Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect
Abstract
:1. Introduction
2. Imaging and Ablation Treatment
3. Complications and Risk Assessment
4. Radiologist: What Do We Expect?
5. Radiologists: How We Should Report
6. RFA, MWA and Other Treatments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ottaiano, A.; Circelli, L.; Santorsola, M.; Savarese, G.; Fontanella, D.; Gigantino, V.; Di Mauro, A.; Capuozzo, M.; Zappavigna, S.; Lombardi, A.; et al. Metastatic colorectal cancer and type 2 diabetes: Prognostic and genetic interactions. Mol. Oncol. 2021, 16, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.; et al. Critical role of bevacizumab scheduling in combination with pre-surgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the branch trial. Oncotarget 2015, 6, 30394–30407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimonte, S.; Leongito, M.; Barbieri, A.; Del Vecchio, V.; Barbieri, M.; Albino, V.; Piccirillo, M.; Amore, A.; Di Giacomo, R.; Nasto, A.; et al. Inhibitory effect of (−)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect. Agents Cancer 2015, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women with Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Filice, S.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Petrillo, A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect. Agents Cancer 2018, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Rega, D.; Pace, U.; Scala, D.; Chiodini, P.; Granata, V.; Bucci, A.F.; Pecori, B.; DelRio, P. Treatment of splenic flexure colon cancer: A comparison of three different surgical procedures: Experience of a high volume cancer center. Sci. Rep. 2019, 9, 10953. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef] [Green Version]
- Petrillo, A.; Fusco, R.; Petrillo, M.; Granata, V.; Delrio, P.; Bianco, F.; Pecori, B.; Botti, G.; Tatangelo, F.; Caracò, C.; et al. Standardized Index of Shape (DCE-MRI) and Standardized Uptake Value (PET/CT): Two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2017, 8, 8143–8153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A.; Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect. Agents Cancer 2017, 12, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commander, C.W.; Wilson, S.B.; Bilaj, F.; Isaacson, A.J.; Burke, C.T.; Yu, H. CT-Guided Percutaneous Drainage Catheter Placement in the Abdomen and Pelvis: Predictors of Outcome and Protocol for Follow-up. J. Vasc. Interv. Radiol. 2020, 31, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, R.; Maruzzelli, L.; Cannataci, C.; Gerasia, R.; Mamone, G.; Cortis, K.; Cimò, B.; Petridis, I.; Volpes, R.; Luca, A. Radiation exposure during transjugular intrahepatic portosystemic shunt creation in patients with complete portal vein thrombosis or portal cavernoma. Radiol. Med. 2020, 125, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; Seoane, E.B.; Cannavale, A.; de Haan, M.W.; Dezman, R.; Kloeckner, R.; O’Sullivan, G.; Ryan, A.; Tsoumakidou, G. CIRSE Clinical Practice Manual. Cardiovasc. Interv. Radiol. 2021, 44, 1323–1353. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Petrillo, M.; Fusco, R.; Setola, S.V.; De Lutio Di Castelguidone, E.; Catalano, O.; Piccirillo, M.; Albino, V.; Izzo, F.; Petrillo, A. Surveillance of HCC Patients after Liver RFA: Role of MRI with Hepatospecific Contrast versus Three-Phase CT Scan—Experience of High Volume Oncologic Institute. Gastroenterol. Res. Pract. 2013, 2013, 469097. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; van Tilborg, A.A.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Vlayen, J. Radiofrequency and Mi- crowave Ablation Compared to Systemic Chemo-therapy and to Partial Hepa- tectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Intervent. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef] [Green Version]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.; Pierie, J.P.E.; Borel-Rinkes, I.; Ledermann, J.A.; Nordlinger, B. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Random-ized Phase II Trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef]
- Ruffino, M.A.; Fronda, M.; Bergamasco, L.; Natrella, M.; Fanelli, G.; Bellosta, R.; Pegorer, M.; Attisani, L.; Ruggiero, M.; Malfa, P.; et al. Prognostic risk factors for loss of patency after femoropopliteal bailout stenting with dual-component stent: Results from the TIGRIS Italian Multicenter Registry. Radiol. Med. 2021, 126, 1129–1137. [Google Scholar] [CrossRef]
- Giurazza, F.; Contegiacomo, A.; Calandri, M.; Mosconi, C.; Modestino, F.; Corvino, F.; Scrofani, A.R.; Marra, P.; Coniglio, G.; Failla, G.; et al. IVC filter retrieval: A multicenter proposal of two score systems to predict application of complex technique and procedural outcome. Radiol. Med. 2021, 126, 1007–1016. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous Ablation Therapy of Hepatocellular Carcinoma with Irreversible Electroporation: MRI Findings. Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Cutolo, C.; Pradella, S.; Grazzini, G.; La Porta, M.; Brunese, M.C.; De Muzio, F.; et al. Diagnostic evaluation and ablation treatments assessment in hepatocellular carcinoma. Infect. Agents Cancer 2021, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Salati, S.; Petrillo, A.; Di Bernardo, E.; Grassi, R.; Palaia, R.; Danti, G.; La Porta, M.; Cadossi, M.; et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. Int. J. Environ. Res. Public Health 2021, 18, 5592. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [Green Version]
