Drug-Related Hypersensitivity Reactions Leading to Emergency Department: Original Data and Systematic Review
Abstract
:1. Introduction
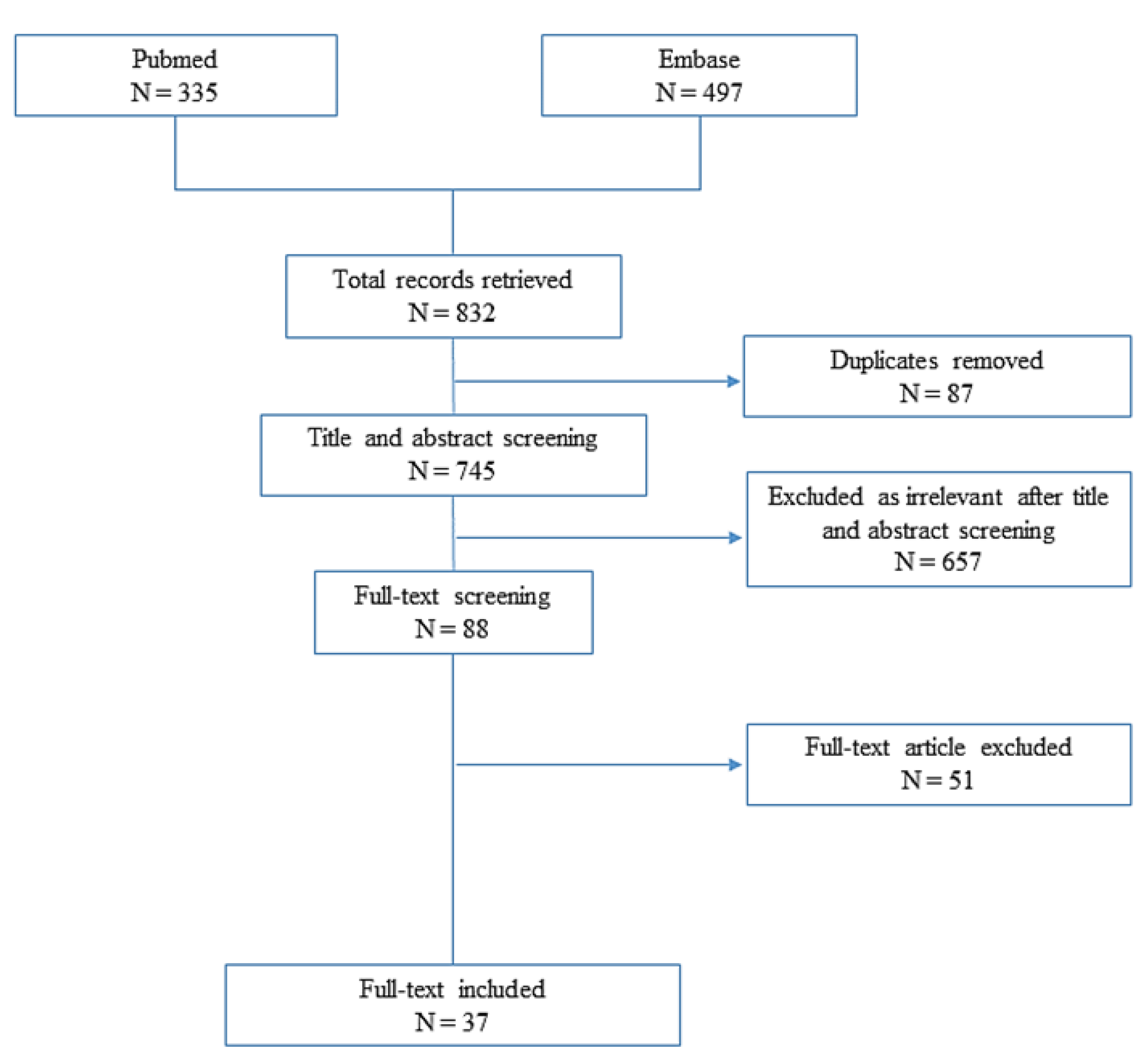
2. Materials and Methods
2.1. Post Hoc Analysis
2.2. Systematic Review
3. Results
3.1. Post Hoc Analysis
3.2. Systematic Review
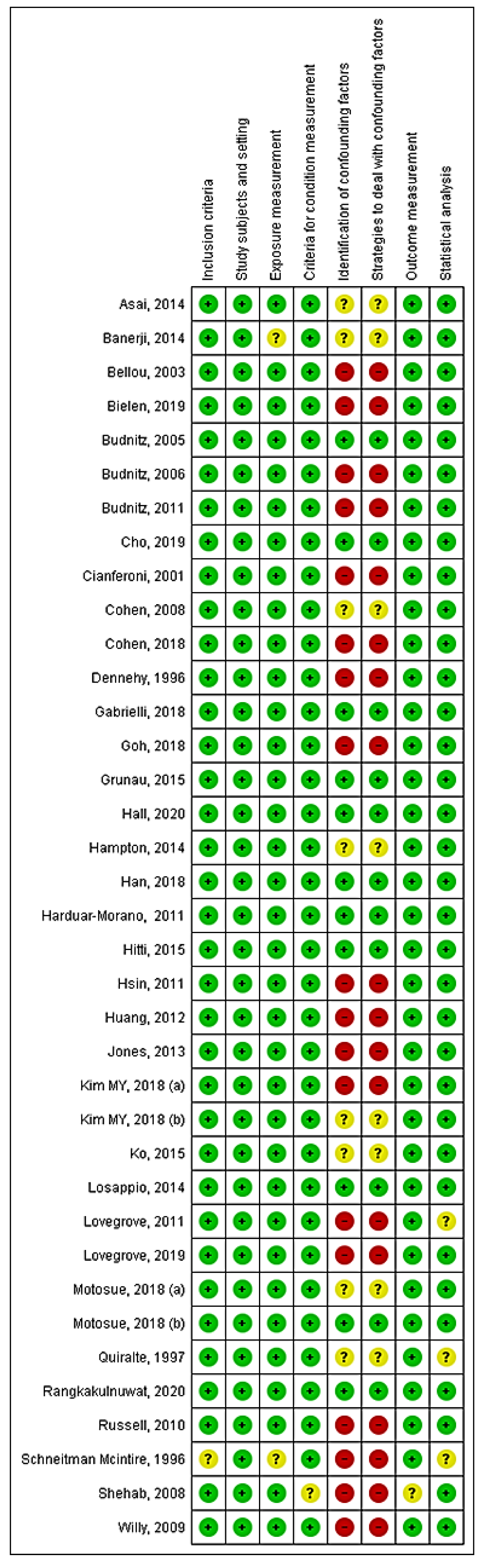
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Aun, M.V.; Kalil, J.; Giavina-Bianchi, P. Drug-Induced Anaphylaxis. Immunol. Allergy Clin. N. Am. 2017, 37, 629–641. [Google Scholar] [CrossRef]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.-S.; Pichler, W.; et al. International Consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, J.P.; Sehouli, J.; Glajzer, J.; Worm, M.; Zuberbier, T.; Maurer, M.; Fluhr, J.W. Olaparib Desensitization in a Patient with Recurrent Peritoneal Cancer. N. Engl. J. Med. 2018, 379, 2176–2177. [Google Scholar] [CrossRef] [PubMed]
- Toletone, A.; Dini, G.; Massa, E.; Bragazzi, N.L.; Pignatti, P.; Voltolini, S.; Durando, P. Chlorhexidine-induced anaphylaxis occurring in the workplace in a health-care worker: Case report and review of the literature. Med. Del Lav. 2018, 109, 68–76. [Google Scholar] [CrossRef]
- Wylon, K.; Dölle, S.; Worm, M. Polyethylene glycol as a cause of anaphylaxis. Allergy Asthma Clin. Immunol. 2016, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, A.; Hashimoto, K.; Ozono, E.; Sasaki, M.; Sakamoto, A.; Tashiro, K.; Moriuchi, H. Anaphylaxis to carboxymethylcellulose: Add food additives to the list of elicitors. Pediatrics 2019, 143, e20181180. [Google Scholar] [CrossRef] [Green Version]
- Tejedor Alonso, M.A.; Moro Moro, M.; Múgica García, M.V. Epidemiology of anaphylaxis. Clin. Exp. Allergy 2015, 45, 1027–1039. [Google Scholar] [CrossRef]
- Turner, P.J.; Campbell, D.E.; Motosue, M.S.; Campbell, R.L. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J. Allergy Clin. Immunol. Pract. 2020, 8, 1169–1176. [Google Scholar] [CrossRef]
- Gomes, E.R.; Kuyucu, S. Epidemiology and Risk Factors in Drug Hypersensitivity Reactions. Curr. Treat Options Allergy 2017, 4, 239–257. [Google Scholar] [CrossRef]
- Crescioli, G.; Bettiol, A.; Bonaiuti, R.; Tuccori, M.; Rossi, M.; Capuano, A.; Pagani, S.; Spada, G.; Venegoni, M.; Vighi, G.D.; et al. Risk of Hospitalization Associated with Cardiovascular Medications in the Elderly Italian Population: A Nationwide Multicenter Study in Emergency Departments. Front. Pharmacol. 2021, 11, 611102. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Lombardi, N.; Crescioli, G.; Vighi, G.V.; Spada, G.; Romoli, I.; Andreetta, P.; Capuano, A.; Marrazzo, E.; Marra, A.; et al. Analysis of fatal adverse drug events recorded in several Italian emergency departments (the MEREAFaPS study). Intern. Emerg. Med. 2021, 16, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Bettiol, A.; Crescioli, G.; Ravaldi, C.; Bonaiuti, R.; Venegoni, M.; Vighi, G.D.; Mugelli, A.; Mannaioni, G.; Vannacci, A.; et al. Risk of hospitalisation associated with benzodiazepines and z-drugs in Italy: A nationwide multicentre study in emergency departments. Intern. Emerg. Med. 2020, 15, 1291–1302. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Bettiol, A.; Tuccori, M.; Capuano, A.; Bonaiuti, R.; Mugelli, A.; Venegoni, M.; Vighi, G.D.; Vannacci, A.; et al. Italian Emergency Department Visits and Hospitalizations for Outpatients’ Adverse Drug Events: 12-Year Active Pharmacovigilance Surveillance (The MEREAFaPS Study). Front. Pharmacol. 2020, 11, 412. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Crescioli, G.; Bettiol, A.; Marconi, E.; Vitiello, A.; Bonaiuti, R.; Calvani, A.M.; Masi, S.; Lucenteforte, E.; Mugelli, A.; et al. Characterization of serious adverse drug reactions as cause of emergency department visit in children: A 5-years active pharmacovigilance study. BMC Pharmacol. Toxicol. 2018, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- MedDRA Hierarchy—Medical Dictionary for Regulatory Activities n.d. Available online: https://www.meddra.org/how-to-use/basics/hierarchy (accessed on 25 March 2022).
- Cremonesi, P.; di Bella, E.; Montefiori, M.; Persico, L. The Robustness and Effectiveness of the Triage System at Times of Overcrowding and the Extra Costs due to Inappropriate Use of Emergency Departments. Appl. Health Econ. Health Policy 2015, 13, 507–514. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Asai, Y.; Yanishevsky, Y.; Clarke, A.; La Vieille, S.; Scott Delaney, J.; Alizadehfar, R.; Joseph, L.; Mill, C.; Morris, J.; Ben-Shoshan, M. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int. Arch. Allergy Immunol. 2014, 164, 246–252. [Google Scholar] [CrossRef]
