CD8+ T Cells in OA Knee Joints Are Differentiated into Subsets Depending on OA Stage and Compartment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Cell Preparation and Isolation
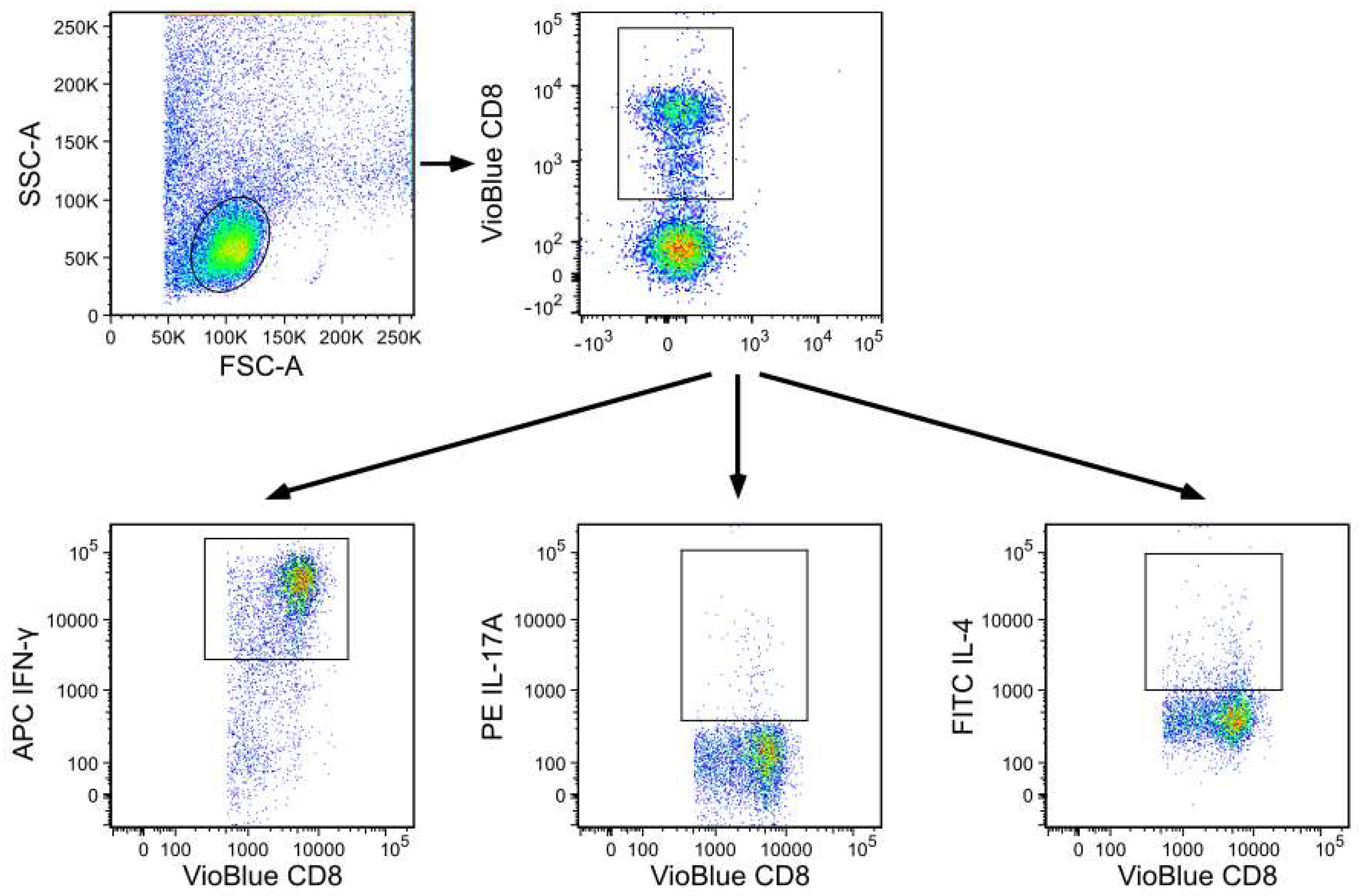
2.4. Flow Cytometry Analysis of Cell Surface Markers and Intracellular Staining
2.5. Statistical Analysis
3. Results
3.1. Differentiation of CD8+ T Cell Subsets in the SF Compared to PB
3.2. Differentiation of CD8+ T Cell Subsets in SM Compared to PB
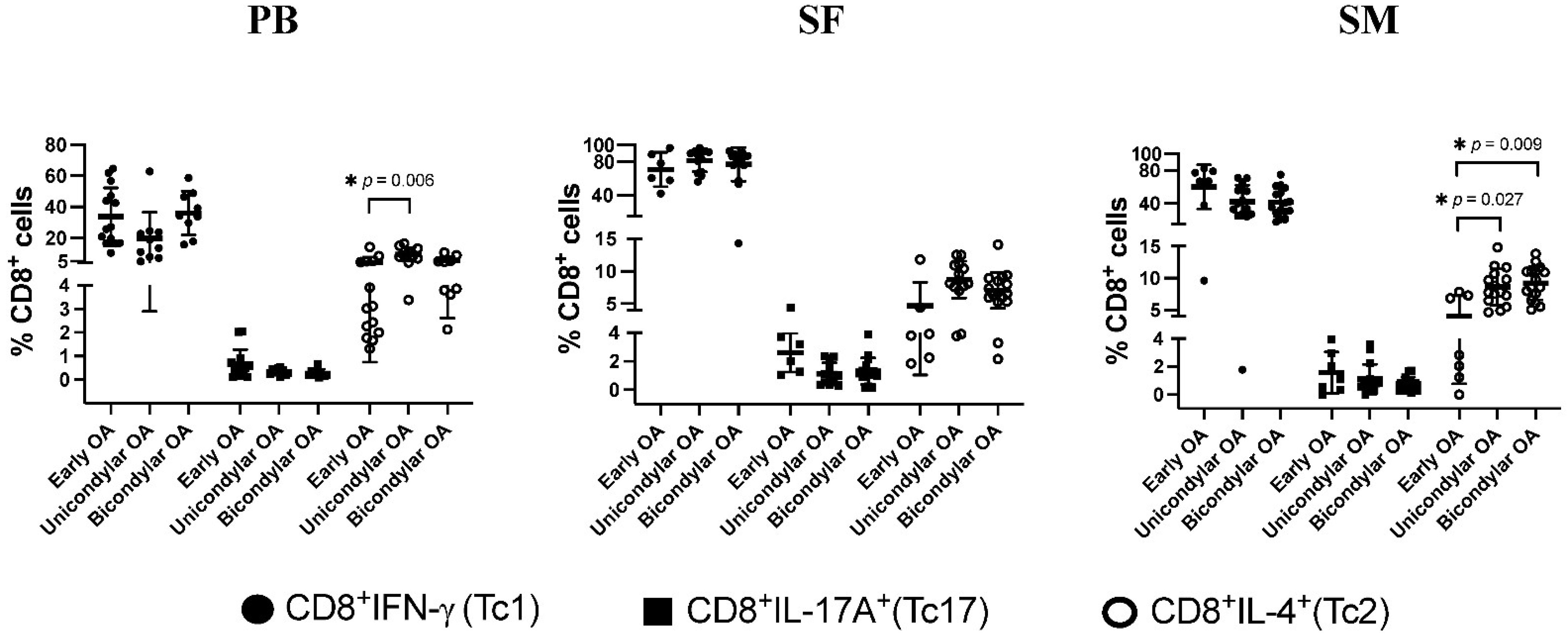
3.3. Comparison of CD8+ T Cell Differentiation between Joint Compartments—The SF versus the SM
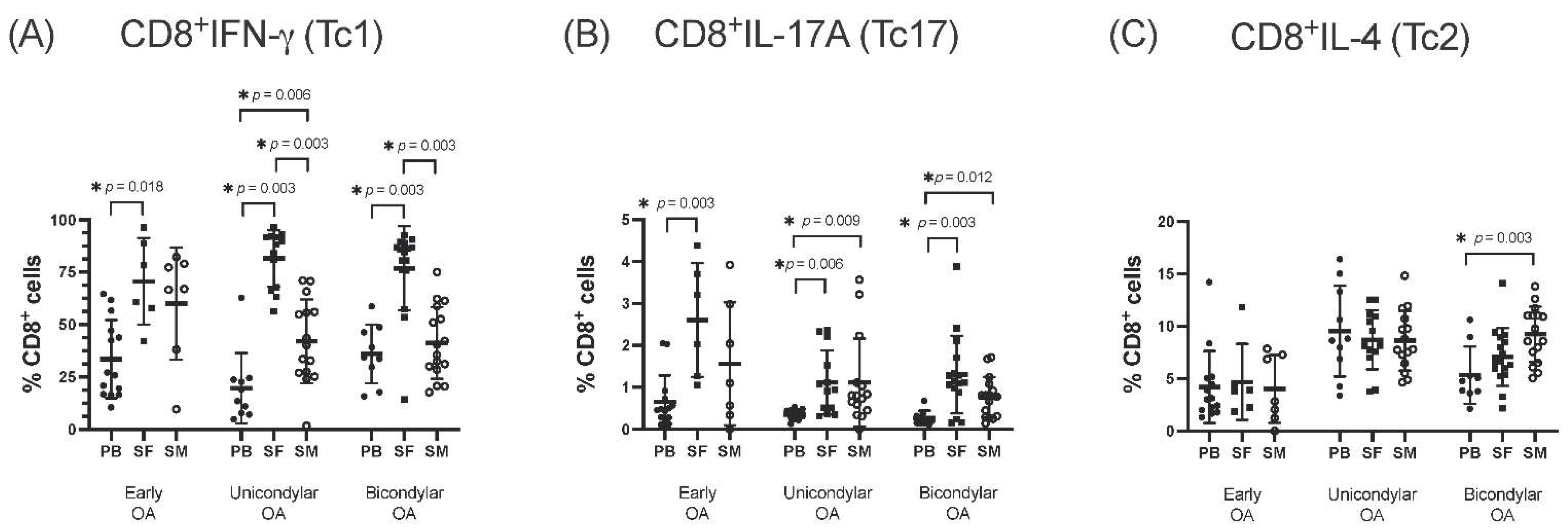
3.4. Differentiation of CD8+ T Cell Subsets in Relation to OA Stage
3.5. Tc1/Tc2 and Tc17/Tc2 Relation Dependent on K&L Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Baar, M.E.; Dekker, J.; Oostendorp, R.A.; Bijl, D.; Voorn, T.B.; Bijlsma, J.W. Effectiveness of exercise in patients with osteoarthritis of hip or knee: Nine months’ follow up. Ann. Rheum. Dis. 2001, 60, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fored, C.M.; Ejerblad, E.; Lindblad, P.; Fryzek, J.P.; Dickman, P.W.; Signorello, L.B.; Lipworth, L.; Elinder, C.G.; Blot, W.J.; McLaughlin, J.K.; et al. Acetaminophen, aspirin, and chronic renal failure. N. Engl. J. Med. 2001, 345, 1801–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, L. The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors. Semin. Arthritis Rheum. 2002, 32, 25–32. [Google Scholar] [CrossRef]
- Platzer, H.; Nees, T.A.; Reiner, T.; Tripel, E.; Gantz, S.; Hagmann, S.; Moradi, B.; Rosshirt, N. Impact of Mononuclear Cell Infiltration on Chondrodestructive MMP/ADAMTS Production in Osteoarthritic Knee Joints—An Ex Vivo Study. J. Clin. Med. 2020, 9, 1279. [Google Scholar] [CrossRef]
- Rosshirt, N.; Trauth, R.; Platzer, H.; Tripel, E.; Nees, T.A.; Lorenz, H.M.; Tretter, T.; Moradi, B. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther. 2021, 23, 37. [Google Scholar] [CrossRef]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef] [Green Version]
