Cardio Protective Effects of Lipid Emulsion against Ropivacaine-Induced Local Anesthetic Systemic Toxicity—An Experimental Study
Abstract
:1. Introduction
2. Materials and Method
3. Histopathological Assessment
4. Statistical Analysis
5. Results
5.1. Clinical Data
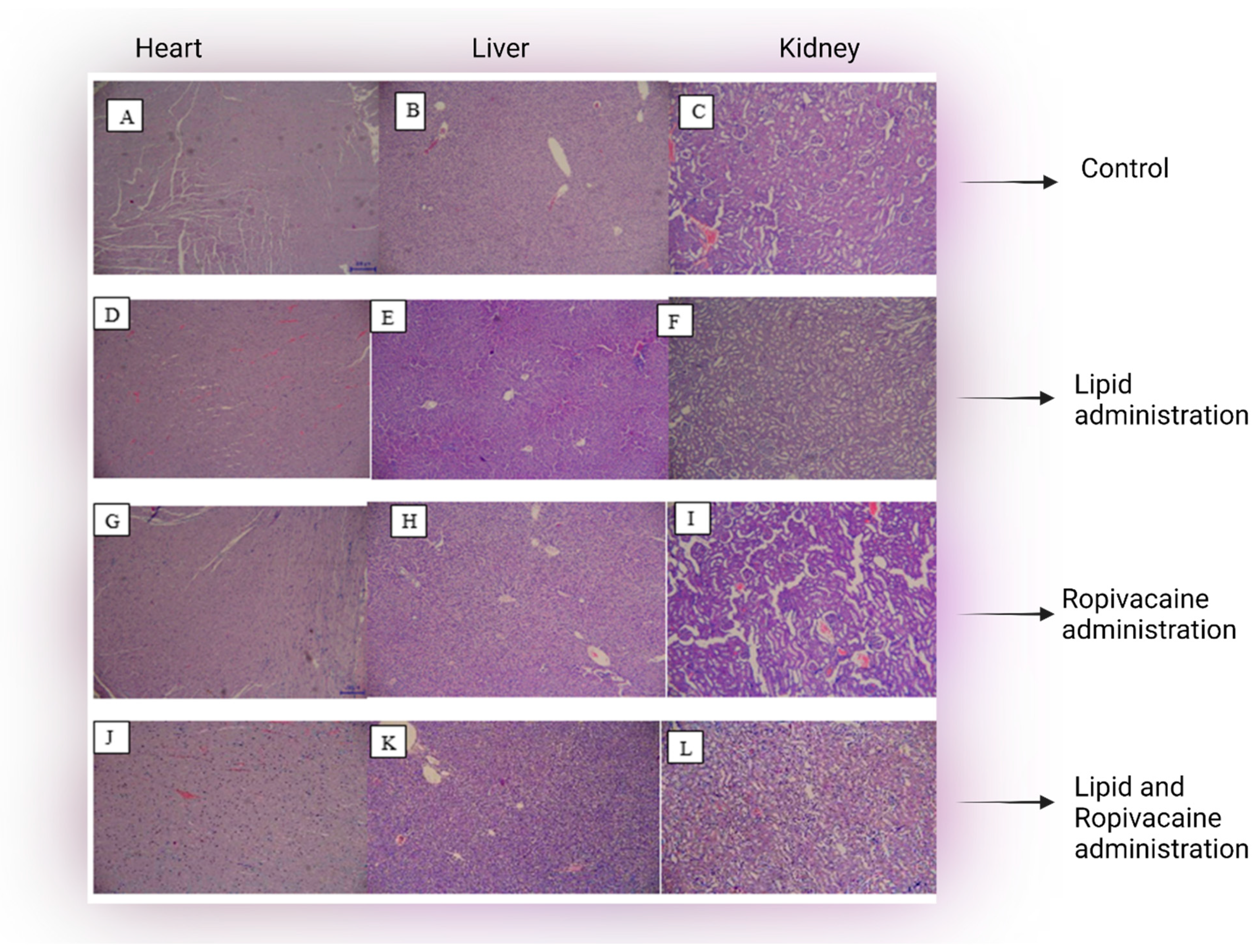
5.2. Microscopy Findings
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Sinha, C.; Kumar, A. Blood-soft tissue barrier breach and soft tissue recoil pressure on local anesthetic: Two physiological mechanisms for local anesthetic systemic toxicity. Braz. J. Anesthesiol. 2021, 71, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Zink, W.; Graf, B.M. The toxicity of local anesthetics: The place of ropivacaine and levobupivacaine. Curr. Opin. Anesthesiol. 2008, 21, 645–650. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Pawa, A.; Chin, K.J. Local anesthetic systemic toxicity: Current perspectives. Local Reg. Anesth. 2018, 11, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolinske, S.; Hoffman, R.S.; Villeneuve, E.; Hoegberg, L.C.G.; Gosselin, S. Utilization of lipid emulsion therapy in fatal overdose cases: An observational study. Clin. Toxicol. 2019, 57, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowicz, S.; Patil, V. Lipid emulsion for local anesthetic systemic toxicity. Anesthesiol. Res. Pract. 2012, 2012, 131784. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O. Use of lipid emulsion therapy in local anesthetic overdose. Saudi Med. J. 2017, 38, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.S.; Høgberg, L.C.G.; Christensen, M.B.; Petersen, T.S.; Dalhoff, K.; Bøgevig, S. Local Anesthetic Systemic Toxicity. Ugeskr Laeger 2020, 182, V11190656. [Google Scholar]