- Puijk, R.S.; Ruarus, A.H.; Scheffer, H.J.; Vroomen, L.G.P.H.; Van Tilborg, A.A.J.M.; De Vries, J.J.; Berger, F.H.; van den Tol, P.M.P.; Meijerink, M.R. Percutaneous Liver Tumour Ablation: Image Guidance, Endpoint Assessment, and Quality Control. Can. Assoc. Radiol. J. 2018, 69, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Cool, D.; Kakani, N.; Wang, G.; Ding, H.; Fenster, A. Automatic Radiofrequency Ablation Planning for Liver Tumors with Multiple Constraints Based on Set Covering. IEEE Trans. Med. Imaging 2020, 39, 1459–1471. [Google Scholar] [CrossRef]
- Cassinotto, C.; Denys, A.; Gay, F.; Duran, R.; Hocquelet, A.; Piron, L.; Guiu, B. Radiofrequency Ablation of Liver Tumors: No Difference in the Ablation Zone Volume Between Cirrhotic and Healthy Liver. Cardiovasc. Interv. Radiol. 2018, 41, 905–911. [Google Scholar] [CrossRef]
- Jiang, A.-N.; Wang, S.; Yang, W.; Zhao, K.; Bai, X.-M.; Zhang, Z.-Y.; Wu, W.; Chen, M.-H.; Yan, K. The Role of a Curved Electrode with Controllable Direction in the Radiofrequency Ablation of Liver Tumors Behind Large Vessels. Cardiovasc. Interv. Radiol. 2019, 42, 893–904. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef]
- Li, J.; Cao, B.; Bi, X.; Chen, W.; Wang, L.; Du, Z.; Zhang, X.; Yu, X. Evaluation of liver function in patients with chronic hepatitis B using Gd-EOB-DTPA-enhanced T1 mapping at different acquisition time points: A feasibility study. Radiol. Med. 2021, 126, 1149–1158. [Google Scholar] [CrossRef]
- Esposito, A.; Buscarino, V.; Raciti, D.; Casiraghi, E.; Manini, M.; Biondetti, P.; Forzenigo, L. Characterization of liver nodules in patients with chronic liver disease by MRI: Performance of the Liver Imaging Reporting and Data System (LI-RADS v.2018) scale and its comparison with the Likert scale. Radiol. Med. 2019, 125, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agents Cancer 2021, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Bicchierai, G.; Fusco, R.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6499–6528. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Petrillo, A. Additional Considerations on Use of Abbreviated Liver MRI in Patients with Colorectal Liver Metastases. Am. J. Roentgenol. 2021, 217, W2–W3. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Piccirillo, M.; Palaia, R.; Nasti, G.; Petrillo, A.; Izzo, F. A radiologist’s point of view in the presurgical and intraoperative setting of colorectal liver metastases. Futur. Oncol. 2018, 14, 2189–2206. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Crocetti, L. Radiofrequency Ablation of Liver Cancer. Tech. Vasc. Interv. Radiol. 2007, 10, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Carrafiello, G.; Laganà, D.; Mangini, M.; Fontana, F.; Dionigi, G.; Boni, L.; Fugazzola, C. Microwave tumors ablation: Principles, clinical applications and review of prelimi-nary experiences. Int. J. Surg. 2008, 6, S65–S69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Palaia, R.; Belli, A.; Miele, V.; Brunese, L.; Petrillo, A.; Izzo, F. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist’s Challenge. Front. Oncol. 2020, 10, 560952. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef]
- Granata, V.; Castelguidone, E.D.L.D.; Fusco, R.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Gallipoli, A.D.; Petrillo, A. Irreversible electroporation of hepatocellular carcinoma: Preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol. Med. 2016, 121, 122–131. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Palaia, R.; Carrafiello, G.; Miele, V.; Petrillo, A.; Izzo, F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J. Gastroenterol. 2021, 27, 3413–3428. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Palaia, R.; Albino, V.; Amore, A.; Di Giacomo, R.; Piccirillo, M.; Leongito, M.; Nasto, A.; Granata, V.; Petrillo, A.; et al. Hepatocellular carcinoma and liver metastases: Clinical data on a new dual-lumen catheter kit for surgical sealant infusion to prevent perihepatic bleeding and dissemination of cancer cells following biopsy and loco-regional treatments. Infect. Agents Cancer 2015, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Maura, C.T.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21, S78–S82. [Google Scholar] [CrossRef]
- Vahldiek, J.L.; Erxleben, C.; Bressem, K.K.; Gemeinhardt, O.; Poch, F.; Hiebl, B.; Lehmann, K.S.; Hamm, B.; Niehues, S.M. Multipolar RFA of the liver: Influence of intrahepatic vessels on ablation zones and appropriateness of CECT in detecting ablation dimensions—Results of an in-vivo porcine liver model. Clin. Hemorheol. Microcirc. 2019, 70, 467–476. [Google Scholar] [CrossRef]
- Pfannenstiel, A.; Sebek, J.; Fallahi, H.; Beard, W.L.; Ganta, C.K.; Dupuy, D.E.; Prakash, P. Directional Microwave Ablation: Experimental Evaluation of a 2.45-GHz Applicator in Ex Vivo and In Vivo Liver. J. Vasc. Interv. Radiol. 2020, 31, 1170–1177.e2. [Google Scholar] [CrossRef]
- Vogel, J.A.; Van Veldhuisen, E.; Alles, L.K.; Busch, O.R.; Dijk, F.; Van Gulik, T.M.; Huijzer, G.M.; Besselink, M.G.; Van Lienden, K.P.; Verheij, J. Time-Dependent Impact of Irreversible Electroporation on Pathology and Ablation Size in the Porcine Liver: A 24-H Experimental Study. Technol. Cancer Res. Treat. 2019, 18, 1533033819876899. [Google Scholar] [CrossRef]
- Sun, J.; Yang, L.; Zhou, Z.; Zhang, D.; Han, W.; Zhang, Q.; Peng, Y. Performance evaluation of two iterative reconstruction algorithms, MBIR and ASIR, in low radiation dose and low contrast dose abdominal CT in children. Radiol. Med. 2020, 125, 918–925. [Google Scholar] [CrossRef]
- Imajo, K.; Ogawa, Y.; Yoneda, M.; Saito, S.; Nakajima, A. A review of conventional and newer generation microwave ablation systems for hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 265–277. [Google Scholar] [CrossRef]
