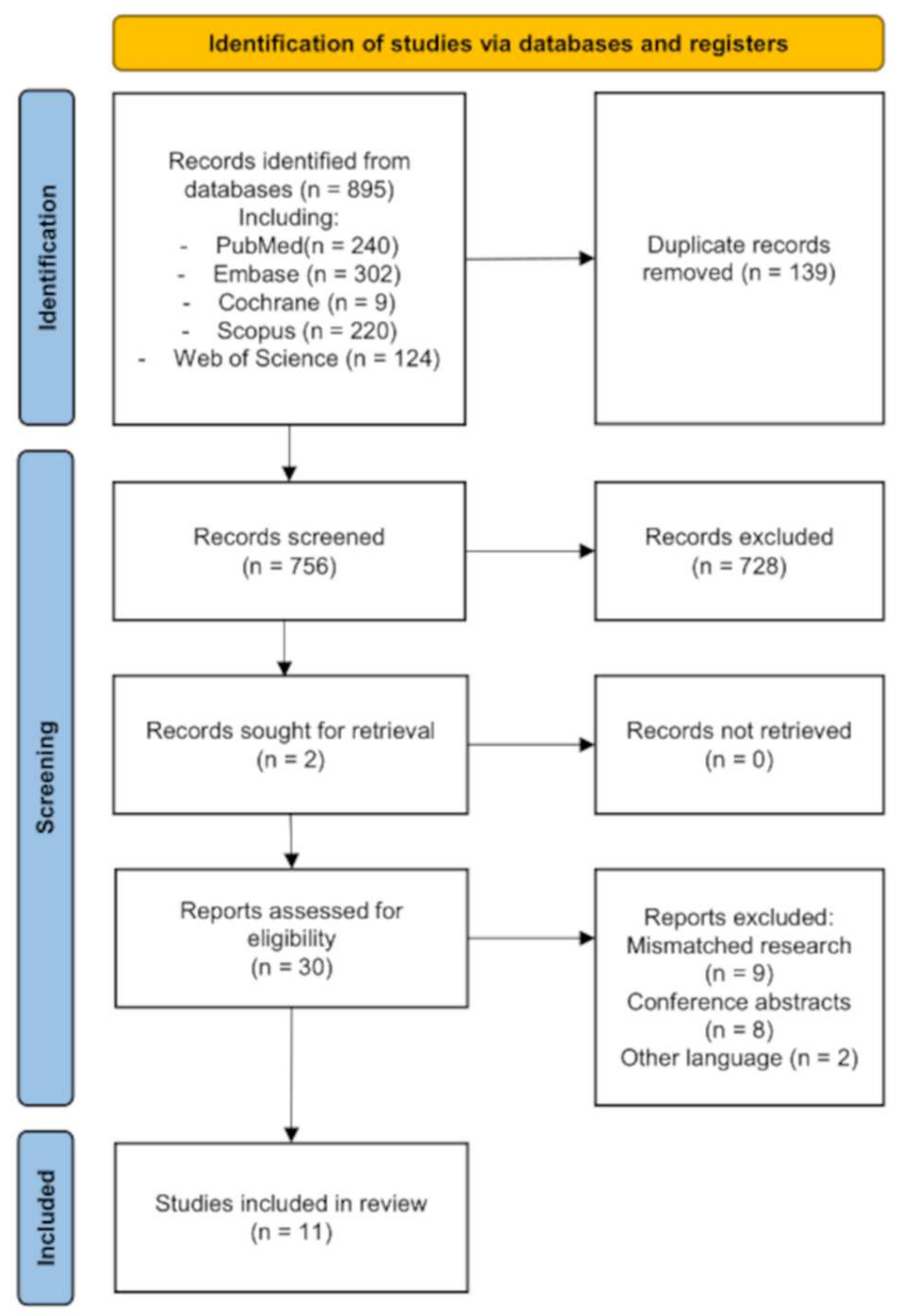
3.1. Results of the Search
3.2. Risk of Bias Assessment
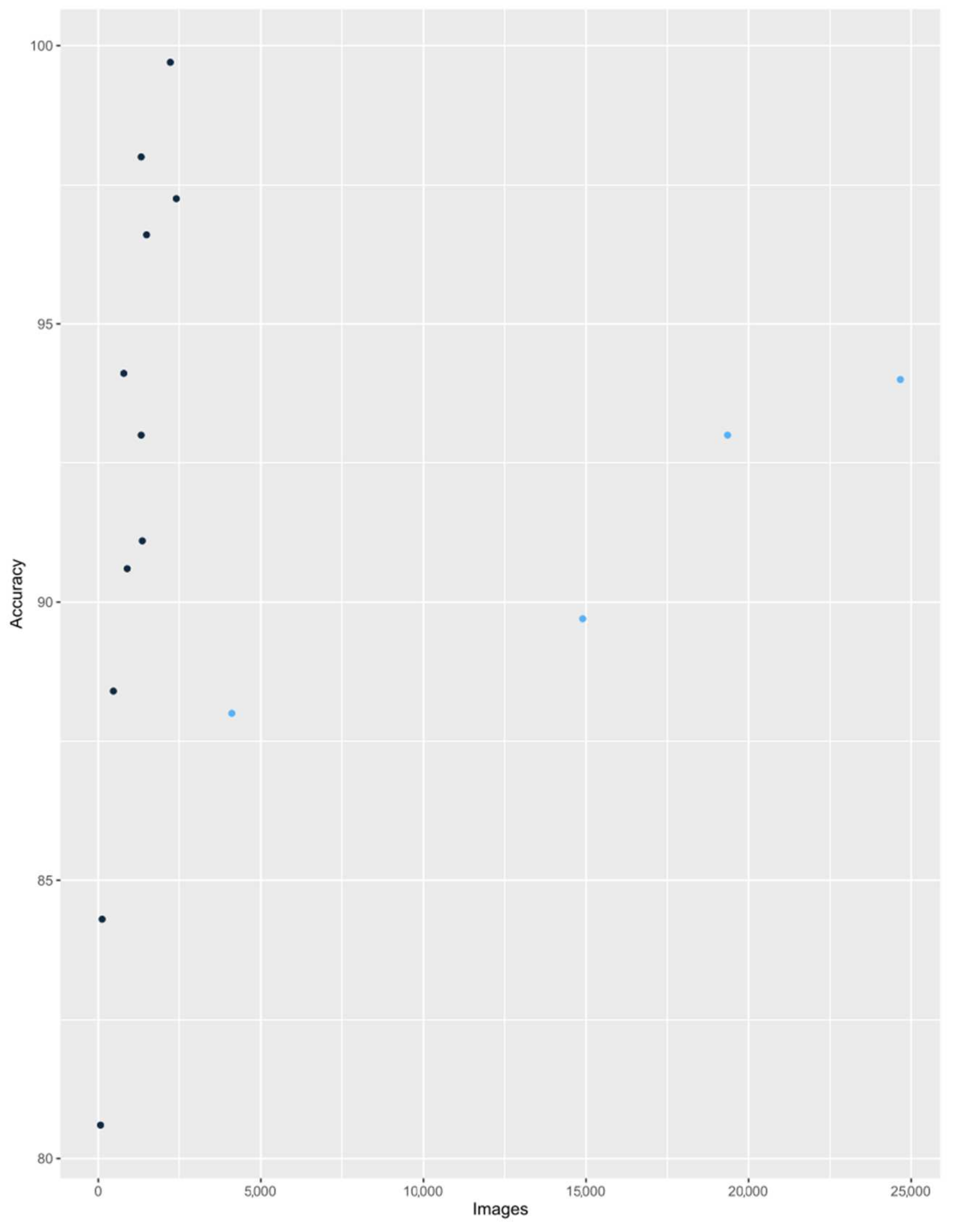
3.3. Diagnostic Accuracy of AI in Assessment of Laryngeal Lesions
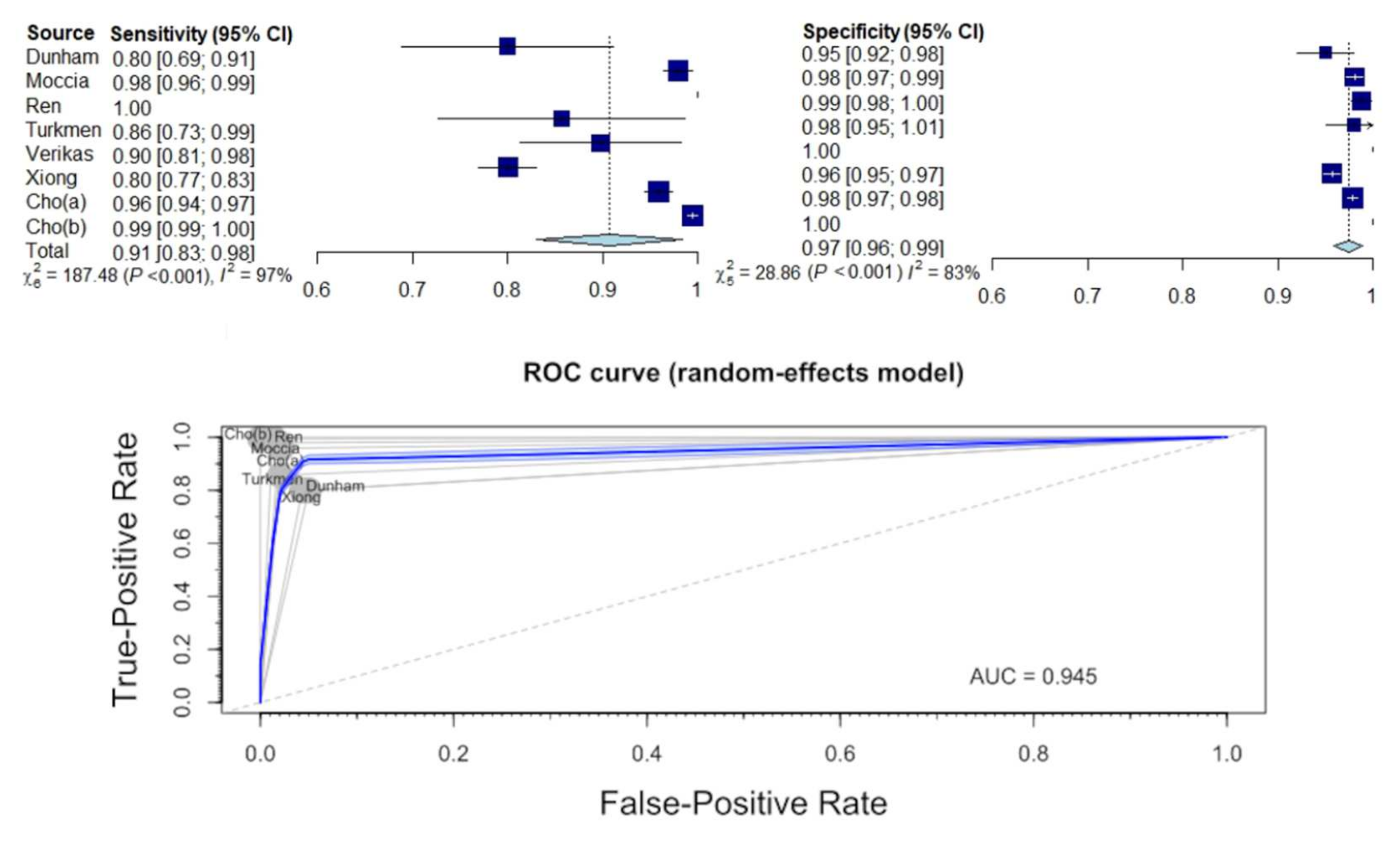
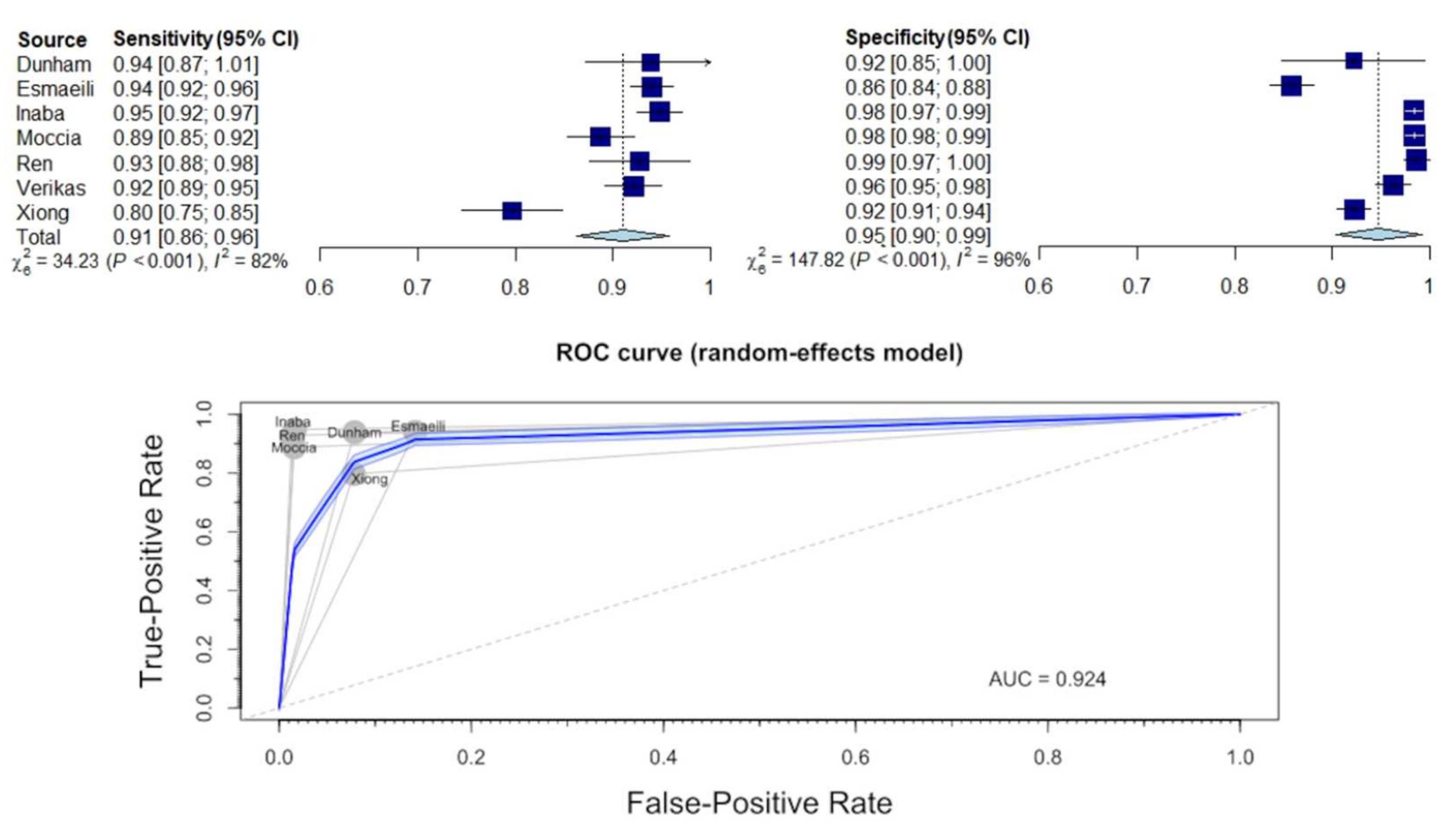
3.4. Diagnostic Sensitivity and Specificity for Identification of Normal Tissue
3.5. Diagnostic Sensitivity and Specificity for Distinguishing between Benign and Malignant Lesions
3.6. Comparison of Diagnostics Using WL and NBI
4. Discussion
4.1. Main Findings
4.2. Association with Other Studies
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hrelec, C. Management of Laryngeal Dysplasia and Early Invasive Cancer. Curr. Treat. Options Oncol. 2021, 22, 90. [Google Scholar] [CrossRef]
- Mannelli, G.; Cecconi, L.; Gallo, O. Laryngeal preneoplastic lesions and cancer: Challenging diagnosis. Qualitative literature review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 106, 64–90. [Google Scholar] [CrossRef]
- Naunheim, M.R.; Carroll, T.L. Benign vocal fold lesions: Update on nomenclature, cause, diagnosis, and treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 453–458. [Google Scholar] [CrossRef]
- Obid, R.; Redlich, M.; Tomeh, C. The Treatment of Laryngeal Cancer. Oral Maxillofac. Surg. Clin. North Am. 2018, 31, 1–11. [Google Scholar] [CrossRef]