- American College of Medical Toxicology. ACMT position statement: Guidance for the use of intravenous lipid emulsion. J. Med. Toxicol. 2017, 13, 124–125. [Google Scholar] [CrossRef]
- Kien, N.T.; Giang, N.T.; van Manh, B.; Cuong, N.M.; van Dinh, N.; Pho, D.C.; Anh, V.T.; Khanh, D.T.; Thuy, L.Q.; van Dong, P. Successful intralipid-emulsion treatment of local anesthetic systemic toxicity following ultrasound-guided brachial plexus block: Case report. Int. Med. Case Rep. J. 2019, 12, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.E.; Reed, K.L. Local anesthetics: Review of pharmacological considerations. Anesth. Prog. 2012, 59, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Dureau, P.; Charbit, B.; Nicolas, N.; Benhamou, D.; Mazoit, J.X. Effect of Intralipid® on the Dose of Ropivacaine or Levobupivacaine Tolerated by Volunteers: A Clinical and Pharmacokinetic Study. Anesthesiology 2016, 125, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, M.R.; Ripper, R.; Lis, K.; Lin, B.; Lang, J.; Zider, B.; Wang, J.; Rubinstein, I.; Weinberg, G. Rapid cardiotonic effects of lipid emulsion infusion. Crit. Care Med. 2013, 41, e156–e162. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.D.; Gosselin, S.; Calello, D.P.; Nacca, N.; Rollins, C.J.; Abourbih, D.; Morris, M.; Nesbitt-Miller, A.; Morais, J.A.; Lavergne, V.; et al. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin. Toxicol. 2016, 54, 365–404. [Google Scholar] [CrossRef] [PubMed]
- Grunbaum, A.M.; Gilfix, B.M.; Hoffman, R.S.; Lavergne, V.; Morris, M.; Miller-Nesbitt, A.; Gosselin, S. Review of the effect of intravenous lipid emulsion on laboratory analyzes. Clin. Toxicol. 2016, 54, 92–102. [Google Scholar] [CrossRef]
- Intralipid® [Package Insert]; Fresenius Kabi: Uppsala, Sweden, 2015.