- Liebl, M.; Schulze-Hagen, M.; Zimmermann, M.; Pedersoli, F.; Kuhl, C.; Bruners, P.; Isfort, P. Microwave Ablation in the Proximity of Surgical Clips: Is there a Safety Issue? Cardiovasc. Interv. Radiol. 2020, 43, 918–923. [Google Scholar] [CrossRef] [Green Version]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. Radiol. Med. 2020, 125, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Knavel, E.M.; Brace, C.L. Tumor Ablation: Common Modalities and General Practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, A.; Gregucci, F.; Bonaparte, I.; Vitulano, N.; Surgo, A.; Mazzola, R.; Di Monaco, A.; Carbonara, R.; Alongi, F.; Langialonga, T.; et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2020, 126, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fersino, S.; Borghesi, S.; Jereczek-Fossa, B.A.; Arcangeli, S.; Mortellaro, G.; Magrini, S.M.; Alongi, F. Uro-Oncology study group of Italian association of Radiotherapy and Clinical Oncology (AIRO) PROACTA: A survey on the actual attitude of the Italian radiation oncologists in the management and prescription of hormonal therapy in prostate cancer patients. Radiol. Med. 2020, 126, 460–465. [Google Scholar] [CrossRef]
- Laimer, G.; Schullian, P.; Jaschke, N.; Putzer, D.; Eberle, G.; Alzaga, A.; Odisio, B.; Bale, R. Minimal ablative margin (MAM) assessment with image fusion: An independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur. Radiol. 2020, 30, 2463–2472. [Google Scholar] [CrossRef] [Green Version]
- Uhlig, J.; Strauss, A.; Rucker, G.; Seif Amir Hosseini, A.; Lotz, J.; Trojan, L.; Kim, H.S.; Uhlig, A. Partial nephrectomy versus ablative techniques for small renal masses: A systematic review and network meta-analysis. Eur. Radiol. 2019, 29, 1293–1307. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bianchi, G.; Formiconi, F.; Palumbo, P.; Zugaro, L.; Gravina, G.L.; Barile, A.; Masciocchi, C. CT-guided cryoablation for management of bone metastases: A single center experience and review of the literature. Radiol. Med. 2022, 127, 199–205. [Google Scholar] [CrossRef]
- Minami, Y.; Minami, T.; Hagiwara, S.; Ida, H.; Ueshima, K.; Nishida, N.; Murakami, T.; Kudo, M. Ultrasound-ultrasound image overlay fusion improves real-time control of radiofrequency ablation margin in the treatment of hepatocellular carcinoma. Eur. Radiol. 2018, 28, 1986–1993. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ahmed, M.; Adam, A.; Arai, Y.; Arellano, R.; de Baère, T.; Bale, R.; Bellera, C.; Binkert, C.A.; Brace, C.L.; et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology 2021, 301, 533–540. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; De Baère, T.; Dodd, G.D., III; et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. Radiology 2014, 273, 241–260. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; de Baère, T.; Dodd, G.D.; et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. J. Vasc. Interv. Radiol. 2014, 25, 1691–1705.e4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, H.; Zhou, L.; Chen, X.; Zheng, S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 8619096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohlman, R.M.; Hinshaw, J.L.; Ziemlewicz, T.J.; Lubner, M.G.; Wells, S.A.; Lee, F.T.; Alexander, M.L.; Wergin, K.L.; Varghese, T. Differential Imaging of Liver Tumors before and after Microwave Ablation with Electrode Displacement Elastography. Ultrasound Med. Biol. 2021, 47, 2138–2156. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Geng, X.; Yang, Y.; Cai, X.; Hu, P.; Xia, L.; Zhang, B.; Wu, P. Dynamic imaging and pathological changes in pig liver after MR-guided microwave ablation. BMC Cancer 2018, 18, 397. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Sirabella, G.; Marano, I.; Granata, V.; Grassi, R.; et al. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Petrillo, A. Introduction to Special Issue of Radiology and Imaging of Cancer. Cancers 2020, 12, 2665. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Galdiero, R.; Setola, S.V.; Palaia, R.; Belli, A.; Silvestro, L.; Cozzi, D.; Brunese, L.; et al. Pancreatic cancer detection and characterization: State of the art and radiomics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3684–3699. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Castelguidone, E.D.L.D.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: The radiologist’s challenge. Radiol. Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.; Belli, A.; Piccirillo, M.; Pradella, S.; Giordano, M.; Cappabianca, S.; Brunese, L.; et al. Abbreviated MRI Protocol for the Assessment of Ablated Area in HCC Patients. Int. J. Environ. Res. Public Health 2021, 18, 3598. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bruno, F.; Gianneramo, C.; Palumbo, P.; Zugaro, L.; Zoccali, C.; Barile, A.; Masciocchi, C. Evolution of the imaging features of osteoid osteoma treated with RFA or MRgFUS during a long-term follow-up: A pictorial review with clinical correlations. Radiol. Med. 2020, 125, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Barile, A. Some thoughts and greetings from the new Editor-in-Chief. Radiol. Med. 2021, 126, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Cannizzaro, E.; Di Cesare, A.; Bruno, F.; Schicchi, N.; Giovagnoni, A.; Splendiani, A.; Barile, A.; Masciocchi, C.; Di Cesare, E. Cardiac magnetic resonance in arrhythmogenic cardiomyopathies. Radiol. Med. 2020, 125, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Mirabile, A.; Lucarelli, N.M.; Sollazzo, E.P.; Ianora, A.A.S.; Sardaro, A.; Mirabile, G.; Lorusso, F.; Racanelli, V.; Maggialetti, N.; Scardapane, A. CT pulmonary angiography appropriateness in a single emergency department: Does the use of revised Geneva score matter? Radiol. Med. 2021, 126, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.R.; Franziska, F.; Philipp, M.; Miriam, K.; Lars, G.; Wolfram, S.; Hans-Ulrich, K.; Stephan, S. Iodine concentration and tissue attenuation in dual-energy contrast-enhanced CT as a potential quantitative parameter in early detection of local pancreatic carcinoma recurrence after surgical resection. Eur. J. Radiol. 2021, 143, 109944. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Wessendorf, J.; König, A.; Heers, H.; Mahnken, A.H. Repeat Percutaneous Radiofrequency Ablation of T1 Renal Cell Carcinomas is Safe in Patients with Von Hippel–Lindau Disease. Cardiovasc. Interv. Radiol. 2021, 44, 2022–2025. [Google Scholar] [CrossRef]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol. Med. 2021, 126, 1388–1395. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Gao, J.; Li, J.; Li, M.; Zhou, Z.; Peng, Y. Performance evaluation of a deep learning image reconstruction (DLIR) algorithm in “double low” chest CTA in children: A feasibility study. Radiol. Med. 2021, 126, 1181–1188. [Google Scholar] [CrossRef]