- Banerji, A.; Rudders, S.; Clark, S.; Wei, W.; Long, A.A.; Camargo, C.A. Retrospective study of drug-induced anaphylaxis treated in the emergency department or hospital: Patient characteristics, management, and 1-year follow-up. J. Allergy Clin. Immunol. Pract. 2014, 2, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Budnitz, D.S.; Pollock, D.A.; Mendelsohn, A.B.; Weidenbach, K.N.; McDonald, A.K.; Annest, J.L. Emergency department visits for outpatient adverse drug events: Demonstration for a national surveillance system. Ann. Emerg. Med. 2005, 45, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Budnitz, D.S.; Pollock, D.A.; Weidenbach, K.N.; Mendelsohn, A.B.; Schroeder, T.J.; Annest, J.L. National surveillance of emergency department visits for outpatient adverse drug events. J. Am. Med. Assoc. 2006, 296, 1858–1866. [Google Scholar] [CrossRef] [Green Version]
- Budnitz, D.S.; Lovegrove, M.C.; Shehab, N.; Richards, C.L. Emergency Hospitalizations for Adverse Drug Events in Older Americans. N. Engl. J. Med. 2011, 365, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Budnitz, D.S.; Weidenbach, K.N.; Jernigan, D.B.; Schroeder, T.J.; Shehab, N.; Pollock, D.A. National Surveillance of Emergency Department Visits for Outpatient Adverse Drug Events in Children and Adolescents. J. Pediatr. 2008, 152, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, C.E.; Kishi, D.T.; Louie, C. Drug-related illness in emergency department patients. Am. J. Heal. Pharm. 1996, 53, 1422–1426. [Google Scholar] [CrossRef]
- Gabrielli, S.; Clarke, A.E.; Eisman, H.; Morris, J.; Joseph, L.; La Vieille, S.; Small, P.; Lim, R.; Enarson, P.; Zelcer, M.; et al. Disparities in rate, triggers, and management in pediatric and adult cases of suspected drug-induced anaphylaxis in Canada. Immun. Inflamm. Dis. 2018, 6, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Grunau, B.E.; Wiens, M.O.; Rowe, B.H.; McKay, R.; Li, J.; Yi, T.W.; Stenstrom, R.; Schellenberg, R.R.; Grafstein, E.; Scheuermeyer, F.X. Emergency Department Corticosteroid Use for Allergy or Anaphylaxis Is Not Associated with Decreased Relapses. Ann. Emerg. Med. 2015, 66, 381–389. [Google Scholar] [CrossRef]
- Olfson, M. Surveillance of adverse psychiatric medication events. JAMA J. Am. Med. Assoc. 2014, 313, 1256–1257. [Google Scholar] [CrossRef]
- Harduar-Morano, L.; Simon, M.R.; Watkins, S.; Blackmore, C. A population-based epidemiologic study of emergency department visits for anaphylaxis in Florida. J. Allergy Clin. Immunol. 2011, 128, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Chawla, K.; Järvinen, K.M.; Nowak-Wȩgrzyn, A. Anaphylaxis in a New York City pediatric emergency department: Triggers, treatments, and outcomes. J. Allergy Clin. Immunol. 2012, 129, 162–168.e3. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.C.; Budnitz, D.S.; Sorbello, A.; Mehta, H. US-based emergency department visits for fluoroquinolone-associated hypersensitivity reactions. Pharmacoepidemiol. Drug Saf. 2013, 22, 1099–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovegrove, M.C.; Shehab, N.; Hales, C.M.; Poneleit, K.; Crane, E.; Budnitz, D.S. Emergency department visits for antiviral adverse events during the 2009 H1N1 influenza pandemic. Public Health Rep. 2011, 126, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovegrove, M.C.; Geller, A.I.; Fleming-Dutra, K.E.; Shehab, N.; Sapiano, M.R.P.; Budnitz, D.S. US Emergency Department visits for adverse drug events from antibiotics in children, 2011–2015. J. Pediatric. Infect. Dis. Soc. 2019, 8, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Motosue, M.S.; Bellolio, M.F.; Van Houten, H.K.; Shah, N.D.; Li, J.T.; Campbell, R.L. Outcomes of Emergency Department Anaphylaxis Visits from 2005 to 2014. J. Allergy Clin. Immunol. Pract 2018, 6, 1002–1009.e2. [Google Scholar] [CrossRef] [PubMed]
- Motosue, M.S.; Bellolio, M.F.; Van Houten, H.K.; Shah, N.D.; Campbell, R.L. Risk factors for recurrent anaphylaxis-related emergency department visits in the United States. Ann. Allergy Asthma Immunol. 2018, 121, 717–721.e1. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Monroe, K.; Losek, J.D. Anaphylaxis management in the pediatric emergency department: Opportunities for improvement. Pediatr. Emerg. Care 2010, 26, 71–76. [Google Scholar] [CrossRef]
- Schneitman-Mcintire, O.; Farnen, T.A.; Gordon, N.; Chaaan, J.; Toy, W.A. Medication misadventures resulting in emergency department visits at an HMO medical center. Am. J. Heal. Pharm. 1996, 53, 1416–1422. [Google Scholar] [CrossRef]