- de Lange-Brokaar, B.J.; Ioan-Facsinay, A.; van Osch, G.J.; Zuurmond, A.M.; Schoones, J.; Toes, R.E.; Huizinga, T.W.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef] [Green Version]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [Green Version]
- Haynes, M.K.; Hume, E.L.; Smith, J.B. Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin. Immunol. 2002, 105, 315–325. [Google Scholar] [CrossRef]
- Ishii, H.; Tanaka, H.; Katoh, K.; Nakamura, H.; Nagashima, M.; Yoshino, S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthr. Cartil. 2002, 10, 277–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlowska, J.; Mikosik, A.; Soroczynska-Cybula, M.; Jozwik, A.; Luczkiewicz, P.; Mazurkiewicz, S.; Lorczynski, A.; Witkowski, J.M.; Bryl, E. Different distribution of CD4 and CD8 T cells in synovial membrane and peripheral blood of rheumatoid arthritis and osteoarthritis patients. Folia Histochem. Cytobiol. 2009, 47, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Aggarwal, A.; Sharma, R.; Randhawa, A.; Sahni, D.; Jacob, J.; Sharma, V.; Aggarwal, A. Chronic inflammation during osteoarthritis is associated with an increased expression of CD161 during advanced stage. Scand. J. Immunol. 2019, 90, e12770. [Google Scholar] [CrossRef]
- Hsieh, J.L.; Shiau, A.L.; Lee, C.H.; Yang, S.J.; Lee, B.O.; Jou, I.M.; Wu, C.L.; Chen, S.H.; Shen, P.C. CD8+ T cell-induced expression of tissue inhibitor of metalloproteinses-1 exacerbated osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19951–19970. [Google Scholar] [CrossRef] [Green Version]
- Apinun, J.; Sengprasert, P.; Yuktanandana, P.; Ngarmukos, S.; Tanavalee, A.; Reantragoon, R. Immune Mediators in Osteoarthritis: Infrapatellar Fat Pad-Infiltrating CD8+ T Cells Are Increased in Osteoarthritic Patients with Higher Clinical Radiographic Grading. Int. J. Rheumatol. 2016, 2016, 9525724. [Google Scholar] [CrossRef] [PubMed]