- Arita, Y.; Yoshida, S.; Kwee, T.C.; Edo, H.; Kufukihara, R.; Shigeta, K.; Nagasaka, M.; Takeshita, R.; Okamura, H.; Ueda, R.; et al. Clinical utility of the Bosniak classification version 2019: Diagnostic value of adding magnetic resonance imaging to computed tomography examination. Eur. J. Radiol. 2022, 148, 110163. [Google Scholar] [CrossRef]
- Scialpi, M.; Scialpi, P.; Martorana, E.; Torre, R.; Mancioli, F.A.; D’Andrea, A.; Di Blasi, A. Biparametric MRI with simplified PI-RADS (S-PI-RADS) for prostate cancer detection and management: What do radiologist need to know. Radiol. Med. 2021, 126, 1660–1661. [Google Scholar] [CrossRef] [PubMed]
- Öcal, O.; Peynircioglu, B.; Loewe, C.; van Delden, O.; Vandecaveye, V.; Gebauer, B.; Zech, C.J.; Sengel, C.; Bargellini, I.; Iezzi, R.; et al. Correlation of liver enhancement in gadoxetic acid–enhanced MRI with liver functions: A multicenter-multivendor analysis of hepatocellular carcinoma patients from SORAMIC trial. Eur. Radiol. 2022, 32, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Dimov, I.P.; Tous, C.; Li, N.; Häfeli, U.O.; Martel, S.; Soulez, G. Future Advances in Diagnosis and Drug Delivery in Interventional Radiology Using MR Imaging–Steered Theranostic Iron Oxide Nanoparticles. J. Vasc. Interv. Radiol. 2021, 32, 1292–1295.e1. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Darvizeh, F.; Cundari, G.; Rovere, G.; Ferrandino, G.; Nicoletti, V.; Cilia, F.; De Vizio, S.; Palumbo, R.; Esposito, A.; et al. Advanced cardiac imaging in athlete’s heart: Unravelling the grey zone between physiologic adaptation and pathology. Radiol. Med. 2021, 126, 1518–1531. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.I.; Soliman, M.M.; Sparapani, J.; Doustaly, R.; Cobb, B.W.; Malhotra, A.; Charalel, R.; May, B.J.; Lee, K.S.; Madoff, D.C.; et al. Conventional Hepatic Volumetry May Lead to Inaccurate Segmental Yttrium-90 Radiation Dosimetry. Cardiovasc. Interv. Radiol. 2021, 44, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. On behalf of the Italian Working Group on Magnetic Resonance Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, Y.; Yuan, M.; Liang, J.; Feng, Y.; Shi, Y.; Zhang, Z.; Shan, F. CT perfusion imaging can detect residual lung tumor early after radiofrequency ablation: A preliminary animal study on both tumoral and peri-tumoral region assessment. J. Thorac. Dis. 2022, 14, 64–75. [Google Scholar] [CrossRef]
- Blazevski, A.; Gondoputro, W.; Scheltema, M.J.; Amin, A.; Geboers, B.; Barreto, D.; Haynes, A.-M.; Shnier, R.; Delprado, W.; Agrawal, S.; et al. Salvage robot-assisted radical prostatectomy following focal ablation with irreversible electroporation: Feasibility, oncological and functional outcomes. BMC Urol. 2022, 22, 28. [Google Scholar] [CrossRef]
- Rohan, T.; Andrasina, T.; Matkulcik, P.; Bernard, V.; Valek, V. Percutaneous Endoluminal Radiofrequency Ablation of Occluded Biliary Metal Stent in Malignancy Using Monopolar Technique: A Feasibility Study. Cardiovasc. Interv. Radiol. 2022, 1–6. [Google Scholar] [CrossRef]
- Baust, J.M.; Santucci, K.L.; Van Buskirk, R.G.; Raijman, I.; Fisher, W.E.; Baust, J.G.; Snyder, K.K. An In Vitro Investigation into Cryoablation and Adjunctive Cryoablation/Chemotherapy Combination Therapy for the Treatment of Pancreatic Cancer Using the PANC-1 Cell Line. Biomedicines 2022, 10, 450. [Google Scholar] [CrossRef]
- Li, W.; Lou, Y.; Wang, G.; Zhang, K.; Xu, L.; Liu, P.; Xu, L.X. A Novel Multi-Mode Thermal Therapy for Colorectal Cancer Liver Metastasis: A Pilot Study. Biomedicines 2022, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.J.J. The role for MRI-guided transurethral ultrasound ablation in the continuum of prostate cancer care. Br. J. Radiol. 2022, 95, 20210959. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.-S.; Abul, A.; Osman, F.H.; Ng, H.H.-L.; Wang, K.; Yuan, Y.; Cartledge, J.; Wah, T.M. Ablative therapies versus partial nephrectomy for small renal masses—A systematic review and meta-analysis. Int. J. Surg. 2022, 97, 106194. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Cannavale, A.; Chisci, E.; Citone, M.; Falcone, G.M.; Michelagnoli, S.; Miele, V. Direct percutaneous embolization of aneurysm sac: A safe and effective procedure to treat post-EVAR type II endoleaks. Radiol. Med. 2021, 126, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, V.; Cervelli, R. Liver investigations: Updating on US technique and contrast-enhanced ultrasound (CEUS). Eur. J. Radiol. 2017, 96, 65–73. [Google Scholar] [CrossRef]
- Faccia, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers 2022, 14, 481. [Google Scholar] [CrossRef]
- Ossola, C.; Curti, M.; Calvi, M.; Tack, S.; Mazzoni, S.; Genesio, L.; Venturini, M.; Genovese, E.A. Role of ultrasound and magnetic resonance imaging in the prognosis and classification of muscle injuries in professional football players: Correlation between imaging and return to sport time. Radiol. Med. 2021, 126, 1460–1467. [Google Scholar] [CrossRef]
