The Combined Value of Type2 Inflammatory Markers in Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. FeNO Measurement
2.3. Blood Eosinophil Count
2.4. Pulmonary Function Test
2.5. Definition of Acute Exacerbation
2.6. Other Information
2.7. Statistical Analyses
3. Results
3.1. Subject Characteristics and Classifications
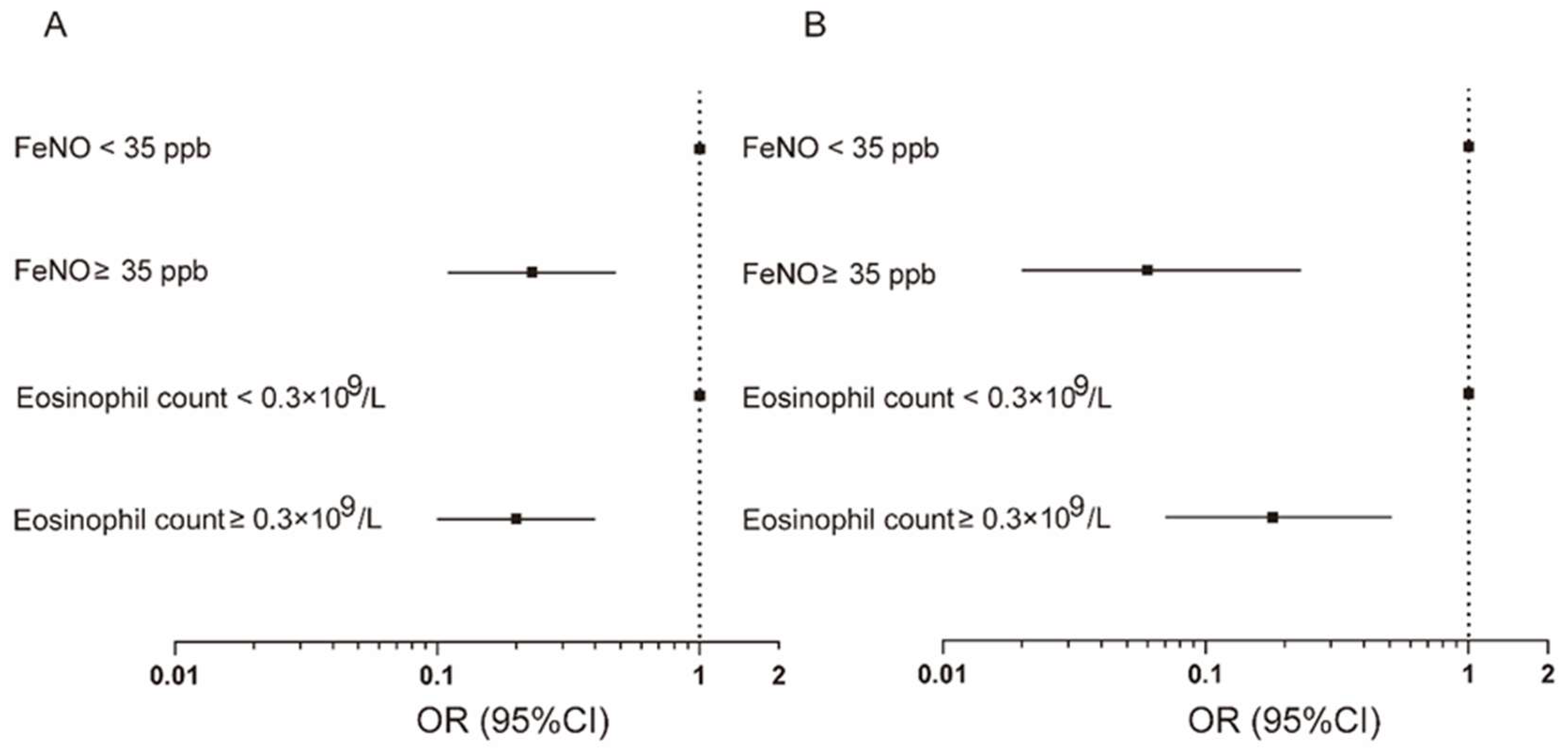
3.2. Increased FeNO Level in Relation to Incidence and Frequency of Acute Exacerbation
3.3. Increased Blood Eosinophil Count in Relation to Incidence and Frequency of Acute Exacerbation
3.4. Simultaneously Increased FeNO Level and Blood Eosinophil Count in Relation to Acute Exacerbation and Their Predictive Values
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: Systematic analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Xu, J.; Yang, L.; Xu, Y.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; Wen, F.; et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 2018, 391, 1706–1717. [Google Scholar] [CrossRef]
- Walsh, J.A.; Barker, R.E.; Kon, S.S.C.; Jones, S.E.; Banya, W.; Nolan, C.M.; Patel, S.; Polgar, O.; Haselden, B.M.; Polkey, M.I.; et al. Gait speed and adverse outcomes following hospitalized exacerbation of COPD. Eur. Respir. J. 2021, 58, 2004047. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.; Chan, K.P.; Hui, D.S.; Goddard, J.R.; Shaw, J.G.; Reid, D.W.; Yang, I.A. Acute exacerbation of COPD. Respirology 2016, 21, 1152–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosío, B.G.; Pérez de Llano, L.; Lopez Viña, A.; Torrego, A.; Lopez-Campos, J.L.; Soriano, J.B.; Martinez Moragon, E.; Izquierdo, J.L.; Bobolea, I.; Callejas, J.; et al. Th-2 signature in chronic airway diseases: Towards the extinction of asthma-COPD overlap syndrome? Eur. Respir. J. 2017, 49, 1602397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, K.; Matsunaga, K.; Shirai, T.; Hirai, K.; Gon, Y. Role of Type2 Inflammatory Biomarkers in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 2670. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.J.; Zhang, X.; Kit, B.K.; Dillon, C.F. Reference values and factors associated with exhaled nitric oxide: U.S. youth and adults. Respir. Med. 2013, 107, 1682–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, S.S.; Ahmed, S.M.; Al Drees, A.M.; Husain, A. Effect of cigarette smoking on fractional exhaled nitric oxide in Saudi medical college students. J. Pak. Med. Assoc. 2011, 61, 120–123. [Google Scholar]
- Tan, W.C.; Bourbeau, J.; Nadeau, G.; Wang, W.; Barnes, N.; Landis, S.H.; Kirby, M.; Hogg, J.C.; Sin, D.D. High eosinophil counts predict decline in FEV(1): Results from the CanCOLD study. Eur. Respir. J. 2021, 57, 2000838. [Google Scholar] [CrossRef]
- Lázár, Z.; Kelemen, Á.; Gálffy, G.; Losonczy, G.; Horváth, I.; Bikov, A. Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J. Breath Res. 2018, 12, 036017. [Google Scholar] [CrossRef]