- Levendoski, E.E.; Leydon, C.; Thibeault, S.L. Vocal fold epithelial barrier in health and injury: A research review. J. Speech Lang. Hearth Res. 2014, 57, 1679–1691. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.; Kim, S.W.; Hwang, S.H. Use of narrowband imaging for the diagnosis and screening of laryngeal cancer: A systematic review and meta-analysis. Head Neck 2020, 42, 2635–2643. [Google Scholar] [CrossRef]
- Alonso-Coello, P.; Rigau, D.; Sanabria, A.J.; Plaza, V.; Miravitlles, M.; Martinez, L. Quality and strength: The GRADE system for formulating recommendations in clinical practice guidelines. Arch. Bronconeumol. 2013, 49, 261–267. [Google Scholar] [CrossRef]
- Krausert, C.R.; Olszewski, A.E.; Taylor, L.N.; McMurray, J.S.; Dailey, S.H.; Jiang, J.J. Mucosal wave measurement and visualization techniques. J. Voice 2011, 25, 395–405. [Google Scholar] [CrossRef]
- Puxeddu, R.; Sionis, S.; Gerosa, C.; Carta, F. Enhanced contact endoscopy for the detection of neoangiogenesis in tumors of the larynx and hypopharynx. Laryngoscope 2015, 125, 1600–1606. [Google Scholar] [CrossRef]
- Eckel, H.E.; Simo, R.; Quer, M.; Odell, E.; Paleri, V.; Klussmann, J.P.; Remacle, M.; Sjögren, E.; Piazza, C. European Laryngological Society position paper on laryngeal dysplasia Part II: Diagnosis, treatment, and follow-up. Eur. Arch. Otorhinolaryngol. 2021, 278, 1723–1732. [Google Scholar] [CrossRef]
