Prevalence and Factors of Osteoporosis and High Risk of Osteoporotic Fracture in Patients with Ankylosing Spondylitis: A Multicenter Comparative Study of Bone Mineral Density and the Fracture Risk Assessment Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Assessments
2.2. Evaluation of Osteoporosis by the WHO Criteria and of Osteoporotic Fracture Risk by the FRAX
2.3. Radiological Assessments
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with AS
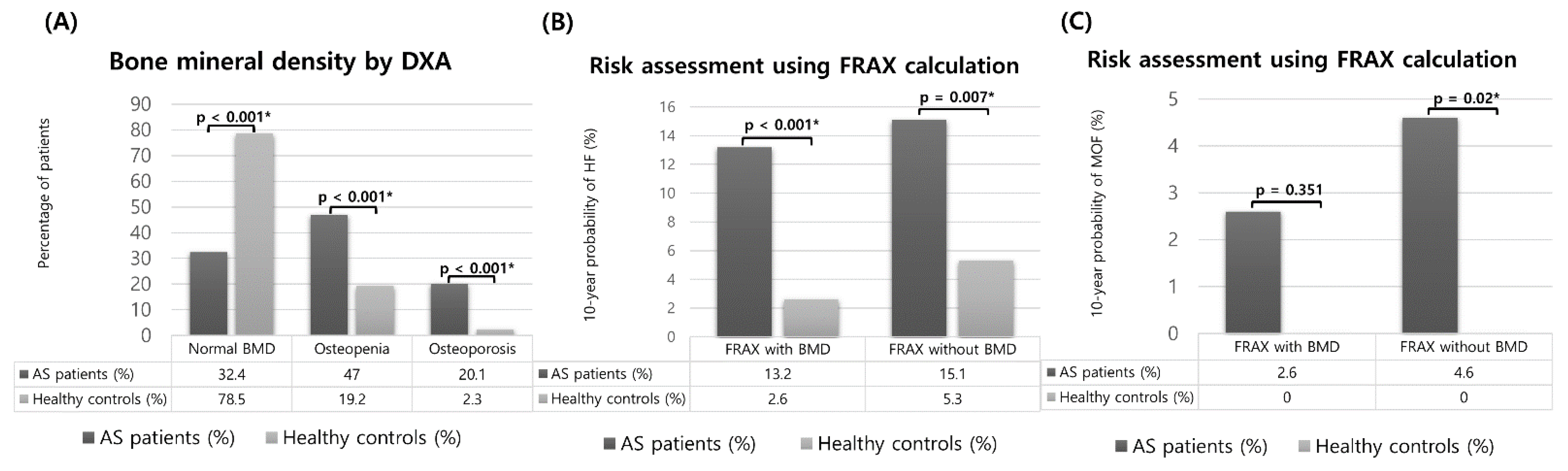
3.2. Evaluation of Osteoporosis by the WHO Criteria and of the Osteoporotic Fracture Risk by the FRAX and Comparison between Patients with AS and Healthy Controls
3.3. Treatment Status of Patients with a High Risk of Osteoporotic Fracture as Determined by the FRAX and of Osteoporosis According to the WHO Criteria
3.4. Clinical Factors Related to a High Risk of Fracture Calculated Using FRAX and Osteoporosis Evaluation Based on the WHO Criteria
3.5. Analysis of Risk Factors Affecting Osteoporotic Fractures in Patients with AS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2016, 390, 73–84. [Google Scholar] [CrossRef]
- Ghasemi-Rad, M.; Attaya, H.; Lesha, E.; Vegh, A.; Maleki-Miandoab, T.; Nosair, E.; Sepehrvand, N.; Davarian, A.; Rajebi, H.; Pakniat, A.; et al. Ankylosing spondylitis: A state of the art factual backbone. World J. Radiol. 2015, 7, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.; Lambrecht, S.; Verheugen, E.; Pauwels, E.; Kollias, G.; Armaka, M.; Verhoye, M.; Van der Linden, A.; Achten, R.; Lories, R.J.; et al. Proof of concept: Enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis. 2013, 73, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosse, D.; Landewe, R.; Van Der Heijde, D.; Van Der Linden, S.; van Staa, T.; Geusens, P. Ankylosing spondylitis and the risk of fracture: Results from a large primary care-based nested case-control study. Ann. Rheum. Dis. 2008, 68, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.M.; Wang, G.S.; Tao, J.H.; Chen, Z.; Ma, Y.; Li, X.P. The association between ankylosing spondylitis and the risk of any, hip, or vertebral fracture: A meta-analysis. Medicine 2017, 96, e8458. [Google Scholar] [CrossRef]
- Sambrook, P.N.; Geusens, P. The epidemiology of osteoporosis and fractures in ankylosing spondylitis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerveld, L.A.; Verlaan, J.J.; Oner, F.C. Spinal fractures in patients with ankylosing spinal disorders: A systematic review of the literature on treatment, neurological status and complications. Eur. Spine J. 2008, 18, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Hinze, A.M.; Louie, G.H. Osteoporosis management in ankylosing spondylitis. Curr. Treat. Options Rheumatol. 2016, 2, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef]
- Klingberg, E.; Lorentzon, M.; Göthlin, J.; Mellström, D.; Geijer, M.; Ohlsson, C.; Atkinson, E.J.; Khosla, S.; Carlsten, H.; Forsblad-D’Elia, H. Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthritis Res. Ther. 2013, 15, R179. [Google Scholar] [CrossRef] [Green Version]
- van der Weijden, M.; Claushuis TA, M.; Nazari, T.; Lems, W.F.; Dijkmans BA, C.; Van Der Horst-Bruinsma, I.E. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis:a systematic review. Clin. Rheumatol. 2012, 31, 1529–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, S.; Doyle, D.V.; Denton, A.; Rolfe, I.; McCloskey, E.V.; Spector, T.D. Bone mineral density and vertebral compression fracture rates in ankylosing spondylitis. Ann. Rheum. Dis. 1994, 53, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.; Oke, I.; Donato, A.; Paudel, A.; Poudel, D.R.; Paudel, P.; Karmacharya, P. Trends in hospitalizations for vertebral compression fracture in ankylosing spondylitis: Data from the National Inpatient Sample 2000–2014. Clin. Rheumatol. 2021, 40, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Magrey, M.N.; Lewis, S.; Khan, M.A. Utility of DXA scanning and risk factors for osteoporosis in ankylosing spondylitis—A prospective study. Semin. Arthritis Rheum. 2016, 46, 88–94. [Google Scholar] [CrossRef]