- Chan, M.C.; Yeung, Y.C.; Yu, E.L.M.; Yu, W.C. Blood Eosinophil and Risk of Exacerbation in Chronic Obstructive Pulmonary Disease Patients: A Retrospective Cohort Analysis. Int. J. Chronic Obstruct. Pulm. Dis. 2020, 15, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Southworth, T.; Higham, A.; Kolsum, U.; Li, J.; Scott, T.; Dungwa, J.; Sridhar, S.; Pham, T.H.; Newbold, P.; Singh, D. The relationship between airway immunoglobulin activity and eosinophils in COPD. J. Cell. Mol. Med. 2021, 25, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.J.; Huang, J.T.J.; Lonergan, M.; Keir, H.R.; Fong, C.J.; Tan, B.; Cassidy, A.J.; Finch, S.; Mullerova, H.; Miller, B.E.; et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2021, 147, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Kjarsgaard, M.; Capaldi, D.; Radford, K.; Aleman, F.; Boylan, C.; Altman, L.C.; Wight, T.N.; Parraga, G.; O’Byrne, P.M.; et al. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur. Respir. J. 2017, 49, 1602486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, T.; Milan, S.J.; Wang, R.; Banchoff, E.; Bradley, P.; Crossingham, I. Anti-IL-5 therapies for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 12, Cd013432. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Ritondo, B.L.; de Marco, P.; Cazzola, M.; Rogliani, P. Evaluating triple ICS/LABA/LAMA therapies for COPD patients: A network meta-analysis of ETHOS, KRONOS, IMPACT, and TRILOGY studies. Expert Rev. Respir. Med. 2021, 15, 143–152. [Google Scholar] [CrossRef]
- Liu, T.; Xiang, Z.J.; Hou, X.M.; Chai, J.J.; Yang, Y.L.; Zhang, X.T. Blood eosinophil count-guided corticosteroid therapy and as a prognostic biomarker of exacerbations of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211028768. [Google Scholar] [CrossRef]
- Greulich, T.; Hohlfeld, J.M.; Neuser, P.; Lueer, K.; Klemmer, A.; Schade-Brittinger, C.; Harnisch, S.; Garn, H.; Renz, H.; Homburg, U.; et al. A GATA3-specific DNAzyme attenuates sputum eosinophilia in eosinophilic COPD patients: A feasibility randomized clinical trial. Respir. Res. 2018, 19, 55. [Google Scholar] [CrossRef]
- Fricker, M.; McDonald, V.M.; Winter, N.A.; Baines, K.J.; Wark, P.A.B.; Simpson, J.L.; Gibson, P.G. Molecular markers of type 2 airway inflammation are similar between eosinophilic severe asthma and eosinophilic chronic obstructive pulmonary disease. Allergy 2021, 76, 2079–2089. [Google Scholar] [CrossRef]
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J.; Dweik, R.A.; Gelb, A.F.; Gibson, P.G.; George, S.C.; Grasemann, H.; Pavord, I.D.; Ratjen, F.; Silkoff, P.E.; Taylor, D.R.; et al. Exhaled nitric oxide in pulmonary diseases: A comprehensive review. Chest 2010, 138, 682–692. [Google Scholar] [CrossRef]
- Singh, D.; Bafadhel, M.; Brightling, C.E.; Sciurba, F.C.; Curtis, J.L.; Martinez, F.J.; Pasquale, C.B.; Merrill, D.D.; Metzdorf, N.; Petruzzelli, S.; et al. Blood Eosinophil Counts in Clinical Trials for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Labaki, W.W.; Rosenberg, S.R. Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 2020, 173, 17–32. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, H.; Paillard-Borg, S.; Zhu, H.; Qi, X.; Rizzuto, D. Prevalence of Overweight and Obesity among Chinese Adults: Role of Adiposity Indicators and Age. Obes. Facts 2016, 9, 17–28. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; McDonnell, K.; Kris, M.G.; Shen, R.; Sima, C.S.; Bach, P.B.; Rizvi, N.A.; Riely, G.J. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer 2010, 116, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Zhou, Y.; Wu, Y.; Yu, Y.; Liang, W.; Ran, P. The Pneumonia Severity Index as a Predictor of In-Hospital Mortality in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. PLoS ONE 2015, 10, e0133160. [Google Scholar] [CrossRef]