- Stanikova, L.; Walderova, R.; Jancatova, D.; Formanek, M.; Zelenik, K.; Kominek, P. Comparison of narrow band imaging and the Storz Professional Image Enhancement System for detection of laryngeal and hypopharyngeal pathologies. Eur. Arch. Otorhinolaryngol. 2018, 275, 1819–1825. [Google Scholar] [CrossRef]
- Bergström, L.W.E.; Finizia, C. The impact of laryngeal biopsy on voice outcomes: A pilot study. Otorhinolaryngol. Head Neck Surg. 2016, 1, 33–37. [Google Scholar] [CrossRef][Green Version]
- Zurek, M.; Rzepakowska, A.; Osuch-Wojcikiewicz, E.; Niemczyk, K. Learning curve for endoscopic evaluation of vocal folds lesions with narrow band imaging. Braz. J. Otorhinolaryngol. 2019, 85, 753–759. [Google Scholar] [CrossRef]
- Kaul, V.; Enslin, S.; Gross, S.A. History of artificial intelligence in medicine. Gastrointest Endosc 2020, 92, 807–812. [Google Scholar] [CrossRef]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Ni, X.G.; He, S.; Xu, Z.G.; Gao, L.; Lu, N.; Yuan, Z.; Lai, S.Q.; Zhang, Y.M.; Yi, J.L.; Wang, X.L.; et al. Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. J. Laryngol. Otol. 2011, 125, 288–296. [Google Scholar] [CrossRef]
- Araújo, T.; Santos, C.P.; De Momi, E.; Moccia, S. Learned and handcrafted features for early-stage laryngeal SCC diagnosis. Med. Biol. Eng. Comput. 2019, 57, 2683–2692. [Google Scholar] [CrossRef]
- Barbalata, C.; Mattos, L.S. Laryngeal Tumor Detection and Classification in Endoscopic Video. IEEE J. Biomed. Health Inf. 2016, 20, 322–332. [Google Scholar] [CrossRef]