- Mittrucker, H.W.; Visekruna, A.; Huber, M. Heterogeneity in the differentiation and function of CD8(+) T cells. Arch. Immunol. Ther. Exp. 2014, 62, 449–458. [Google Scholar] [CrossRef]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Luyten, F.P.; Denti, M.; Filardo, G.; Kon, E.; Engebretsen, L. Definition and classification of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Luyten, F.P.; Bierma-Zeinstra, S.; Dell’Accio, F.; Kraus, V.B.; Nakata, K.; Sekiya, I.; Arden, N.K.; Lohmander, L.S. Toward classification criteria for early osteoarthritis of the knee. Semin. Arthritis Rheum. 2018, 47, 457–463. [Google Scholar] [CrossRef]
- Rosshirt, N.; Hagmann, S.; Tripel, E.; Gotterbarm, T.; Kirsch, J.; Zeifang, F.; Lorenz, H.M.; Tretter, T.; Moradi, B. A predominant Th1 polarization is present in synovial fluid of end-stage osteoarthritic knee joints—Analysis of peripheral blood, synovial fluid & synovial membrane. Clin. Exp. Immunol. 2018, 195, 395–406. [Google Scholar] [CrossRef]
- Kriegova, E.; Manukyan, G.; Mikulkova, Z.; Gabcova, G.; Kudelka, M.; Gajdos, P.; Gallo, J. Gender-related differences observed among immune cells in synovial fluid in knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubberts, E.; Joosten, L.A.; van de Loo, F.A.; van den Gersselaar, L.A.; van den Berg, W.B. Reduction of interleukin-17-induced inhibition of chondrocyte proteoglycan synthesis in intact murine articular cartilage by interleukin-4. Arthritis Rheum. 2000, 43, 1300–1306. [Google Scholar] [CrossRef]
- Benderdour, M.; Tardif, G.; Pelletier, J.P.; Di Battista, J.A.; Reboul, P.; Ranger, P.; Martel-Pelletier, J. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: Differential activation of AP-1 members by IL-17 and IL-1beta. J. Rheumatol. 2002, 29, 1262–1272. [Google Scholar]