- Ho, T.W.; Tsai, Y.J.; Ruan, S.Y.; Huang, C.T.; Lai, F.; Yu, C.J. In-hospital and one-year mortality and their predictors in patients hospitalized for first-ever chronic obstructive pulmonary disease exacerbations: A nationwide population-based study. PLoS ONE 2014, 9, e114866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai-Ping, C.; Lee, K.H.; Lim, T.K. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: A retrospective study. Chest 2005, 128, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, K.E.J.; Ulrik, C.S.; Godtfredsen, N.S.; Kallemose, T.; Andersen, O.; Eugen-Olsen, J.; Marsaa, K.; Rasmussen, L.J.H. High suPAR and Low Blood Eosinophil Count are Risk Factors for Hospital Readmission and Mortality in Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 733–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcázar-Navarrete, B.; Ruiz Rodríguez, O.; Conde Baena, P.; Romero Palacios, P.J.; Agusti, A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur. Respir. J. 2018, 51, 1701457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Marott, J.L.; Lange, P. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: The Copenhagen General Population Study. Eur. Respir. J. 2018, 52, 1800616. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Cowan, J.O.; Brassett, K.P.; Herbison, G.P.; Taylor, D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005, 352, 2163–2173. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Wenzel, S.E.; Casale, T.B.; FitzGerald, J.M.; Rice, M.S.; Daizadeh, N.; Deniz, Y.; Patel, N.; Harel, S.; Rowe, P.J.; et al. Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the LIBERTY ASTHMA QUEST study: A post-hoc analysis. Lancet Respir. Med. 2021, 9, 1165–1173. [Google Scholar] [CrossRef]
- Chung, K.F. Increasing utility of FeNO as a biomarker of type-2 inflammation in severe asthma. Lancet Respir. Med. 2021, 9, 1083–1084. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, Y.; Su, X.; Lei, T.; Yu, H.; Liu, J. Diagnostic value of fractional exhaled nitric oxide in differentiating the asthma-COPD overlap from COPD: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2021, 1–9. [Google Scholar] [CrossRef]
- Ye, M.; Li, Q.; Xiao, L.; Zheng, Z. Serum Magnesium and Fractional Exhaled Nitric Oxide in Relation to the Severity in Asthma-Chronic Obstructive Pulmonary Disease Overlap. Biol. Trace Elem. Res. 2021, 199, 1771–1777. [Google Scholar] [CrossRef]
- Li, M.; Yang, T.; He, R.; Li, A.; Dang, W.; Liu, X.; Chen, M. The Value of Inflammatory Biomarkers in Differentiating Asthma COPD Overlap from COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3025–3037. [Google Scholar] [CrossRef] [PubMed]