- Lazar, A.; Perian, M.; Cordoș, B.; Gherghinescu, M.; Grigorescu, B.L. How we did it—An easy and feasible experimental rat model of protective role of Lipid Emulsion in Ropivacaine induced Local Anesthetic Systemic Toxicity—Technique presentation and preliminary results. Acta Marisiensis-Ser. Med. 2021, 67, 90–94. [Google Scholar] [CrossRef]
- Ibrahim, M.; Elnabtity, A.M.; Hegab, A.; Alnujaidi, O.A.; El Sanea, O. Combined opioid free and loco-regional anaesthesia enhances the quality of recovery in sleeve gastrectomy done under ERAS protocol: A randomized controlled trial. BMC Anesthesiol. 2022, 22, 29. [Google Scholar] [CrossRef]
- Ok, S.H.; Hong, J.M.; Lee, S.H.; Sohn, J.T. Lipid Emulsion for Treating Local Anesthetic Systemic Toxicity. Int. J. Med. Sci. 2018, 15, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Mariano, K.C.F.; Papini, J.Z.B.; de Faria, N.C.; Heluany, D.N.C.; Botega, A.L.L.; Cereda, C.M.S.; de Paula, E.; Tófoli, G.R.; de Araujo, D.R. Ropivacaine-Loaded Poloxamer Binary Hydrogels for Prolonged Regional Anesthesia: Structural Aspects, Biocompatibility, and Pharmacological Evaluation. Biomed. Res. Int. 2021, 2021, 7300098. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Horiguchi, T.; Kimura, T.; Nishikawa, T. Recovery from Ropivacaine-Induced or Levobupivacaine-Induced Cardiac Arrest in Rats: Comparison of Lipid Emulsion Effects. Anesth. Analg. 2017, 125, 1496–1502. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xia, Y.; Liu, L.; Shi, T.; Shi, K.; Wang, Q.; Chen, L.; Papadimos, T.J.; Xu, X. Lipid emulsion reverses bupivacaine-induced asystole in isolated rat hearts: Concentration-response and time-response relationships. Anesthesiology 2010, 113, 1320–1325. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Xu, Y.; Liao, L. Comparison of the Fat Elimination Between Long-Chain Triglycerides and Medium-Chain Triglycerides in Rats with Ischemic Acute Renal Failure. Ren. Fail. 2002, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]