- Cho, W.K.; Lee, Y.J.; Joo, H.A.; Jeong, I.S.; Choi, Y.; Nam, S.Y.; Kim, S.Y.; Choi, S.H. Diagnostic Accuracies of Laryngeal Diseases Using a Convolutional Neural Network-Based Image Classification System. Laryngoscope 2021, 131, 2558–2566. [Google Scholar] [CrossRef]
- Dunham, M.E.; Kong, K.A.; McWhorter, A.J.; Adkins, L.K. Optical Biopsy: Automated Classification of Airway Endoscopic Findings Using a Convolutional Neural Network. Laryngoscope 2020, 132, S1–S8. [Google Scholar] [CrossRef]
- Esmaeili, N.; Illanes, A.; Boese, A.; Davaris, N.; Arens, C.; Friebe, M. Novel automated vessel pattern characterization of larynx contact endoscopic video images. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1751–1761. [Google Scholar] [CrossRef]
- Inaba, A.; Hori, K.; Yoda, Y.; Ikematsu, H.; Takano, H.; Matsuzaki, H.; Watanabe, Y.; Takeshita, N.; Tomioka, T.; Ishii, G.; et al. Artificial intelligence system for detecting superficial laryngopharyngeal cancer with high efficiency of deep learning. Head Neck 2020, 42, 2581–2592. [Google Scholar] [CrossRef]
- Moccia, S.; De Momi, E.; Guarnaschelli, M.; Savazzi, M.; Laborai, A.; Guastini, L.; Peretti, G.; Mattos, L.S. Confident texture-based laryngeal tissue classification for early stage diagnosis support. J. Med. Imaging 2017, 4, 034502. [Google Scholar] [CrossRef]
- Ren, J.J.; Jing, X.P.; Wang, J.; Ren, X.; Xu, Y.; Yang, Q.Y.; Ma, L.Z.; Sun, Y.; Xu, W.; Yang, N.; et al. Automatic Recognition of Laryngoscopic Images Using a Deep-Learning Technique. Laryngoscope 2020, 130, E686–E693. [Google Scholar] [CrossRef]