- Nadif, R.; Matran, R.; Maccario, J.; Bechet, M.; Le Moual, N.; Scheinmann, P.; Bousquet, J.; Kauffmann, F.; Pin, I. Passive and active smoking and exhaled nitric oxide levels according to asthma and atopy in adults. Ann. Allergy Asthma Immunol. 2010, 104, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.C.; Bake, B.; Torén, K. Fraction of exhaled nitric oxide at 50 mL/s: Reference values for adult lifelong never-smokers. Chest 2007, 131, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Monteiro, W.; Ward, R.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, I.D. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2000, 356, 1480–1485. [Google Scholar] [CrossRef]
- Brightling, C.E.; McKenna, S.; Hargadon, B.; Birring, S.; Green, R.; Siva, R.; Berry, M.; Parker, D.; Monteiro, W.; Pavord, I.D.; et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005, 60, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Tang, B.; Huang, D.; Wang, J.; Luo, L.L.; Li, Q.G. Relationship of Blood Eosinophils with Fractional Exhaled Nitric Oxide and Pulmonary Function Parameters in Chronic Obstructive Pulmonary Disease (COPD) Exacerbation. Med. Sci. Monit. 2020, 26, e921182. [Google Scholar] [CrossRef]
- Antus, B.; Barta, I. Relationship between exhaled nitric oxide and the frequency of severe acute exacerbation of COPD: 3-year follow-up. Acta Physiol. Hung. 2013, 100, 469–477. [Google Scholar] [CrossRef]
- Ge, H.; Liu, X.; Gu, W.; Feng, X.; Zhang, F.; Han, F.; Qian, Y.; Jin, X.; Gao, B.; Yu, L.; et al. Distribution of COPD Comorbidities and Creation of Acute Exacerbation Risk Score: Results from SCICP. J. Inflamm. Res. 2021, 14, 3335–3348. [Google Scholar] [CrossRef]
- Alcázar-Navarrete, B.; Díaz-Lopez, J.M.; García-Flores, P.; Ortega-Antelo, M.; Aguilar-Cruz, I.; Ruiz-Rodríguez, O.; Santiago-Diaz, P.; Romero Palacios, P.J. T2 Biomarkers as Predictors of Exacerbations of Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2021. [Google Scholar] [CrossRef]
- Hamad, G.A.; Cheung, W.; Crooks, M.G.; Morice, A.H. Eosinophils in COPD: How many swallows make a summer? Eur. Respir. J. 2018, 51, 1702177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kolsum, U.; Ravi, A.; Hitchen, P.; Maddi, S.; Southworth, T.; Singh, D. Clinical characteristics of eosinophilic COPD versus COPD patients with a history of asthma. Respir. Res. 2017, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Annangi, S.; Nutalapati, S.; Sturgill, J.; Flenaugh, E.; Foreman, M. Eosinophilia and fractional exhaled nitric oxide levels in chronic obstructive lung disease. Thorax 2021, 77, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Sá-Sousa, A.; Jacinto, T.; Azevedo, L.F.; Morais-Almeida, M.; Robalo-Cordeiro, C.; Bugalho-Almeida, A.; Bousquet, J.; Fonseca, J.A. Operational definitions of asthma in recent epidemiological studies are inconsistent. Clin. Transl. Allergy 2014, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Characteristics | COPD |
|---|---|
| Age (years) | 72 (65.50–80.00) |
| Males/females | 233 (87.59)/33 (12.41) |
| BMI | 23.50 (21.26–24.91) |
| Smoking status | |
| Never/former/current smoker | 89 (33.46)/148 (55.64)/29 (10.90) |
| Pack-years | |
| Never smoker | NA |
| Former/current smoker | 25 (9–47.5) |