- Honorati, M.C.; Bovara, M.; Cattini, L.; Piacentini, A.; Facchini, A. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthr. Cartil. 2002, 10, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Schuerwegh, A.J.; Dombrecht, E.J.; Stevens, W.J.; Van Offel, J.F.; Bridts, C.H.; De Clerck, L.S. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr. Cartil. 2003, 11, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [Green Version]
- Ene, R.; Sinescu, R.D.; Ene, P.; Cirstoiu, M.M.; Cirstoiu, F.C. Synovial inflammation in patients with different stages of knee osteoarthritis. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2015, 56, 169–173. [Google Scholar]
- Zhu, W.; Zhang, X.; Jiang, Y.; Liu, X.; Huang, L.; Wei, Q.; Huang, Y.; Wu, W.; Gu, J. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin. Rheumatol. 2020, 39, 523–532. [Google Scholar] [CrossRef]
- Moradi, B.; Hagmann, S.; Zahlten-Hinguranage, A.; Caldeira, F.; Putz, C.; Rosshirt, N.; Schonit, E.; Mesrian, A.; Schiltenwolf, M.; Neubauer, E. Efficacy of multidisciplinary treatment for patients with chronic low back pain: A prospective clinical study in 395 patients. J. Clin. Rheumatol. 2012, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Rosshirt, N.; Tripel, E.; Kirsch, J.; Barie, A.; Zeifang, F.; Gotterbarm, T.; Hagmann, S. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin. Exp. Immunol. 2015, 180, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arntz, O.J.; Geurts, J.; Veenbergen, S.; Bennink, M.B.; van den Brand, B.T.; Abdollahi-Roodsaz, S.; van den Berg, W.B.; van de Loo, F.A. A crucial role for tumor necrosis factor receptor 1 in synovial lining cells and the reticuloendothelial system in mediating experimental arthritis. Arthritis Res. Ther. 2010, 12, R61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total Study Population | Early OA | Unicondylar OA | Bicondylar OA | |
|---|---|---|---|---|
| Number of patients, n | 55 | 14 | 19 | 22 |
| Gender, n (%) | ||||
| Male | 23 (41.8%) | 4 (28.6%) | 11 (58.0%) | 8 (36.4%) |