- Turkmen, H.I.; Karsligil, M.E.; Kocak, I. Classification of laryngeal disorders based on shape and vascular defects of vocal folds. Comput. Biol. Med. 2015, 62, 76–85. [Google Scholar] [CrossRef]
- Cho, W.K.; Choi, S.H. Comparison of Convolutional Neural Network Models for Determination of Vocal Fold Normality in Laryngoscopic Images. J. Voice 2020, in press. [Google Scholar] [CrossRef]
- Xiong, H.; Lin, P.L.; Yu, J.G.; Ye, J.; Xiao, L.C.; Tao, Y.; Jiang, Z.B.; Lin, W.; Liu, M.Y.; Xu, J.J.; et al. Computer-aided diagnosis of laryngeal cancer via deep learning based on laryngoscopic images. Ebiomedicine 2019, 48, 92–99. [Google Scholar] [CrossRef]
- Davaris, N.; Voigt-Zimmermann, S.; Kropf, S.; Arens, C. Flexible transnasal endoscopy with white light or narrow band imaging for the diagnosis of laryngeal malignancy: Diagnostic value, observer variability and influence of previous laryngeal surgery. Eur. Arch. Otorhinolaryngol. 2019, 276, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Guo, L.; Nie, J.; Zhu, C.; Ma, X. The value of narrow band imaging in diagnosis of head and neck cancer: A meta-analysis. Sci. Rep. 2018, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Pietruszewska, W.; Morawska, J.; Rosiak, O.; Leduchowska, A.; Klimza, H.; Wierzbicka, M. Vocal Fold Leukoplakia: Which of the Classifications of White Light and Narrow Band Imaging Most Accurately Predicts Laryngeal Cancer Transformation? Proposition for a Diagnostic Algorithm. Cancers 2021, 13, 3273. [Google Scholar] [CrossRef] [PubMed]