- Güldoğan, E.S.; Ergun, O.; Türkmenoğlu, T.T.; Yılmaz, K.B.; Akdağ, T.; Güneş, S.; Durmaz, H.A.; Hekimoğlu, B. The impact of TI-RADS in detecting thyroid malignancies: A prospective study. Radiol. Med. 2021, 126, 1335–1344. [Google Scholar] [CrossRef]
- Celletti, I.; Fresilli, D.; De Vito, C.; Bononi, M.; Cardaccio, S.; Cozzolino, A.; Durante, C.; Grani, G.; Grimaldi, G.; Isidori, A.M.; et al. TIRADS, SRE and SWE in INDETERMINATE thyroid nodule characterization: Which has better diagnostic performance? Radiol. Med. 2021, 126, 1189–1200. [Google Scholar] [CrossRef]
- Rosa, F.; Martinetti, C.; Veirana, M.A.; Attieh, A.; Trisoglio, A.; Sabattini, R.; Gandolfo, N.; Gastaldo, A. How embryology knowledge can help radiologists in the differential diagnosis of canal of Nuck pathologies. Radiol. Med. 2021, 126, 910–924. [Google Scholar] [CrossRef]
- Wang, X.; Liang, P.; Yu, J.; Yao, J.-D.; Fan, F.-Y.; Yu, X.; Cheng, Z.-G.; Han, Z.-Y.; Liu, F.-Y.; Dou, J.-P. Contrast-enhanced ultrasound features predict the prognosis of percutaneous microwave ablation of intrahepatic cholangiocarcinoma. Br. J. Radiol. 2022, 95, 20211379. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, X.; Shi, S.; Gao, J.; Li, X.; Wang, Y.; Jiang, M.; Zheng, C.; Liu, H. Evaluation of 3D-CEUS in the Recurrence of Liver Cancer after Radiofrequency Ablation. J. Heal. Eng. 2021, 2021, 3123553. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ruan, S.-M.; Xian, M.-F.; Li, M.-D.; Cheng, M.-Q.; Li, W.; Huang, Y.; Xie, X.-Y.; Kuang, M.; Wang, W.; et al. Contrast-enhanced ultrasound–based ultrasomics score: A potential biomarker for predicting early recurrence of hepatocellular carcinoma after resection or ablation. Br. J. Radiol. 2022, 95, 20210748. [Google Scholar] [CrossRef]
- Bao, H.; Chen, T.; Zhu, J.; Xie, H.; Chen, F. CEUS-Based Radiomics Can Show Changes in Protein Levels in Liver Metastases After Incomplete Thermal Ablation. Front. Oncol. 2021, 11, 3294. [Google Scholar] [CrossRef] [PubMed]
- Barabino, M.; Gurgitano, M.; Fochesato, C.; Angileri, S.A.; Franceschelli, G.; Santambrogio, R.; Mariani, N.M.; Opocher, E.; Carrafiello, G. LI-RADS to categorize liver nodules in patients at risk of HCC: Tool or a gadget in daily practice? Radiol. Med. 2020, 126, 5–13. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, M.; Ziglioli, F.; Russo, U.; Pagano, P.; Brunese, L.; Bertelli, E.; Pagnini, F.; Maestroni, U. Radiofrequency ablation (RFA) of T1a renal cancer with externally cooled multitined expandable electrodes. Radiol. Med. 2020, 125, 709–797. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, M.; Ninatti, G.; Cozzi, L.; Voulaz, E.; Gennaro, N.; Barajon, I.; Ricci, F.; Carlo-Stella, C.; Zucali, P.; Sollini, M.; et al. Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol. Med. 2020, 125, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, M.; Di Nasso, M.; Morganti, R.; Faggioni, L.; Masi, G.; Falcone, A.; Neri, E. Application of the ESR iGuide clinical decision support system to the imaging pathway of patients with hepatocellular carcinoma and cholangiocarcinoma: Preliminary findings. Radiol. Med. 2020, 125, 531–537. [Google Scholar] [CrossRef]
- Gatti, M.; Calandri, M.; Bergamasco, L.; Darvizeh, F.; Grazioli, L.; Inchingolo, R.; Ippolito, D.; Rousset, S.; Veltri, A.; Fonio, P.; et al. Characterization of the arterial enhancement pattern of focal liver lesions by multiple arterial phase magnetic resonance imaging: Comparison between hepatocellular carcinoma and focal nodular hyperplasia. Radiol. Med. 2020, 125, 348–355. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Schullian, P.; Johnston, E.; Laimer, G.; Putzer, D.; Eberle, G.; Scharll, Y.; Ianetti-Hackl, C.; Bale, R. Stereotactic Radiofrequency Ablation of Breast Cancer Liver Metastases: Short- and Long-Term Results with Predicting Factors for Survival. Cardiovasc. Interv. Radiol. 2021, 44, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, P.; Donati, F.; Cervelli, R.; Pacciardi, F.; Tarantini, G.; Castagna, M.; Urbani, L.; Lencioni, R. Colorectal liver metastases: ADC as an imaging biomarker of tumor behavior and therapeutic response. Eur. J. Radiol. 2021, 137, 109609. [Google Scholar] [CrossRef] [PubMed]
- Laimer, G.; Jaschke, N.; Schullian, P.; Putzer, D.; Eberle, G.; Solbiati, M.; Solbiati, L.; Goldberg, S.N.; Bale, R. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur. Radiol. 2021, 31, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Raso, M.M.; Avallone, A.; De Stefano, A.; Nasti, G.; Palaia, R.; Delrio, P.; Petrillo, A.; et al. Liver radiologic findings of chemotherapy-induced toxicity in liver colorectal metastases patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9697–9706. [Google Scholar]
- Cannataci, C.; Cimo’, B.; Mamone, G.; Tuzzolino, F.; D’Amico, M.; Cortis, K.; Maruzzelli, L.; Miraglia, R. Portal vein puncture-related complications during transjugular intrahepatic portosystemic shunt creation: Colapinto needle set vs Rösch-Uchida needle set. Radiol. Med. 2021, 126, 1487–1495. [Google Scholar] [CrossRef]
- Peisen, F.; Ekert, K.; Bitzer, M.; Bösmüller, H.; Fritz, J.; Horger, M. CT hepatic arterial perfusion index does not allow stratification of the degree of esophageal varices and bleeding risk in cirrhotic patients in Child–Pugh classes A and B. Abdom. Radiol. 2021, 46, 5586–5597. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Ledda, R.E.; Milanese, G.; Cademartiri, F.; Maffei, E.; Benedetti, G.; Goldoni, M.; Silva, M.; Sverzellati, N. Association of hepatic steatosis with epicardial fat volume and coronary artery disease in symptomatic patients. Radiol. Med. 2021, 126, 652–660. [Google Scholar] [CrossRef]