| Female | 32 (58.2%) | 10 (71.4%) | 8 (42.1%) | 14 (63.6%) |
| Age at surgery, years | 60.6 ± 15.2 | 41.9 ± 13.1 | 65.0 ± 9.6 | 68.8 ± 9.3 |
| Mean ± s.d. (IQR) | (52.5–72) | (31.3–47.8) | (60.5–69.5) | (61.3–74.8) |
| BMI, kg/m2 | 29.1 ± 5.8 | 25.6 ± 3.6 | 29.9 ± 5.5 | 30.6 ± 6.4 |
| Mean ± s.d. (IQR) | (25.2–32.4) | (22.6–28.1) | (26.9- 32.8) | (25.6–34.8) |
| Leukocyte cells/nl | 7.0 ± 1.8 | 6.6 ± 1.7 | 7.1 ± 1.3 | 7.1 ± 2.2 |
| Mean ± s.d. (IQR) | (5.9–7.6) | (5.5–7.5) | (6.4–7.5) | (5.8–8.6) |
| C-reactive protein, mg/L | 3.7 ± 2.5 | 3.4 ± 2.0 | 3.9 ± 2.4 | 3.8 ± 3.0 |
| Mean ± s.d. (IQR) | (2.0–4.4) | (2.0–4.0) | (2.0–5.7) | (2.0–4.4) |
| K&L Score, n (%) | ||||
| 1 | 9 (16%) | 9 (64%) | ||
| 2 | 5 (9%) | 5 (36%) | ||
| 3 | 29 (53%) | 19 (100%) | 10 (45%) | |
| 4 | 12 (22%) | 12 (55%) |
| Pearson-Correlation Coefficient | ||
|---|---|---|
| (CD8+IFN-γ+/CD8+IL-4+)/K&L Score | (CD8+IL-17A+/CD8+IL-4+)/K&L Score | |
| SF | −0.290 | −0.504 |
| p-value | 0.091 | 0.002 * |
| SM | −0.429 | −0.497 |
| p-value | 0.008 * | 0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platzer, H.; Trauth, R.; Nees, T.A.; Tripel, E.; Gantz, S.; Schiltenwolf, M.; Moradi, B.; Rosshirt, N. CD8+ T Cells in OA Knee Joints Are Differentiated into Subsets Depending on OA Stage and Compartment. J. Clin. Med. 2022, 11, 2814. https://doi.org/10.3390/jcm11102814
Platzer H, Trauth R, Nees TA, Tripel E, Gantz S, Schiltenwolf M, Moradi B, Rosshirt N. CD8+ T Cells in OA Knee Joints Are Differentiated into Subsets Depending on OA Stage and Compartment. Journal of Clinical Medicine. 2022; 11(10):2814. https://doi.org/10.3390/jcm11102814
Chicago/Turabian StylePlatzer, Hadrian, Richard Trauth, Timo A. Nees, Elena Tripel, Simone Gantz, Marcus Schiltenwolf, Babak Moradi, and Nils Rosshirt. 2022. "CD8+ T Cells in OA Knee Joints Are Differentiated into Subsets Depending on OA Stage and Compartment" Journal of Clinical Medicine 11, no. 10: 2814. https://doi.org/10.3390/jcm11102814