- Satankova, J.; Stanikova, L.; Svejdova, A.; Cerny, M.; Laco, J.; Chrobok, V. Diagnostic Value of Narrow Band Imaging in Visualization of Pathological Lesions in Larynx and Hypopharynx. Acta Med. 2021, 64, 22–28. [Google Scholar]
- Rzepakowska, A.; Sielska-Badurek, E.; Cruz, R.; Sobol, M.; Osuch-Wojcikiewicz, E.; Niemczyk, K. Narrow band imaging versus laryngovideostroboscopy in precancerous and malignant vocal fold lesions. Head Neck 2018, 40, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Zwakenberg, M.A.; Halmos, G.B.; Wedman, J.; van der Laan, B.; Plaat, B.E.C. Evaluating Laryngopharyngeal Tumor Extension Using Narrow Band Imaging Versus Conventional White Light Imaging. Laryngoscope 2021, 131, E2222–E2231. [Google Scholar] [CrossRef] [PubMed]
- Popek, B.; Bojanowska-Pozniak, K.; Tomasik, B.; Fendler, W.; Jeruzal-Swiatecka, J.; Pietruszewska, W. Clinical experience of narrow band imaging (NBI) usage in diagnosis of laryngeal lesions. Otolaryngol. Pol. 2019, 73, 18–23. [Google Scholar] [CrossRef]
- Nogues-Sabate, A.; Aviles-Jurado, F.X.; Ruiz-Sevilla, L.; Lehrer, E.; Santamaria-Gadea, A.; Valls-Mateus, M.; Vilaseca, I. Intra and interobserver agreement of narrow band imaging for the detection of head and neck tumors. Eur. Arch. Otorhinolaryngol. 