- Lombardi, A.F.; Afsahi, A.M.; Gupta, A.; Gholamrezanezhad, A. Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), influenza, and COVID-19, beyond the lungs: A review article. Radiol. Med. 2021, 126, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Johnston, E.; Laimer, G.; Putzer, D.; Eberle, G.; Amann, A.; Effenberger, M.; Maglione, M.; Freund, M.C.; Loizides, A.; et al. Frequency and risk factors for major complications after stereotactic radiofrequency ablation of liver tumors in 1235 ablation sessions: A 15-year experience. Eur. Radiol. 2021, 31, 3042–3052. [Google Scholar] [CrossRef]
- Powerski, M.; Drewes, R.; Omari, J.; Relja, B.; Surov, A.; Pech, M. Intra-hepatic Abscopal Effect Following Radioembolization of Hepatic Metastases. Cardiovasc. Interv. Radiol. 2020, 43, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Schullian, P.; Putzer, D.; Eberle, G.; Laimer, G.; Bale, R. Simultaneous Stereotactic Radiofrequency Ablation of Multiple (≥4) Liver Tumors: Feasibility, Safety, and Efficacy. J. Vasc. Interv. Radiol. 2020, 31, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Corvino, F.; Cavaglià, E.; Silvestre, M.; Cangiano, G.; Amodio, F.; De Magistris, G.; Niola, R. Emborrhoid in patients with portal hypertension and chronic hemorrhoidal bleeding: Preliminary results in five cases with a new coiling release fashion “Spaghetti technique”. Radiol. Med. 2020, 125, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Aberle, S.; Kenkel, D.; Becker, A.S.; Puippe, G.; Burger, I.; Schaefer, N.; Pfammatter, T. Outpatient Yttrium-90 microsphere radioembolization: Assessment of radiation safety and quantification of post-treatment adverse events causing hospitalization. Radiol. Med. 2020, 125, 971–980. [Google Scholar] [CrossRef]
- Abe, T.; Shinzawa, H.; Wakabayashi, H.; Aoki, M.; Sugahara, K.; Iwaba, A.; Haga, H.; Miyano, S.; Terui, Y.; Mitsuhashi, H.; et al. Value of Laparoscopic Microwave Coagulation Therapy for Hepatocellular Carcinoma in Relation to Tumor Size and Location. Laryngo-Rhino-Otologie 2000, 32, 598–603. [Google Scholar] [CrossRef]
- Simo, K.A.; Sereika, S.E.; Newton, K.N.; Gerber, D.A. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: Safety and efficacy in comparison with radiofrequency ablation. J. Surg. Oncol. 2011, 104, 822–829. [Google Scholar] [CrossRef]
- Fang, C.; Cortis, K.; Yusuf, G.T.; Gregory, S.; Lewis, D.; Kane, P.; Peddu, P. Complications from percutaneous microwave ablation of liver tumours: A pictorial review. Br. J. Radiol. 2019, 92, 20180864. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G.; For the Collaborative Italian Group using AMICA system. Complications of Microwave Ablation for Liver Tumors: Results of a Multicenter Study. Cardiovasc. Interv. Radiol. 2012, 35, 868–874. [Google Scholar] [CrossRef]
- Liang, P.; Wang, Y.; Yu, X.; Dong, B. Malignant Liver Tumors: Treatment with Percutaneous Microwave Ablation—Complications among Cohort of 1136 Patients. Radiology 2009, 251, 933–940. [Google Scholar] [CrossRef]
- Marano, R.; Pontone, G.; Agricola, E.; Alushi, B.; Bartorelli, A.; Cameli, M.; Carrabba, N.; Esposito, A.; Faletti, R.; Francone, M.; et al. Recommendations in pre-procedural imaging assessment for TAVI intervention: SIC-SIRM position paper part 2 (CT and MR angiography, standard medical reporting, future perspectives). Radiol. medica 2022, 127, 277–293. [Google Scholar] [CrossRef]
- Masselli, G.; Almberger, M.; Tortora, A.; Capoccia, L.; Dolciami, M.; D’Aprile, M.R.; Valentini, C.; Avventurieri, G.; Bracci, S.; Ricci, P. Role of CT angiography in detecting acute pulmonary embolism associated with COVID-19 pneumonia. Radiol. Med. 2021, 126, 1553–1560. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Li, J.; Peng, Y. Dual-energy spectral CT imaging of pulmonary embolism with Mycoplasma pneumoniae pneumonia in children. Radiol. Med. 2022, 127, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Moroni, C.; Cavigli, E.; Bindi, A.; Caviglioli, C.; Nazerian, P.; Vanni, S.; Miele, V.; Bartolucci, M. Prognostic value of CT pulmonary angiography parameters in acute pulmonary embolism. Radiol. Med. 2021, 126, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Buffa, V.; Fedeli, S.; Vallone, A.; Ruopoli, R.; Luzietti, M.; Miele, V.; Rengo, M.; Enrici, M.M.; Fina, P.; et al. Preliminary experience with abdominal dual-energy CT (DECT): True versus virtual nonenhanced images of the liver. Radiol. Med. 2010, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, M.A.; Saade, C. Radiation dose reduction considerations and imaging patterns of ground glass opacities in coronavirus: Risk of over exposure in computed tomography. Radiol. Med. 2021, 126, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Trinci, M.; Cirimele, V.; Cozzi, D.; Galluzzo, M.; Miele, V. Diagnostic accuracy of pneumo-CT-cystography in the detection of bladder rupture in patients with blunt pelvic trauma. Radiol. Med. 2020, 125, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Ferorelli, D.; Donno, F.; de Giorgio, G.; Mele, F.; Favia, M.; Riefoli, F.; Andresciani, S.; Melodia, R.; Zotti, F.; Dell’Erba, A. Head CT scan in emergency room: Is it still abused? Quantification and causes analysis of overprescription in an Italian Emergency Department. Radiol. Medica 2020, 125, 595–599. [Google Scholar] [CrossRef]
- Masjedi, H.; Zare, M.H.; Siahpoush, N.K.; Razavi-Ratki, S.K.; Alavi, F.; Shabani, M. European trends in radiology: Investigating factors affecting the number of examinations and the effective dose. Radiol. Med. 2020, 125, 296–305. [Google Scholar] [CrossRef]
- Shankar, P.R.; Parikh, K.R.; Heilbrun, M.E.; Sweeney, B.M.; Flake, A.N.; Herbstman, E.A.; Hoffman, T.J.; Havey, R.; Kronick, S.; Davenport, M.S. Cost Implications of Oral Contrast Administration in the Emergency Department: A Time-Driven Activity-Based Costing Analysis. J. Am. Coll. Radiol. 2019, 16, 30–38. [Google Scholar] [CrossRef]