- Shehab, N.; Patel, P.R.; Srinivasan, A.; Budnitz, D.S. Emergency department visits for antibiotic-associated adverse events. Clin. Infect. Dis. 2008, 47, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Willy, M.E.; Kelly, J.P.; Nourjah, P.; Kaufman, D.W.; Budnitz, D.S.; Staffa, J. Emergency department visits attributed to selected analgesics, United States, 2004–2005. Pharmacoepidemiol. Drug Saf. 2009, 18, 188–195. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Choo, Y.; Park, J.; Choi, J.; Jang, D.; Kim, T.; Jeong, J.W.; Kwon, J.-W. Common causes of emergency department visits for anaphylaxis in Korean community hospitals: A cross-sectional study. Medicine 2019, 98, e14114. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Capua, T.; Pivko, D.; Ben-Shoshan, M.; Benor, S.; Rimon, A. Trends in the diagnosis and management of anaphylaxis in a tertiary care pediatric emergency department. Ann. Allergy Asthma Immunol. 2018, 121, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.H.; Soh, J.Y.; Loh, W.; Lee, K.P.; Tan, S.C.; Heng, W.J.K.; Ibrahim, I.; Lee, B.W.; Chiang, W.C. Cause and clinical presentation of anaphylaxis in Singapore: From infancy to old age. Int. Arch. Allergy Immunol. 2018, 175, 91–98. [Google Scholar] [CrossRef]
- Han, J.E.; Ye, Y.M.; Lee, S. Epidemiology of drug hypersensitivity reactions using 6-year national health insurance claim data from Korea. Int. J. Clin. Pharm. 2018, 40, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Hitti, E.A.; Zaitoun, F.; Harmouche, E.; Saliba, M.; Mufarrij, A. Acute allergic reactions in the emergency department: Characteristics and management practices. Eur. J. Emerg. Med. 2015, 22, 253–259. [Google Scholar] [CrossRef]
- Hsin, Y.C.; Hsin, Y.C.; Huang, J.L.; Yeh, K.W. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pacific J. Allergy Immunol. 2011, 29, 307–312. [Google Scholar]
- Kim, M.Y.; Park, C.S.; Jeong, J.W. Management and educational status of adult anaphylaxis patients at emergency department. Korean J. Intern. Med. 2018, 33, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, M.H.; Cho, Y.J. Different clinical features of anaphylaxis according to cause and risk factors for severe reactions. Allergol. Int. 2018, 67, 96–102. [Google Scholar] [CrossRef]
- Ko, B.S.; Kim, W.Y.; Ryoo, S.M.; Ahn, S.; Sohn, C.H.; Seo, D.W.; Lee, Y.-S.; Lim, K.S.; Kim, T.-B. Biphasic reactions in patients with anaphylaxis treated with corticosteroids. Ann. Allergy Asthma Immunol. 2015, 115, 312–316. [Google Scholar] [CrossRef]
- Rangkakulnuwat, P.; Sutham, K.; Lao-Araya, M. Anaphylaxis: Ten-year retrospective study from a tertiary-care hospital in Asia. Asian Pacific J. Allergy Immunol. 2020, 38, 31–39. [Google Scholar] [CrossRef]
- Bellou, A.; Manel, J.; Samman-Kaakaji, H.; De Korwin, J.D.; Moneret-Vautrin, D.A.; Bollaert, P.E.; Lambert, H. Spectrum of acute allergic diseases in an emergency department: An evaluation of one years’ experience. Emerg. Med. 2003, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Bielen, C.; Bielen, L.; Likić, R. Incidence, etiology, pred.dictors and outcomes of suspected drug hypersensitivity reactions in a tertiary care university hospital’s emergency department: A retrospective study. Wien. Klin. Wochenschr. 2019, 131, 329–336. [Google Scholar] [CrossRef]
- Cianferoni, A.; Novembre, E.; Mugnaini, L.; Lombardi, E.; Bernardini, R.; Pucci, N.; Vierucci, A. Clinical features of acute anaphylaxis in patients admitted to a university hospital: An 11-year retrospective review (1985–1996). Ann. Allergy Asthma Immunol. 2001, 87, 27–32. [Google Scholar] [CrossRef]
- Losappio, L.; Heffler, E.; Bussolino, C.; Cannito, C.D.; Carpentiere, R.; Raie, A.; Di Biase, M.; Bugiani, M.; Rolla, G. Acute urticaria presenting in the emergency room of a general hospital. Eur. J. Intern. Med. 2014, 25, 147–150. [Google Scholar] [CrossRef]
- Quiralte, J.; Blanco, C.; Castillo, R.; Ortega, N.; Carrillo, T. Anaphylactoid reactions due to nonsteroidal antiinflammatory drugs: Clinical and cross-reactivity studies. Ann. Allergy Asthma Immunol. 1997, 78, 293–296. [Google Scholar] [CrossRef]
- Hall, V.; Wong, M.; Munsif, M.; Stevenson, B.R.; Elliott, K.; Lucas, M.; Baird, A.J.; Athan, E.; Young, M.; Pickles, R. Antimicrobial anaphylaxis: The changing face of severe antimicrobial allergy. J. Antimicrob. Chemother. 2020, 75, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, G.; Boscia, E.; Bettiol, A.; Pagani, S.; Spada, G.; Vighi, G.V.; Bonaiuti, R.; Venegoni, M.; Vighi, G.D.; Vannacci, A.; et al. Risk of Hospitalization for Adverse Drug Events in Women and Men: A Post Hoc Analysis of an Active Pharmacovigilance Study in Italian Emergency Departments. Pharmaceuticals 2021, 14, 678. [Google Scholar] [CrossRef]
- Thong, B.Y.H.; Tan, T.C. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharmacol. 2011, 71, 684–700. [Google Scholar] [CrossRef]
- Bernard, T.; Daniel, V.; Maria, J.T.J. Drug Allergies; WAO: Milwaukee, WI, USA, 2021. [Google Scholar]
- Chen, W.; Mempel, M.; Schober, W.; Behrendt, H.; Ring, J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 1418–1427. [Google Scholar] [CrossRef]
- Romano, A.; Blanca, M.; Mayorga, C.; Venuti, A.; Gasbarrini, G. Immediate hypersensitivity to penicillins. Stud. Ital. Subj. Allergy Eur. J. Allergy Clin. Immunol. 1997, 52, 89–93. [Google Scholar] [CrossRef]