2018, 275, 2349–2354. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A.; GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group. 2013. Available online: https://guidelinedevelopment.org/handbook (accessed on 31 October 2021).


| PICOS Framework | |
|---|---|
| Population | Patients (without any age limit) who underwent laryngeal endoscopic examination |
| Intervention | Evaluation of endoscopy images by AI |
| Comparison | Histopathology or histopathology with specialist assessment |
| Outcome | Classification of laryngeal lesions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurek, M.; Jasak, K.; Niemczyk, K.; Rzepakowska, A. Artificial Intelligence in Laryngeal Endoscopy: Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2752. https://doi.org/10.3390/jcm11102752
Żurek M, Jasak K, Niemczyk K, Rzepakowska A. Artificial Intelligence in Laryngeal Endoscopy: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(10):2752. https://doi.org/10.3390/jcm11102752
Chicago/Turabian StyleŻurek, Michał, Kamil Jasak, Kazimierz Niemczyk, and Anna Rzepakowska. 2022. "Artificial Intelligence in Laryngeal Endoscopy: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 10: 2752. https://doi.org/10.3390/jcm11102752
APA StyleŻurek, M., Jasak, K., Niemczyk, K., & Rzepakowska, A. (2022). Artificial Intelligence in Laryngeal Endoscopy: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(10), 2752. https://doi.org/10.3390/jcm11102752