- European Society of Radiology (Esr), E.S.O.R. ESR paper on structured reporting in radiology. Insights into Imaging 2018, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-T.; Shan, Q.-Y.; Chen, S.-L.; Li, B.; Feng, S.-T.; Xu, E.-J.; Li, X.; Long, J.-Y.; Xie, X.-Y.; Lu, M.-D.; et al. CT-based radiomics for preoperative prediction of early recurrent hepatocellular carcinoma: Technical reproducibility of acquisition and scanners. Radiol. Med. 2020, 125, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Shiri, I.; Hajianfar, G.; Oveisi, N.; Abdollahi, H.; Deevband, M.R.; Oveisi, M.; Zaidi, H. Noninvasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. Radiol. Med. 2020, 125, 754–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farchione, A.; Larici, A.R.; Masciocchi, C.; Cicchetti, G.; Congedo, M.T.; Franchi, P.; Gatta, R.; Cicero, S.L.; Valentini, V.; Bonomo, L.; et al. Exploring technical issues in personalized medicine: NSCLC survival prediction by quantitative image analysis—usefulness of density correction of volumetric CT data. Radiol. Med. 2020, 125, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Rampado, O.; Depaoli, A.; Marchisio, F.; Gatti, M.; Racine, D.; Ruggeri, V.; Ruggirello, I.; Darvizeh, F.; Fonio, P.; Ropolo, R. Effects of different levels of CT iterative reconstruction on low-contrast detectability and radiation dose in patients of different sizes: An anthropomorphic phantom study. Radiol. Med. 2021, 126, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Pirani, P.E.; Di Cesare, E.; Giovagnoni, A. The sub-millisievert era in CTCA: The technical basis of the new radiation dose approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Cannizzaro, E.; Bruno, F.; Schicchi, N.; Fogante, M.; Agostini, A.; De Donato, M.C.; De Cataldo, C.; Giovagnoni, A.; Barile, A.; et al. Coronary artery disease (CAD) extension-derived risk stratification for asymptomatic diabetic patients: Usefulness of low-dose coronary computed tomography angiography (CCTA) in detecting high-risk profile patients. Radiol. Med. 2020, 125, 1249–1259. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Gong, L.; Jiang, S.; Wang, L.; Zhang, H. Classification of lung nodules based on CT images using squeeze-and-excitation network and aggregated residual transformations. Radiol. Med. 2020, 125, 374–383. [Google Scholar] [CrossRef]
- Granata, V.; Caruso, D.; Grassi, R.; Cappabianca, S.; Reginelli, A.; Rizzati, R.; Masselli, G.; Golfieri, R.; Rengo, M.; Regge, D.; et al. Structured Reporting of Rectal Cancer Staging and Restaging: A Consensus Proposal. Cancers 2021, 13, 2135. [Google Scholar] [CrossRef]
- Faggioni, L.; Coppola, F.; Ferrari, R.; Neri, E.; Regge, D. Usage of structured reporting in radiological practice: Results from an Italian online survey. Eur. Radiol. 2017, 27, 1934–1943. [Google Scholar] [CrossRef]
- Neri, E.; Coppola, F.; Larici, A.R.; Sverzellati, N.; Mazzei, M.A.; Sacco, P.; Dalpiaz, G.; Feragalli, B.; Miele, V.; Grassi, R. Structured reporting of chest CT in COVID-19 pneumonia: A consensus proposal. Insights Into Imaging 2020, 11, 92. [Google Scholar] [CrossRef]
- Granata, V.; Coppola, F.; Grassi, R.; Fusco, R.; Tafuto, S.; Izzo, F.; Reginelli, A.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; et al. Structured Reporting of Computed Tomography in the Staging of Neuroendocrine Neoplasms: A Delphi Consensus Proposal. Front. Endocrinol. 2021, 12, 748944. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Morana, G.; D’Onofrio, M.; Fusco, R.; Coppola, F.; Grassi, F.; Cappabianca, S.; Reginelli, A.; Maggialetti, N.; Buccicardi, D.; et al. Structured Reporting of Computed Tomography and Magnetic Resonance in the Staging of Pancreatic Adenocarcinoma: A Delphi Consensus Proposal. Diagnostics 2021, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Med. 2021, 127, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Miele, V.; Larici, A.R.; Sverzellati, N.; Cappabianca, S.; Brunese, L.; Maggialetti, N.; Borghesi, A.; Fusco, R.; et al. Structured Reporting of Lung Cancer Staging: A Consensus Proposal. Diagnostics 2021, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Pradella, S.; Cozzi, D.; Fusco, R.; Faggioni, L.; Coppola, F.; Grassi, R.; Maggialetti, N.; Buccicardi, D.; Lacasella, G.V.; et al. Computed Tomography Structured Reporting in the Staging of Lymphoma: A Delphi Consensus Proposal. J. Clin. Med. 2021, 10, 4007. [Google Scholar] [CrossRef]
- Available online: www.sirm.org (accessed on 20 April 2022).
- Asiyanbola, B.; Chang, D.; Gleisner, A.L.; Nathan, H.; Choti, M.A.; Schulick, R.D.; Pawlik, T.M. Operative mortality after hepatic resection: Are literature-based rates broadly ap-plicable? J. Gastrointest. Surg. 2008, 12, 842–851. [Google Scholar] [CrossRef]
- Mullen, J.T.; Ribero, D.; Reddy, S.K.; Donadon, M.; Zorzi, D.; Gautam, S.; Abdalla, E.K.; Curley, S.A.; Capussotti, L.; Clary, B.M.; et al. Hepatic Insufficiency and Mortality in 1,059 Noncirrhotic Patients Undergoing Major Hepatectomy. J. Am. Coll. Surg. 2007, 204, 854–862. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Ergün, E.L.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef]
- Mathur, A.K.; Ghaferi, A.A.; Osborne, N.H.; Pawlik, T.M.; Campbell, D.A.; Englesbe, M.J.; Welling, T.H. Body mass index and adverse perioperative outcomes following hepatic resec-tion. J. Gastrointest. Surg. 2010, 14, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Benzoni, E.; Cojutti, A.; Lorenzin, D.; Adani, G.L.; Baccarani, U.; Favero, A.; Uzzau, A. Liver resective surgery: A multivariate analysis of postoperative outcome and compli-cation. Langenbecks Arch. Surg. 2007, 392, 45–54. [Google Scholar] [CrossRef]