- International Collaborative Study of Severe Anaphylaxis. Risk of anaphylaxis in a hospital population in relation to the use of various drugs: An international study. Pharmacoepidemiol. Drug Saf. 2003, 12, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Regateiro, F.S.; Marques, M.L.; Gomes, E.R. Drug-Induced Anaphylaxis: An Update on Epidemiology and Risk Factors. Int. Arch. Allergy Immunol. 2020, 181, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.C. Capturing Drug-Induced Anaphylaxis Through Electronic Health Records: A Step Forward. J. Allergy Clin. Immunol. Pract. 2019, 7, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Warrington, R.; Silviu-Dan, F.; Wong, T. Drug allergy. Allergy Asthma Clin. Immunol. 2018, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Gowland, M.H.; Sharma, V.; Ierodiakonou, D.; Harper, N.; Garcez, T.; Pumphrey, R.; Boyle, R.J. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: An analysis of United Kingdom national anaphylaxis data, 1992–2012. J. Allergy Clin. Immunol. 2015, 135, 956–963.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oussalah, A.; Mayorga, C.; Blanca, M.; Barbaud, A.; Nakonechna, A.; Cernadas, J.; Gotua, M.; Brockow, K.; Caubet, J.-C.; Bircher, A. Genetic variants associated with drugs-induced immediate hypersensitivity reactions: A PRISMA-compliant systematic review. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 443–462. [Google Scholar] [CrossRef] [Green Version]
- Jerschow, E.; Lin, R.Y.; Scaperotti, M.M.; McGinn, A.P. Fatal anaphylaxis in the United States, 1999–2010, Temporal patterns and demographic associations. J. Allergy Clin. Immunol. 2014, 134, 1318–1328.e7. [Google Scholar] [CrossRef] [Green Version]
- Fabbian, F.; Melandri, R.; Borsetti, G.; Micaglio, E.; Pala, M.; De Giorgi, A.; Menegatti, A.M.; Boccafogli, A.; Manfredini, R. Color-coding triage and allergic reactions in an Italian ED. Am. J. Emerg Med. 2012, 30, 826–829. [Google Scholar] [CrossRef]
- D’Antonio, C.D.; Galimberti, M.; Barbone, B.; Calamari, M.; Airoldi, G.; Campanini, M.; Di Pietrantonj, C.; Avanzi, G.C. Suspected acute allergic reactions: Analysis of admissions to the Emergency Department of the AOU Maggiore della Carità Hospital in Novara from 2003 to 2007. Eur. Ann. Allergy Clin. Immunol. 2008, 40, 122–129. [Google Scholar]
- Khan, D.A.; Solensky, R. Drug allergy. J. Allergy Clin. Immunol. 2010, 125, S126–S137. [Google Scholar] [CrossRef]
- Tanno, L.K.; Chalmers, R.; Bierrenbach, A.L.; Simons, F.E.R.; Martin, B.; Molinari, N.; Annesi-Maesano, I.; Worm, M.; Cardona, V.; Papadopoulos, N.G. Changing the history of anaphylaxis mortality statistics through the World Health Organization’s International Classification of Diseases–11. J. Allergy Clin. Immunol. 2019, 144, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, P.S.; Li, J.T.-C. Cephalosporin Allergy. N. Engl. J. Med. 2001, 345, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Tanno, L.K.; Simons, F.E.R.; Sanchez-Borges, M.; Cardona, V.; Moon, H.B.; Calderon, M.A.; Sisul, J.C.; Muraro, A.; Casale, T.; Demoly, P.; et al. Applying prevention concepts to anaphylaxis: A call for worldwide availability of adrenaline auto-injectors. Clin. Exp. Allergy 2017, 47, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundquist, B.K.; Jose, J.; Pauze, D.; Pauze, D.; Wang, H.; Järvinen, K.M. Anaphylaxis risk factors for hospitalization and intensive care: A comparison between adults and children in an upstate New York emergency department. Allergy Asthma Proc. 2019, 40, 41–47. [Google Scholar] [CrossRef]
- Martelli, A.; Ghiglioni, D.; Sarratud, T.; Calcinai, E.; Veehof, S.; Terracciano, L.; Fiocchi, A. Anaphylaxis in the emergency department: A paediatric perspective. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 321–329. [Google Scholar] [CrossRef]
- Clark, S.; Wei, W.; Rudders, S.A.; Camargo, C.A. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J. Allergy Clin. Immunol. 2014, 134, 1125–1130. [Google Scholar] [CrossRef]
- Worm, M.; Francuzik, W.; Renaudin, J.M.; Bilo, M.B.; Cardona, V.; Scherer Hofmeier, K.; Köhli, A.; Bauer, A.; Christoff, G.; Cichocka-Jarosz, E.; et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 1322–1330. [Google Scholar] [CrossRef]
- Triggiani, M.; Patella, V.; Staiano, R.I.; Granata, F.; Marone, G. Allergy and the cardiovascular system. Clin. Exp. Immunol. 2008, 153, 7–11. [Google Scholar] [CrossRef]
| Overall N = 94,073 (%) | Non-Allergy N = 79,993 (%) | Allergy N = 13,532 (%) | Anaphylaxis N = 548 (%) | Adjusted ROR of Allergy (95% CI) | Adjusted ROR of Anaphylaxis (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Sex | Male | 41,075 (43.66) | 35,307 (44.14) | 5507 (40.70) | 261 (47.60) | 1 | 1 |
| Female | 52,832 (56.16) | 44,531 (55.67) | 8014 (59.22) | 287 (52.40) | 0.88 (0.84–0.91) | 1.20 (1.01–1.42) | |
| Missing | 166 (0.18) | 155 (0.19) | 11 (0.08) | - | |||
| Age, years | 0–1 | 2174 (2.31) | 1816 (2.27) | 358 (2.70) | - | 1.19 (1.06–1.34) | - |