| Pulmonary function | |
| FEV1 post BD (L) | 1.24 (1.09–1.35) |
| FVC post BD (L) | 1.96 (1.79–2.34) |
| FEV1/FVC post BD (%) | 63.25 (50.83–66.52) |
| FEV1/predict post BD (%) | 59.30 (43.30–70.10) |
| mMRC | |
| Grade 0 | 21 (7.89) |
| Grade 1 | 121 (45.49) |
| Grade 2 | 74 (27.82) |
| Grade 3 | 45 (16.92) |
| Grade 4 | 5 (1.88) |
| SGRQ | 37 (32–59) |
| 6-min walking distance (m) | 320 (300–350) |
| Exacerbations in previous year | |
| Incidence | 139 (52.85) |
| Total frequency of exacerbation | 1.29 ± 2.24 |
| FeNO (ppb) | 35 (23–50) |
| Eosinophil count × 109/L | 0.21 (0.10–0.40) |
| Groups | |||
|---|---|---|---|
| Groups | A | B | C |
| Characteristics | Eosinophil count < 0.3 × 109/L and FeNO < 35 ppb | Eosinophil count ≥ 0.3 × 109/L or FeNO ≥ 35 ppb | Eosinophil count ≥ 0.3 × 109/L and FeNO ≥ 35 ppb |
| N | 120 | 90 | 56 |
| Age (years) | 69 (63–78) | 72 (65–81) | 76 (72–82) |
| Gender (males) | 106 (88.33) | 76 (84.44) | 51 (91.07) |
| BMI | 22.85 (20.58–25.70) | 23.51 (21.74–24.97) | 24.22 (22.12–24.97) |
| Underweight | 12 (10.00) | 7 (7.78) | 2 (3.57) |
| Normal weight | 67 (55.83) | 44 (48.89) | 22 (39.29) |
| Overweight | 32 (26.67) | 30 (33.33) | 25 (44.64) |
| Obese | 9 (7.50) | 9 (10.00) | 7 (12.50) |
| Smoking status | |||
| Former and current smoker | 81 (67.5) | 64 (71.11) | 32 (57.14) |
| Pack-years | 40 (20–60) | 35 (15–50) | 9 (2.75–30) |
| ≥15 pack-years | 56 (46.67) | 50 (55.56) | 13 (23.21) |
| Lung function | |||
| FEV1 post-BD (L) | 1.20 (0.80–1.67) | 1.22 (1.05–1.37) | 1.25 (1.24–1.3) |
| FVC post-BD (L) | 2.25 (1.89–2.98) | 1.98 (1.88–2.48) | 1.96 (1.91–1.99) |
| FEV1/FVC post-BD (%) | 55.70 (42.20–65.31) | 62.12 (46.61–66.85) | 63.30 (63.20–63.85) |
| FEV1 post-BD% pred (%) | 48.90 (37.70–60.00) | 50.35 (39.90–67.78) | 67.66 (61.40–72.37) |
| Quality of Life | |||
| SGRQ | 39 (27–52) | 37 (32–47) | 35 (32–37) |
| Motor capacity | |||
| 6-minute walking distance (m) | 378 (276–420) | 340 (310–373) | 320 (310–330) |
| Previous exacerbations | |||
| Incidence | 67 (56.30) | 61 (68.54) | 11 (20.00) |
| Total frequency | 1.69 ± 1.33 | 1.39 ± 1.86 | 0.69 ± 1.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ma, G.; Mou, Y.; Liu, X.; Qiu, W.; Zheng, Y.; Zhu, H.; Ge, H. The Combined Value of Type2 Inflammatory Markers in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2022, 11, 2791. https://doi.org/10.3390/jcm11102791
Liu Y, Ma G, Mou Y, Liu X, Qiu W, Zheng Y, Zhu H, Ge H. The Combined Value of Type2 Inflammatory Markers in Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2022; 11(10):2791. https://doi.org/10.3390/jcm11102791
Chicago/Turabian StyleLiu, Yunhuan, Guanhua Ma, Yan Mou, Xuanqi Liu, Wenjia Qiu, Yang Zheng, Huili Zhu, and Haiyan Ge. 2022. "The Combined Value of Type2 Inflammatory Markers in Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 11, no. 10: 2791. https://doi.org/10.3390/jcm11102791
APA StyleLiu, Y., Ma, G., Mou, Y., Liu, X., Qiu, W., Zheng, Y., Zhu, H., & Ge, H. (2022). The Combined Value of Type2 Inflammatory Markers in Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 11(10), 2791. https://doi.org/10.3390/jcm11102791