- Sadamori, H.; Yagi, T.; Shinoura, S.; Umeda, Y.; Yoshida, R.; Satoh, D.; Nobuoka, D.; Utsumi, M.; Fujiwara, T. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br. J. Surg. 2012, 100, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kim, J.-S.; Kim, K.H.; Moon, H.J.; Kim, S. Clinical significance of radiation dose–volume parameters and functional status on the patient-reported quality of life changes after thoracic radiotherapy for lung cancer: A prospective study. Radiol. Med. 2020, 126, 466–473. [Google Scholar] [CrossRef]
- Mathew, R.P.; Sam, M.; Raubenheimer, M.; Patel, V.; Low, G. Hepatic hemangiomas: The various imaging avatars and its mimickers. Radiol. Med. 2020, 125, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Song, J.; Ma, Z.; Chen, T. Combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging to predict neoadjuvant chemotherapy effect in FIGO stage IB2–IIA2 cervical cancers. Radiol. Med. 2020, 125, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-N.; Ge, X.-L.; Liu, X.-S.; Xu, L.-L. Histogram analysis of DCE-MRI for chemoradiotherapy response evaluation in locally advanced esophageal squamous cell carcinoma. Radiol. Med. 2019, 125, 165–176. [Google Scholar] [CrossRef]
- Shannon, B.A.; Ahlawat, S.; Morris, C.D.; Levin, A.S.; Fayad, L.M. Do contrast-enhanced and advanced MRI sequences improve diagnostic accuracy for indeterminate lipomatous tumors? Radiol. Med. 2021, 127, 90–99. [Google Scholar] [CrossRef]
- Rubinsky, B.; Onik, G.; Mikus, P. Irreversible Electroporation: A New Ablation Modality—Clinical Implications. Technol. Cancer Res. Treat. 2007, 6, 37–48. [Google Scholar] [CrossRef]
- Edd, J.F.; Horowitz, L.; Dávalos, R.; Mir, L.; Rubinsky, B. In Vivo Results of a New Focal Tissue Ablation Technique: Irre-versible Electroporation. IEEE Trans. Biomed. Eng. 2006, 53, 1409–1415. [Google Scholar] [CrossRef]
- Onik, G.; Mikus, P.; Rubinsky, B. Irreversible Electroporation: Implications for Prostate Ablation. Technol. Cancer Res. Treat. 2007, 6, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Izzo, F.; Piccirillo, M.; Albino, V.; Palaia, R.; Belli, A.; Granata, V.; Setola, S.; Fusco, R.; Petrillo, A.; Orlando, R.; et al. Prospective screening increases the detection of potentially curable hepatocellular carcinoma: Results in 8900 high-risk patients. HPB 2013, 15, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Palaia, R.; Albino, V.; Piccirillo, M.; Setola, S.V.; Petrillo, A.; Izzo, F. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A case report. Eur. Rev. Med. Pharmacol Sci. 2020, 24, 7051–7057. [Google Scholar] [PubMed]
- Tarantino, L.; Busto, G.; Nasto, A.; Nasto, R.A.; Tarantino, P.; Fristachi, R.; Cacace, L.; Bortone, S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Tsoumakidou, G.; Saltiel, S.; Villard, N.; Duran, R.; Meuwly, J.-Y.; Denys, A. Image-guided marking techniques in interventional radiology: A review of current evidence. Diagn. Interv. Imaging 2021, 102, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Yamakado, K.; Nakatsuka, A.; Yamada, T.; Uraki, J.; Kashima, M.; Yamanaka, T.; Shiraki, K.; Takei, Y.; Takeda, K. Computed tomography fluoroscopy-guided radiofrequency ablation following intra-arterial iodized-oil injection for hepatocellular carcinomas invisible on ultrasonographic images. Int. J. Clin. Oncol. 2013, 18, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Schembri, V.; Piron, L.; Le Roy, J.; Hermida, M.; Lonjon, J.; Escal, L.; Pierredon, M.-A.; Belgour, A.; Cassinotto, C.; Guiu, B. Percutaneous ablation of obscure hypovascular liver tumours in challenging locations using arterial CT-portography guidance. Diagn. Interv. Imaging 2020, 101, 707–713. [Google Scholar] [CrossRef] [PubMed]
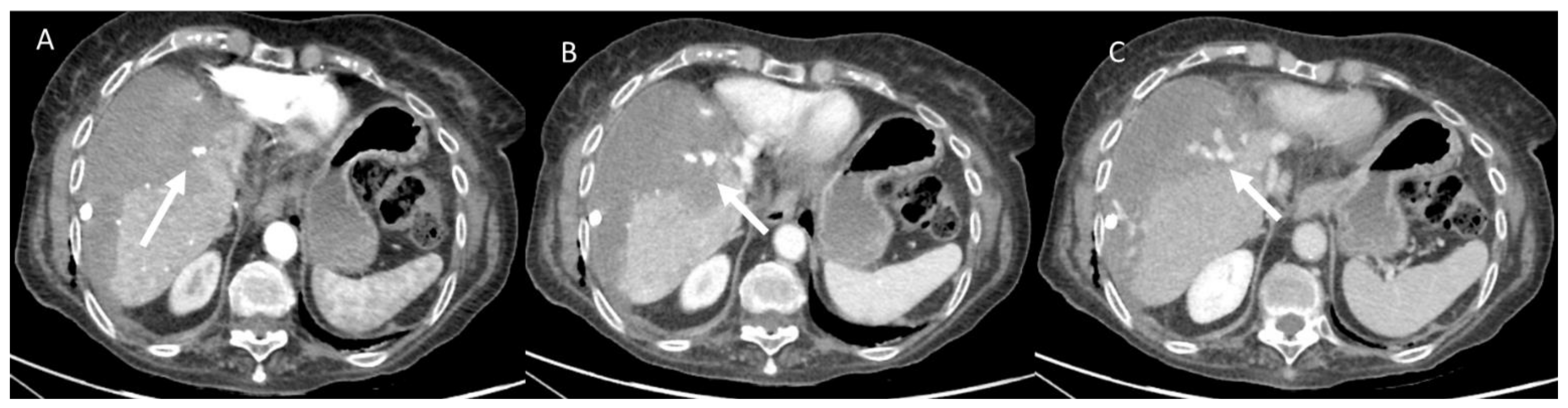

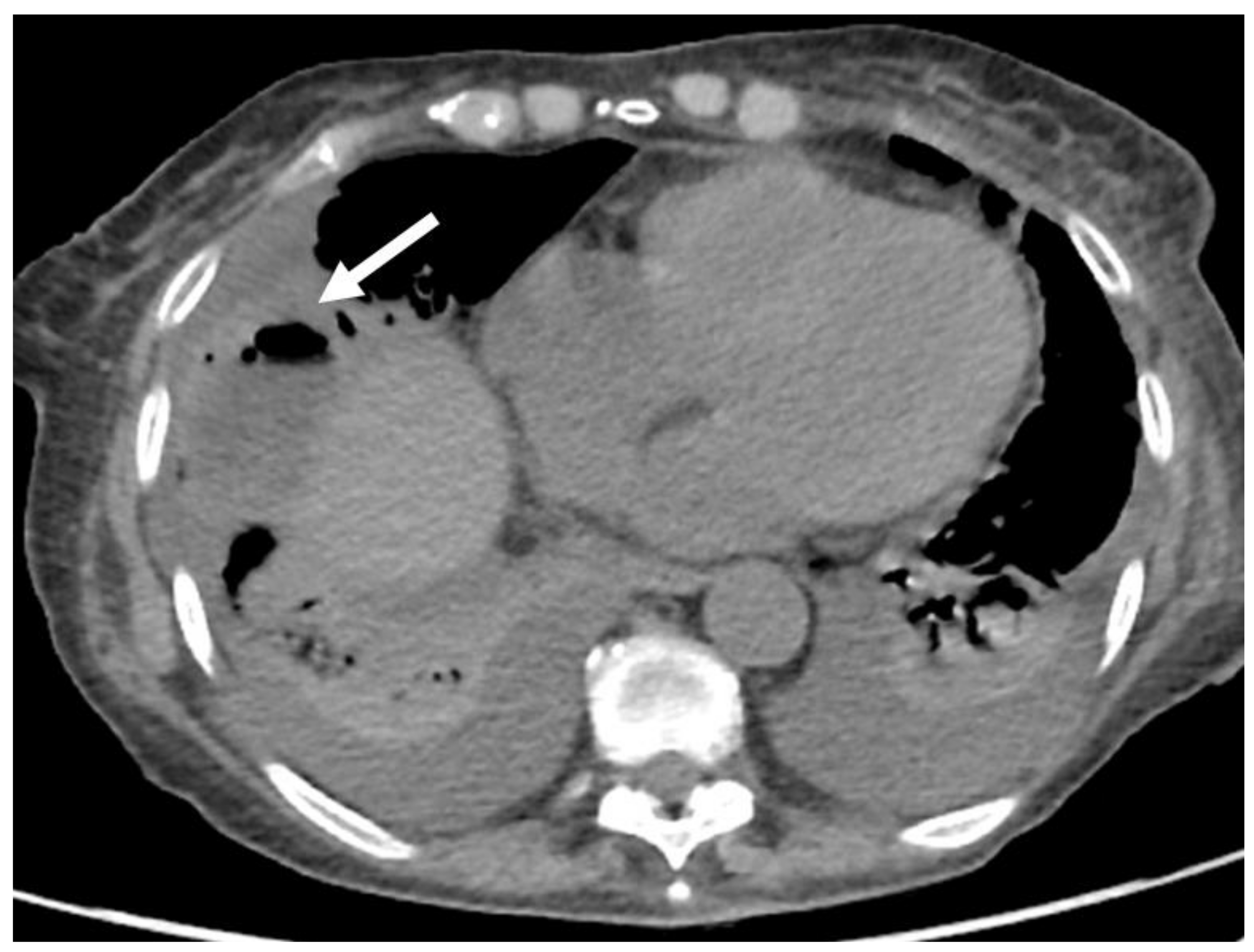
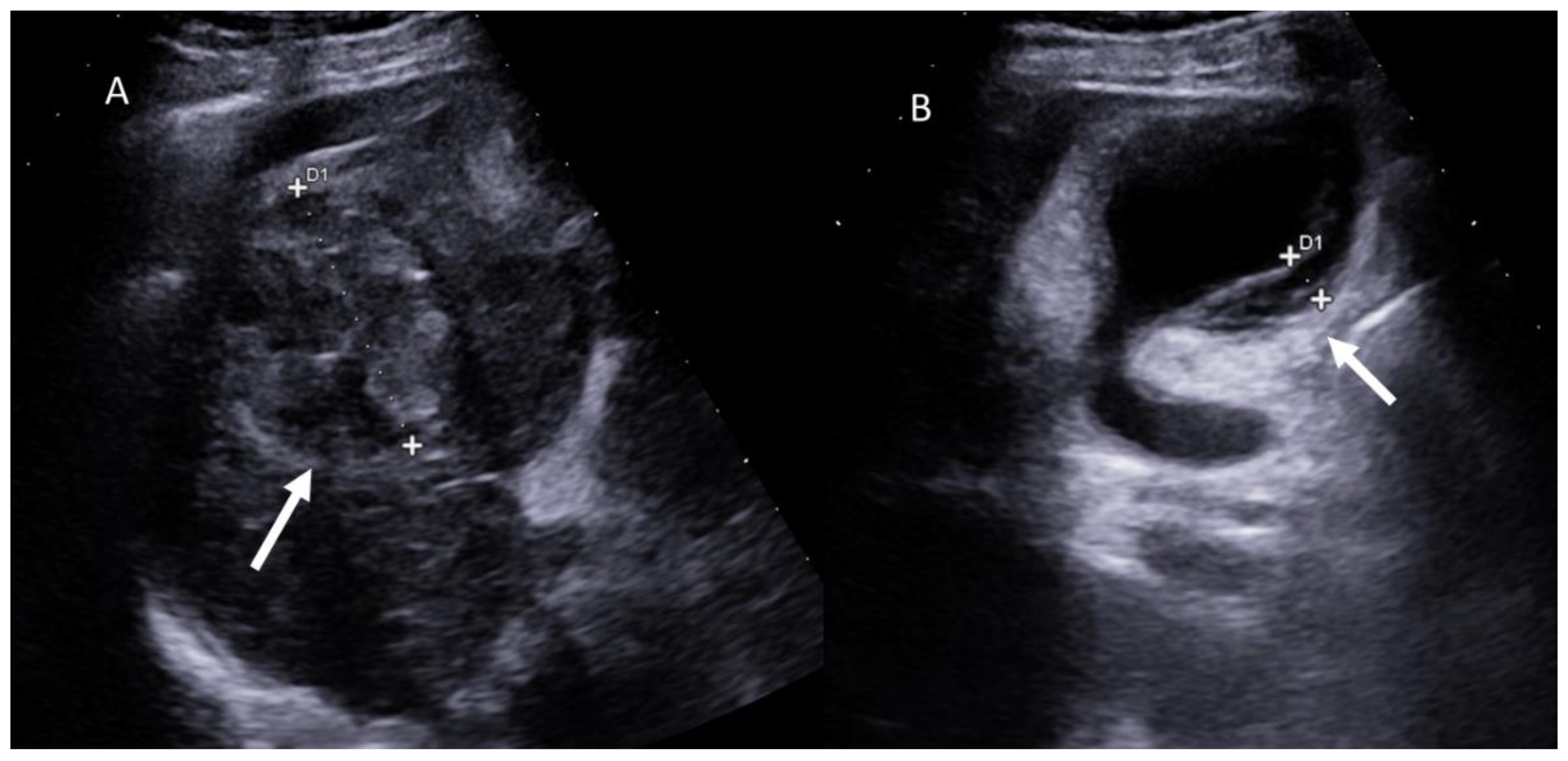

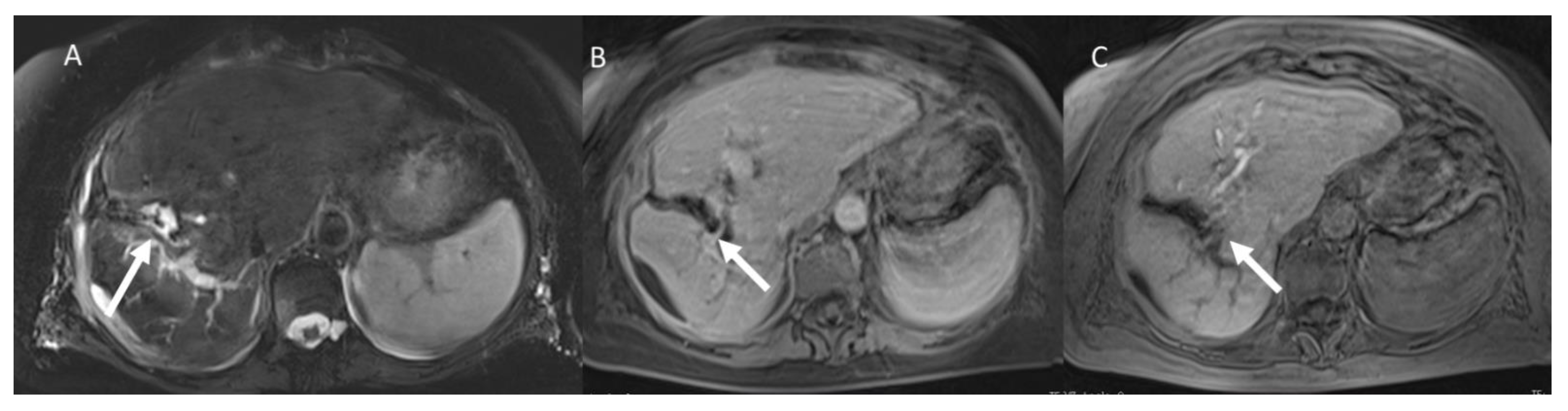
| Treatment | RFA | MWA |
|---|---|---|
| Physical phenomenon to generate heat | Thermocoagulation necrosis | Dielectric heating |
| Necrosis volume | Restricted to areas of high current density; the zone of active tissue heating is limited to a few millimeters surrounding the active electrode, with the remainder of the ablation zone being heated via thermal conduction | Volume around the applicator antenna; up to 2 cm surrounding the antenna |
| Heat-sink effect | Yes | No |
| Benefits | Safety, tolerability, efficacy, ease of use, and cost-effectiveness | Similar benefits to RFA, with several advantages, such as a greater volume of cellular necrosis, procedure time reduction, and higher temperatures delivered to the target lesion, and reduced susceptibility to variation in the morphology of the treatment zone because of heat-sink effects from adjacent vasculature |
| Metastasis complication rates | Between 1.1% and 24% | Between 3.1% and 27% |
| HCC complication rates | Between 0% vs. 45.4% | Between 2.2% and 61.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Simonetti, I.; Dell’Aversana, F.; Grassi, F.; Bruno, F.; Belli, A.; et al. Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect. J. Clin. Med. 2022, 11, 2766. https://doi.org/10.3390/jcm11102766
Granata V, Fusco R, De Muzio F, Cutolo C, Setola SV, Simonetti I, Dell’Aversana F, Grassi F, Bruno F, Belli A, et al. Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect. Journal of Clinical Medicine. 2022; 11(10):2766. https://doi.org/10.3390/jcm11102766
Chicago/Turabian StyleGranata, Vincenza, Roberta Fusco, Federica De Muzio, Carmen Cutolo, Sergio Venanzio Setola, Igino Simonetti, Federica Dell’Aversana, Francesca Grassi, Federico Bruno, Andrea Belli, and et al. 2022. "Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect" Journal of Clinical Medicine 11, no. 10: 2766. https://doi.org/10.3390/jcm11102766