| 2–11 | 2954 (3.14) | 2003 (2.50) | 948 (7.00) | 3 (0.55) | 2.97 (2.74–3.22) | 0.21 (0.07–0.66) | |
| 12–17 | 1616 (1.72) | 1222 (1.53) | 386 (2.90) | 8 (1.46) | 1.88 (1.68–2.12) | 0.97 (0.48–1.95) | |
| 18–65 | 42,303 (44.97) | 33,289 (41.61) | 8657 (64.00) | 357 (65.14) | 2.48 (2.39–2.58) | 2.63 (2.21–3.14) | |
| >65 | 44,866 (47.69) | 41,518 (51.90) | 3170 (23.40) | 178 (32.48) | 0.28 (0.27–0.29) | 0.44 (0.37–0.53) | |
| Missing | 160 (0.17) | 145 (0.19) | 13 (0.09) | 2 (0.37) | |||
| Mean (±SD) | 58.50 ± 23.90 | 60.59 ± 23.46 | 46.28 ± 22.98 | 55.70 ± 17.67 | |||
| Ethnicity | Caucasian | 75,668 (80.44) | 64,158 (80.20) | 11,059 (81.72) | 451 (82.30) | 1.12 (1.07–1.78) | 1.16 (0.93–1.45) |
| Other | 2953 (3.14) | 2330 (2.92) | 603 (4.46) | 20 (3.65) | - | - | |
| Missing | 15,452 (16.42) | 13,505 (16.88) | 1870 (13.82) | 77 (14.05) | |||
| Triage codes | Red | 2672 (2.84) | 2251 (2.81) | 292 (2.16) | 129 (23.54) | 0.86 (0.76–0.97) | 10.68 (8.69–13.13) |
| Yellow | 18,596 (19.77) | 16,037 (20.05) | 2376 (17.56) | 183 (33.39) | 0.92 (0.88–0.97) | 2.00 (1.68–2.39) | |
| Green | 36,045 (38.32) | 29,748 (37.19) | 6235 (46.07) | 62 (11.31) | 1.29 (1.24–1.34) | 0.22 (0.17–0.28) | |
| White | 3436 (3.65) | 2719 (3.40) | 717 (5.30) | - | 1.25 (1.15–1.37) | - | |
| Missing | 33,324 (35.42) | 29,238 (36.55) | 3912 (28.91) | 174 (31.76) | |||
| Hospitalization | Yes | 26,644 (28.32) | 24,168 (30.20) | 2105 (15.60) | 371 (67.70) | 0.53 (0.50–0.55) | 5.62 (4.66–6.79) |
| No | 67,429 (71.68) | 55,825 (69.80) | 11,427 (84.40) | 177 (32.30) | - | - |
| Year | Allergy Events N = 13,532 | Anaphylaxis Events N = 548 | Adrenaline Use N = 323 (% Row) |
|---|---|---|---|
| 2012 | 1610 | 59 | 37 (62.71) |
| 2013 | 2552 | 108 | 61 (56.48) |
| 2014 | 2737 | 101 | 50 (49.50) |
| 2015 | 1609 | 52 | 36 (69.23) |
| 2016 | 804 | 36 | 19 (52.78) |
| 2017 | 1206 | 65 | 41 (63.08) |
| 2018 | 1838 | 64 | 45 (70.31) |
| 2019 | 1176 | 63 | 34 (53.97) |
| Anaphylaxis N = 608 (%) | Non-Allergy N = 104,366 (%) | Unadjusted ROR (95% CI) | Adjusted ROR (95% CI) | |
|---|---|---|---|---|
| Antibacterials | 327 (53.78) | 10,744 (10.29) | 9.99 (8.51–11.75) | 8.75 (7.47–10.25) |
| Penicillins | 218 (66.67) | 5503 (51.22) | 9.91 (8.37–11.73) | 8.98 (7.57–10.65) |
| Cephalosporins | 69 (21.10) | 1291 (12.02) | 10.09 (7.80–13.05) | 10.75 (8.32–13.88) |
| Fluoroquinolones | 28 (8.56) | 1881 (17.51) | 2.60 (1.77–3.80) | 2.30 (1.57–3.38) |
| Macrolides | 6 (1.83) | 1155 (10.75) | 0.88 (0.39–1.97) | 0.75 (0.34–1.68) |
| Glycopeptides | 5 (1.53) | 347 (3.23) | 2.45 (1.01–5.96) | 2.28 (0.94–5.53) |
| Sulfamet./Trimetop. | 1 (0.31) | 337 (3.14) | 0.50 (0.07–3.58) | 0.42 (0.06–2.99) |
| NSAIDs | 78 (12.83) | 4980 (4.77) | 2.88 (2.27–3.66) | 2.18 (1.71–2.78) |
| Diclofenac | 32 (41.03) | 1058 (21.24) | 5.33 (3.71–7.65) | 4.45 (3.11–6.39) |
| Ketoprofen | 24 (30.77) | 1727 (34.68) | 2.40 (1.59–3.62) | 1.65 (1.08–2.52) |
| Ketorolac | 5 (6.41) | 291 (5.84) | 2.91 (1.20–7.08) | 2.26 (0.93–5.49) |
| Flurbiprofen | 4 (5.13) | 101 (2.03) | 6.72 (2.47–18.31) | 5.21 (1.91–14.25) |
| Indomethacin | 3 (3.85) | 191 (3.84) | 2.66 (0.85–8.34) | 2.09 (0.66–6.60) |
| Nimesulide | 2 (2.56) | 581 (11.67) | 0.58 (0.14–2.33) | 0.43 (0.11–1.74) |
| Etoricoxib | 2 (2.56) | 234 (4.70) | 1.44 (0.36–5.82) | 1.30 (0.32–5.26) |
| Dexibuprofen | 2 (2.56) | 39 (0.78) | 8.68 (2.09–36.02) | 7.88 (1.90–32.68) |
| Radiology contrast agents | 42 (6.92) | 554 (0.53) | 13.73 (9.92–19.00) | 11.52 (8.33–15.92) |
| Iomeprol | 16 (38.10) | 201 (36.28) | 13.83 (8.26–23.17) | 11.52 (6.89–19.25) |
| Iopromide | 12 (28.57) | 88 (15.88) | 25.56 (12.81–43.33) | 20.26 (11.04–37.19) |
| Iobitridol | 4 (9.52) | 40 (7.22) | 17.06 (6.08–47.84) | 14.96 (5.41–41.41) |
| Iodixanol | 3 (7.14) | 52 (9.39) | 9.82 (3.06–31.54) | 8.62 (2.70–27.53) |
| Iopamidol | 3 (7.14) | 36 (6.50) | 14.91 (4.36–46.22) | 13.40 (4.05–44.40) |
| Other contrast agents | 4 (9.52) | 68 (12.27) | 10.03 (3.65–27.59) | 7.44 (2.71–20.47) |
| Analgesic drugs | 32 (5.26) | 11,131 (10.67) | 0.46 (0.33–0.66) | 0.47 (0.33–0.67) |
| Paracetamol * | 15 (46.88) | 2152 (19.33) | 1.19 (0.71–1.98) | 1.02 (0.61–1.71) |
| Acetylsalicylic acid | 11 (34.38) | 5589 (50.21) | 0.32 (0.18–0.58) | 0.38 (0.21–0.68) |
| Tramadol | 2 (6.25) | 859 (7.72) | 0.39 (0.10–1.58) | 0.34 (0.09–1.39) |
| Pethidine | 2 (6.25) | 12 (0.11) | 28.34 (6.33–126.95) | 22.99 (4.92–107.37) |
| Other analgesics | 2 (6.25) | 254 (2.28) | 1.34 (0.33–5.38) | 1.44 (0.36–5.86) |
| Antineoplastic drugs | 23 (3.78) | 7887 (7.56) | 0.47 (0.31–0.72) | 0.41 (0.27–0.63) |
| Paclitaxel | 7 (30.43) | 645 (8.18) | 1.85 (0.87–3.91) | 1.56 (0.73–3.30) |
| Oxaliplatin | 5 (21.74) | 486 (6.16) | 1.75 (0.72–4.24) | 1.53 (0.63–3.72) |
| Cetuximab | 2 (8.70) | 101 (1.28) | 3.36 (0.83–13.67) | 3.35 (0.83–13.54) |
| Trastuzumab | 2 (8.70) | 166 (2.10) | 2.05 (0.51–8.27) | 1.78 (0.44–7.15) |