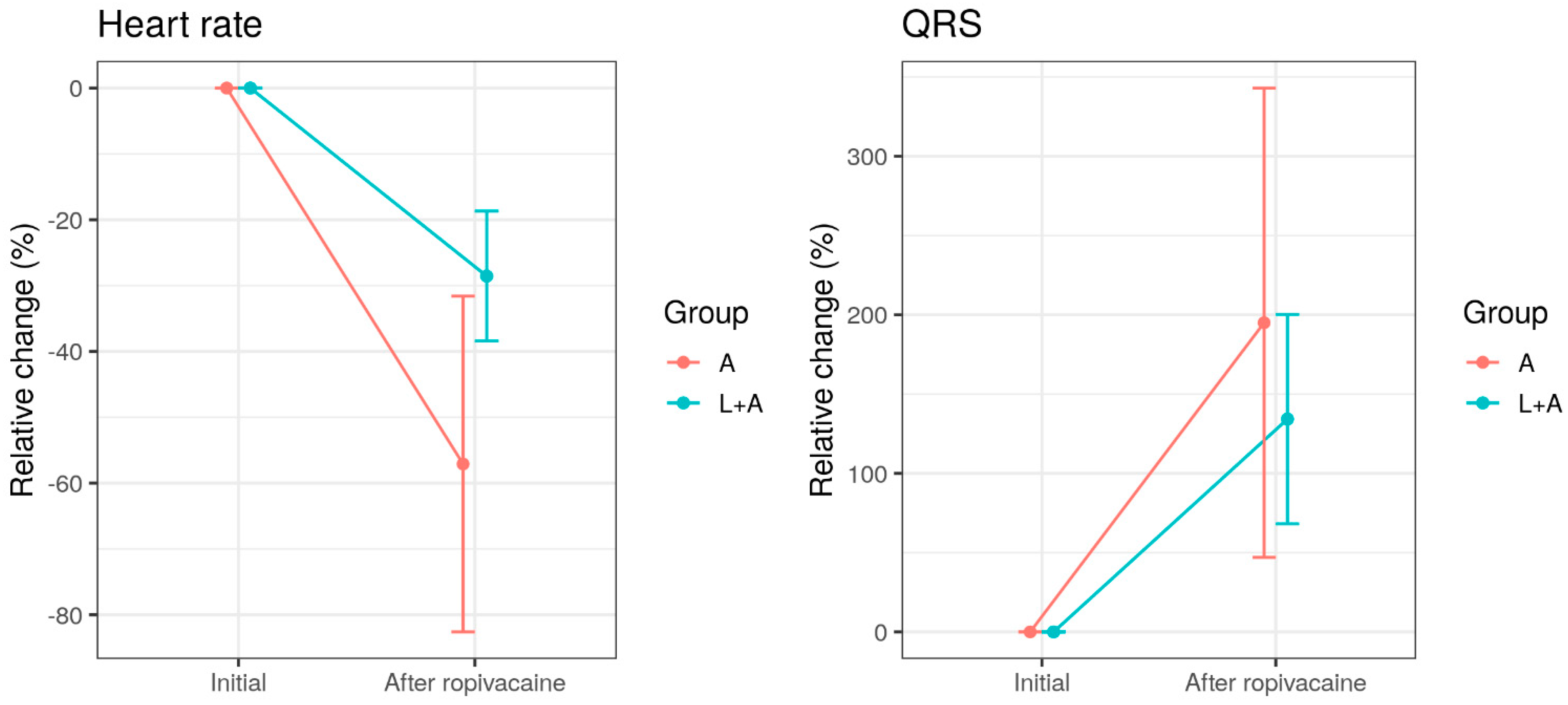
| Group A n = 10 | Group B n = 9 | |
|---|---|---|
| Baseline cardiac parameters | ||
| Heart rate (min−1) | 380 (66.0) | 321 (68.3) |
| QRS duration (ms) | 20.6 (5.44) | 19.7 (5.48) |
| Cardiac parameters after intravenous Lipid emulsion | ||
| Heart rate (min−1) | - | 296 (78.4) |
| QRS duration (ms) | - | 22.9 (4.26) |
| Cardiac parameters after intravenous Ropivacaine | ||
| Heart rate (min−1) | 156 (93.2) | 226 (37.5) |
| QRS duration (ms) | 55.6 (21.1) | 45.0 (14.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, A.E.; Gurzu, S.; Kovecsi, A.; Perian, M.; Cordos, B.; Gherghinescu, M.C.; Enache, L.S. Cardio Protective Effects of Lipid Emulsion against Ropivacaine-Induced Local Anesthetic Systemic Toxicity—An Experimental Study. J. Clin. Med. 2022, 11, 2784. https://doi.org/10.3390/jcm11102784
Lazar AE, Gurzu S, Kovecsi A, Perian M, Cordos B, Gherghinescu MC, Enache LS. Cardio Protective Effects of Lipid Emulsion against Ropivacaine-Induced Local Anesthetic Systemic Toxicity—An Experimental Study. Journal of Clinical Medicine. 2022; 11(10):2784. https://doi.org/10.3390/jcm11102784
Chicago/Turabian StyleLazar, Alexandra Elena, Simona Gurzu, Attila Kovecsi, Marcel Perian, Bogdan Cordos, Mircea Constantin Gherghinescu, and Liviu Sorin Enache. 2022. "Cardio Protective Effects of Lipid Emulsion against Ropivacaine-Induced Local Anesthetic Systemic Toxicity—An Experimental Study" Journal of Clinical Medicine 11, no. 10: 2784. https://doi.org/10.3390/jcm11102784
APA StyleLazar, A. E., Gurzu, S., Kovecsi, A., Perian, M., Cordos, B., Gherghinescu, M. C., & Enache, L. S. (2022). Cardio Protective Effects of Lipid Emulsion against Ropivacaine-Induced Local Anesthetic Systemic Toxicity—An Experimental Study. Journal of Clinical Medicine, 11(10), 2784. https://doi.org/10.3390/jcm11102784