| Rituximab | 2 (8.70) | 546 (6.92) | 0.62 (0.15–2.49) | 0.59 (0.14–2.37) |
| Other antineoplas. drugs | 5 (21.74) | 1226 (15.54) | 0.69 (0.28–1.66) | 0.59 (0.25–1.43) |
| Author, Year | Country | Period of Observation and Study Design | Participating Centres | Patients’ Selection | Age and Sex | Total of Patients | ED Visits for Allergy or Anaphylaxis | Drug-Related Allergy or Anaphylaxis | Hospitalization for Drug-Related Allergy or Anaphylaxis | Causative Drug Classes |
|---|---|---|---|---|---|---|---|---|---|---|
| Asai, 2014 [21] | Canada | 2011–2012 Retrospective single centre study | Adult tertiary care ED | Diagnosis of anaphylaxis or allergic reactions (ICD-10 codes) | Median (IQR): 31.5 (26.4–44.0) years Females 66.3% | 37,730 | 98 | 18 | NR | Amoxicillin 16.7% |
| Banerji, 2014 [22] | USA | 2006–2008 Retrospective database analysis | Truven Health MarketScan Commercial and Medicare Supplemental Databases (Truven, Ann Arbor, Mich) | Diagnosis of anaphylaxis (ICD9-CM codes) | Mean ± SD 48 ± 19 years Females 71% | 716 | 716 | 716 | 205 | NR |
| Bellou, 2003 [52] | France | 1 year (1998) Retrospective single centre study | General hospital ED | Cases of suspected allergic reaction | Mean ± SD 55 ± 18.5 years Females 51% | 324 | 324 | 25 | Overall, 90 | Beta-lactams 28% Macrolides 20% NSAIDs 52% |
| Bielen, 2019 [53] | Croatia | 2012–2015 Retrospective single centre study | Tertiary care university hospital ED | Cases of hypersensitivity (SMQ) | <29 years: 8005 30–39 years: 7875 40–49 years: 8095 50–64 years; 17611 65–74 years: 13414 >75 years: 16982 Females 54.6% | 71,982 | 3039 | 627 | 38 | Antibiotics 44.7% Analgesics and NSAIDs 18.7% |
| Budnitz, 2005 [23] | USA | 3 months Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of ADE | <2 years: 56; 2–9 years: 62; 10–19 years: 44; 20–29: 66; 30–39 years: 59; 40–49 years: 84; 50–59 years: 65; 60–69 years: 57; 70–79: 58; ≥80 years: 47 Females 63.9% | 598 | 155 | 155 | 4 | Antibiotics 42.9% Non-opioid analgesics 29.3% Cardiovascular agents 24% |
| Budnitz, 2006 [24] | USA | 2004–2005 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of ADE | 0–4 years: 104,185; 5–17 years: 225,082; 18–44 years: 362,044; 45–64: 147,178; ≥65 years: 83,549 Females 44.7% | 701,547 estimated annual ED visits | 235,202 estimated annual ED visits | 235,202 estimated annual ED visits | 13,232 estimated annual ED visits | NR |
| Budnitz, 2011 [25] | USA | 2007–2009 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of ADE | 65–69 years: 2470; 70–74 years: 1840; 75–79 years: 2629; 80–84 years: 2476; ≥85 years: 2621 Females 59% | 265,802 estimated annual ED visits | 39,455 estimated annual ED visits | 39,455 estimated annual ED visits | 5617 estimated annual hospitalization | Cardiovascular agents Antibiotics |
| Cho, 2019 [42] | Korea | 2012–2016 Cross-sectional multicentre study | 7 community hospitals EDs | Cases of anaphylaxis (ICD-10 codes) | Mean ± SD 51.5 ± 16.0 Females 34.7% | 325,857 | 1021 | 135 | NR | NSAIDs 28.1% Antibiotics 15.6% Antibiotics and NSAIDs 3.7% Radiocontrast media 2.2% |
| Cianferoni, 2001 [54] | Italy | 1985–1996 Retrospective chart review | University hospital ED | Diagnosis of acute anaphylaxis | Mean ± SD 42 ± 18 Females 45% | 113 | 113 | 52 | NR | Antibiotics 48% NSAIDs 35% |
| Cohen, 2008 [26] | USA | 2004–2005 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of ADE | <1 year: 386; 1–4 years: 703; 5–8 years: 302; 9–12 years: 216; 13–18 years: 475 Females NR | 6681 | 2802 | 2802 | Overall, 5.1 | Antibiotics 60.8% Analgesics 9.2% Multiple agents 6.7% Respiratory medications 5.9% Psychotropic medications 2.2% |
| Cohen, 2018 [43] | Israel | 2013–2016 Retrospective single centre study | Paediatric hospital ED | Cases of allergic reactions or anaphylaxis (Anaphylaxis Criteria, Sampson et al.) | Mean 6.8 years (range 0–16 years) Females 34.7% | 113,067 | 428 | 10 | 8 (1 of which in ICU) | NR |
| Dennehy, 1996 [27] | USA | 30 days (1994) Single centre study | General hospital ED | Cases of drug-related illness | Mean ± SD 41.7 ± 22.5 years Females 50% | 50 | 7 | 7 | Overall, 8 | NR |
| Gabrielli, 2018 [28] | Canada | 2012–2016 Retrospective/prospective multicentre study | 3 paediatric hospital and 1 general hospital EDs | Cases of anaphylaxis (diagnosis at ED presentation or ICD codes) | Median 49.4 (IQR 40.1–62.9) adults; median 8.00 (IQR 3.79–15.36) children Females: 71.9% adults; 47.1% children | 884,000 | 1913 | 115 (64 adults; 51 children) | Admitted (5/51 = 9.8% children, 3/64 = 4.7% adults) Admitted ICU (1/51 = 2.0% children, 1/64 = 1.6% adults) Admitted hospital ward (4/51 = 7.8% children, 2/64 = 3.1% adults) | Beta-lactams (28.1% adults, 31.4% children) Quinolones (20.3% adults, 2% children) Other antibiotics (6.3% adults, 0% children) NSAIDs (20.3% adults, 21.6% children) Radiocontrast media (3.1% adults, 3.9% children) |
| Goh, 2018 [44] | Singapore | 2014–2015 Prospective multicentre study | 3 general hospital EDs | Cases of anaphylaxis (ICD-9 codes) | Median 23 years (range 3 months to 88 years and 9 months) Females 49.1% | 7373 | 426 | 85 (66 adults; 19 children) | 3 | NSAIDs (24.2% adults, 52.6% children) Antibiotics (21.2% adults, 5.3% children) Paracetamol (3.0% adults, 10.5% children) |
| Grunau, 2015 [29] | USA and Canada | 2007–2012 Retrospective multicentre cohort study | 2 teaching hospital EDs | Diagnosis of allergic reaction | Median (IQR): 34 (27–47) years patients treated with steroids; 35 (26–49) years patients treated without steroids Females 60.9% | 2701 | 2701 | 702 | 11 | Anti-infective agents 48.9% Nervous system agents 10.3% (analgesics 2%) Radiocontrast media 3.7% NSAIDs 2.4% |
| Hall, 2020 [57] | Australia | 2010–2015 Retrospective multicentre cohort study | 5 university tertiary hospital EDs | Cases of antimicrobial anaphylaxis (ICD-10 codes) | Median 51 years (IQR 36–67) Females 61% | 293 | 185 | 185 | 7 ICU admission | Overall (out of 185) Penicillins 39.9% Cephalosporins 35.1% Amino-penicillins 18.5% Amino-cephalosporins 17.0% |
| Hampton, 2014 [30] | USA | 2009–2011 Retrospective multicentre study; database analysis | Administrative database 63 centres | Cases of psychiatric medication-related ADE | 19–44 years: 49.4 (46.5–52.4) 45–64 years: 33.3 (30.7–35.9) ≥65 years: 17.3 (14.7–19.8) Females 61.9% | 89,094 estimated annual ED visits | 11,493 estimated annual ED visits | 11,493 estimated annual ED visits | Overall, 17,188 estimated annual hospitalization | Zolpidem Quetiapine Alprazolam Lorazepam Haloperidol Clonazepam Trazodone Citalopram Lithium Risperidone |
| Han, 2018 [45] | Korea | 2009–2014 Retrospective cohort study; database analysis | National insurance claim database of the Health Insurance Review and Assessment (HIRA) | Cases of drug hypersensitivity reactions (ICD-10 codes) | 88,003 ≤19 years 169,103 20–44 years 180,535 45–64 years 97,408 ≥65 years Females 57.5% | 535,049 | 3984 (T88.6 code) | 3984 (T88.6 code) | 184 (T88.6 code) | NR |
| Harduar-Morano, 2011 [31] | USA | 2005–2006 Retrospective multicentre study | General hospital EDs | Diagnosis of anaphylaxis (ICD9-CM codes) | Mean ± SD 38.7 ± 21.46 Females 57% | 2751 | 2751 | 228 | 54 | NR |
| Hitti, 2015 [46] | Lebanon | July–December 2009 Retrospective single centre study | Tertiary care centre ED | Cases of acute allergic reaction (ICD-9 codes) | Mean ± SD 31.8 ± 19.2 years Females 42% | 293 | 245 | 58 | Overall, 1 patient was hospitalized | Antibiotics 8.2% NSAIDs 4.9% |
| Hsin, 2011 [47] | India | 2000–2010 Retrospective single centre cohort study | General hospital ED | Diagnosis of anaphylaxis (ICD9 codes) | Mean age overall 43.3 years Female 47% | 201 | 86 | Overall, 161 | NR | NSAIDs Antibiotics Chemotherapy Anti-epileptics Contrast media Immunotherapy Biologics H1N1 Vaccine Anaesthesia |
| Huang, 2012 [32] | USA | 2004–2008 Retrospective single centre study | Paediatric hospital ED | Cases of anaphylaxis | Median (IQR) 8 (4 months–18 years) years Females 49% | 192 (20 had multiple reactions) | 192 | 19 | Overall, 28 | NR |
| Jones, 2013 [33] | USA | 2004–2013 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of fluoroquinolone-associated hypersensitivity ADEs | Mean age overall 48.22 years Females 73.7% | 102,536 | 1659 | 1422 | 96 | Ciprofloxacin Levofloxacin Moxifloxacin Gemifloxacin Ofloxacin |
| Kim MY, 2018 (a) [48] | Korea | 2011–2013 Retrospective multicentre study | 2 tertiary hospitals and 1 secondary hospital EDs | Cases of anaphylaxis (ICD codes) | Mean ± SD 46 ± 17.1 Females 55.2% | 194 | 194 | 151 | NR | Antibiotics Acetylsalicylic acid Radiocontrast media NSAIDs |
| Kim MY, 2018 (b) [49] | South Korea | 2003–2016 Retrospective single centre study | Tertiary university hospital ED | Cases of anaphylaxis (Korean Standard Classification of Disease) | Mean ± SD 41.1 ± 23.4 Females 48.2% | 199 | 199 | 72 | 13 | Overall (out of 199) Antibiotics 40.2% NSAIDs 33.3% Radiocontrast media 11.1% |
| Ko, 2015 [50] | Korea | 2007–2014 Single centre study | Tertiary teaching hospital ED | Cases of anaphylaxis (Skin or mucosal tissue involvement; Respiratory compromise; Systolic blood pressure <90 mmHg or syncope; Gastrointestinal symptoms) | Mean ± SD 48.4 ± 15.7 years Females 54.9% | 655 | 415 | 187 | Overall, 3 patients were hospitalized | Radiocontrast media 70 NSAIDs 39 Cephalosporins 34 Anticancer agents 16 |
| Losappio, 2014 [55] | Italy | 2011 Retrospective single centre study | General hospital ED | Cases of allergic urticaria (ICD-9 codes) | Mean 35.4 years (range 0–90 years) Females 49.2% | 44,112 | 459 | 92 (79 adults; 13 children) | NR | NSAIDs Beta-lactams |
| Lovegrove, 2011 [34] | USA | 2006–2009 Retrospective multicentre study | Drug Abuse Warning Network (DAWN), 250 non-federal, short-stay general hospitals | Cases of antivirals-related ADE | <6 years: 139; 6–11 years: 103; 12–17 years: 58; 18–44 years: 332; 45–64 years: 161; ≥65 years: 89 Female 59.3% | 879 | 274 | 274 | Overall, 125 | Amantadine Rimantadine Oseltamivir Zanamivir |
| Lovegrove, 2019 [35] | USA | 2011–2015 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of antibiotics-related ADE in children (MedDRA) | 2870 < 1–2 years 743 3–4 years 1187 5–9 years 1742 10–19 years Females 52.1% | 6542 | 5763 | 5763 | Overall, 265 | Overall (out of 6542) Penicillins 59.7% Cephalosporins 11.2% Sulfonamides 9.5% |
| Motosue, 2018 (a) [36] | USA | 2005–2014 Prospective observational study; database analysis | OLDW administrative database | Cases of anaphylaxis (ICD-9 codes) | Median 36 years (interquartile range 17–52) Females 57.5% | 56,212 | 56,212 | 6720 | Inpatient 717 and ICU 409 | NR |
| Motosue, 2018 (b) [37] | USA | 2008–2012 Retrospective study; database analysis | Administrative claims database (OptumLabs Data Warehouse) | Cases of anaphylaxis and anaphylactic shock (ICD-9 codes) | Median 42 years (range 1–87 years) Females 58.3% | 7367 | 7367 | 1076 | Overall, 532 ICU admission | NR |
| Quiralte, 1997 [56] | Spain | 1992–1995 Prospective single centre study | University hospital ED | Cases of NSAIDs-related anaphylaxis | Mean ± SD 35.7 ± 13.9 Females 71% | 21 | 21 | 21 | NR | Dipyrone 57.1% Propyphenazone 14.2% Acetic derivatives (diclofenac and indomethacin) 14.2% |
| Rangkakulnuwat, 2020 [51] | Thailand | 2007–2016 Retrospective single centre study | University hospital ED | Cases of anaphylaxis (ICD-10 codes) | Median 24.0 years (IQR 19.0–43.0) Females 57.2% | 10,848,695 | 441 | 79 | NR | NSAIDs 7.4% Antimicrobial Agents 4.0% Radiocontrast media 0.9% |
| Russell, 2010 [38] | USA | 2002–2006 Retrospective single centre cross-sectional study | Tertiary care paediatric hospital ED | Diagnosis of anaphylaxis (ICD-9 codes) | Mean ± SD 9.49 ± 5.56 Females 36% | 103 | 103 | 15 | 4 | Antibiotics Intravenous contrast |
| Schneitman Mcintire, 1996 [39] | USA | 1992–1993 Retrospective single centre study | General hospital ED | Patients who experienced medication misadventures | 15–44 years 38% 65 years or older 33% Females 62% | 62,216 | 221 | 204 | 7 | Trimetoprim sulfametoxazol 34% Amoxicillin 21% Ibuprofen 5.4% |
| Shehab, 2008 [40] | USA | 2004–2005 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of antibiotics-related ADE | <1 years: 545; 1–4 years: 976; 5–14 years: 656; 15–44 years: 2577; 45–64 years: 1143; 65–79 years: 507; ≥80 years: 210 Females 64.4% | 142,505 estimated annual ED visits | 112,116 estimated annual ED visits | 112,116 estimated annual ED visits | 8738 estimated annual hospitalization | Penicillins 36.9% Cephalosporins 12.2% Fluoroquinolones 13.5% Sulfonamide trimethoprim 11.8% Macrolides and Ketolides 6.9% Tetracyclines 3.1% Vancomycin Linezolid 0.8% |
| Willy, 2009 [41] | USA | 2004–2005 Retrospective multicentre study; database analysis | NEISS-CADES database | Cases of analgesics-related ADE | 0–9 years: 32,222; 10–19 years: 17,012; 20–29 years: 28,298; 30–39 years: 23,165; 40–49 years: 22,706; 50–59 years: 18,767; 60–69 years: 14,590; 70–79 years: 15,030; 80–89 years: 14,933; ≥90 years: 1998 Females 57% | 188,721 | 58,101 | 58,101 | Overall, 22,646 | Acetaminophen Non-narcotic-acetaminophen combination Narcotic- acetaminophen combination Acetylsalicylic acid Ibuprofen Naproxen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagani, S.; Lombardi, N.; Crescioli, G.; Vighi, V.G.; Spada, G.; Andreetta, P.; Capuano, A.; Vannacci, A.; Venegoni, M.; Vighi, G.D.; et al. Drug-Related Hypersensitivity Reactions Leading to Emergency Department: Original Data and Systematic Review. J. Clin. Med. 2022, 11, 2811. https://doi.org/10.3390/jcm11102811
Pagani S, Lombardi N, Crescioli G, Vighi VG, Spada G, Andreetta P, Capuano A, Vannacci A, Venegoni M, Vighi GD, et al. Drug-Related Hypersensitivity Reactions Leading to Emergency Department: Original Data and Systematic Review. Journal of Clinical Medicine. 2022; 11(10):2811. https://doi.org/10.3390/jcm11102811
Chicago/Turabian StylePagani, Silvia, Niccolò Lombardi, Giada Crescioli, Violetta Giuditta Vighi, Giulia Spada, Paola Andreetta, Annalisa Capuano, Alfredo Vannacci, Mauro Venegoni, Giuseppe Danilo Vighi, and et al. 2022. "Drug-Related Hypersensitivity Reactions Leading to Emergency Department: Original Data and Systematic Review" Journal of Clinical Medicine 11, no. 10: 2811. https://doi.org/10.3390/jcm11102811
APA StylePagani, S., Lombardi, N., Crescioli, G., Vighi, V. G., Spada, G., Andreetta, P., Capuano, A., Vannacci, A., Venegoni, M., Vighi, G. D., & on behalf of the MEREAFaPS Study Group. (2022). Drug-Related Hypersensitivity Reactions Leading to Emergency Department: Original Data and Systematic Review. Journal of Clinical Medicine, 11(10), 2811. https://doi.org/10.3390/jcm11102811