- Kim, J.-W.; Chung, M.K.; Lee, J.; Kwok, S.-K.; Kim, W.-U.; Park, S.-H.; Ju, J.H. Low bone mineral density of vertebral lateral projections can predict spinal radiographic damage in patients with ankylosing spondylitis. Clin. Rheumatol. 2019, 38, 3567–3574. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Muñoz-Ortego, J.; De Vries, F.; Vosse, D.; Arden, N.K.; Bowness, P.; Cooper, C.; Diez-Perez, A.; Vestergaard, P. Ankylosing spondylitis confers substantially increased risk of clinical spine fractures: A nationwide case-control study. Osteoporos. Int. 2014, 26, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, G.E.; O’Dwyer, T.; Mockler, D.; O’Shea, F.D.; Wilson, F. Pharmacological treatment for managing bone health in axial spondyloarthropathy: Systematic review and meta-analysis. Rheumatol. Int. 2020, 40, 1369–1384. [Google Scholar] [CrossRef]
- Van Der Heijde, D.; Ramiro, S.; Landewé, R.; Baraliakos, X.; Van den Bosch, F.; Sepriano, A.; Regel, A.; Ciurea, A.; Dagfinrud, H.; Dougados, M.; et al. Faculty Opinions recommendation of 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 2018, 76, 978–991. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; McCloskey, E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Van Der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Care Res. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Creemers, M.C.W.; Franssen, M.J.A.M.; Van’t Hof, M.A.; Gribnau, F.W.J.; Van de Putte, L.B.A.; Van Riel, P.L.C.M. Assessment of outcome in ankylosing spondylitis: An extended radiographic scoring system. Ann. Rheum. Dis. 2005, 64, 127–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.J.; Nimarpreet, K.; Ashima, S.D.; Kumar, A.; Prakash, S. Study of bone mineral density in patients with ankylosing spondylitis. J. Clin. Diagn. Res. 2013, 7, 2832–2835. [Google Scholar] [CrossRef] [PubMed]
- Bessant, R.; Keat, A. How should clinicians manage osteoporosis in ankylosing spondylitis? J. Rheumatol. 2002, 29, 1511–1519. [Google Scholar] [PubMed]
- Choi, S.T.; Kwon, S.-R.; Jung, J.-Y.; Kim, H.-A.; Kim, S.-S.; Kim, S.H.; Kim, J.-M.; Park, J.-H.; Suh, C.-H. Prevalence and fracture risk of osteoporosis in patients with rheumatoid arthritis: A multicenter comparative study of the FRAX and WHO criteria. J. Clin. Med. 2018, 7, 507. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.Y.; Kim, H.A.; Jung, J.Y.; Choi, S.T.; Kim, J.M.; Kim, S.H.; Kwon, S.R.; Suh, C.H.; Kim, S.S. Clinical impact of the fracture risk assessment tool on the treatment decision for osteoporosis in patients with knee osteoarthritis: A multicenter comparative study of the fracture risk assessment tool and World Health Organization criteria. J. Clin. Med. 2019, 8, 918. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-Y.; Choi, S.T.; Park, S.-H.; Kwon, S.-R.; Kim, H.-A.; Kim, S.-S.; Kim, S.H.; Suh, C.-H. Prevalence of osteoporosis in patients with systemic lupus erythematosus: A multicenter comparative study of the World Health Organization and fracture risk assessment tool criteria. Osteoporos. Sarcopenia 2020, 6, 173–178. [Google Scholar] [CrossRef]
- Klingberg, E.; Lorentzon, M.; Mellström, D.; Geijer, M.; Göthlin, J.; Hilme, E.; Hedberg, M.; Carlsten, H.; Forsblad-d’Elia, H. Osteoporosis in ankylosing spondylitis-prevalence, risk factors and methods of assessment. Arthritis Res. Ther. 2012, 14, R108. [Google Scholar] [CrossRef] [Green Version]
- Henchiri, I.; Hamdi, W.; Maatallah, K.; Ferjani, H.; Kasraoui, A.; Kaffel, D.; Kchir, M.M. Assessment of fracture risk in patients with spondyloarthritis using the FRAX scores. Tunis. Med. 2019, 97, 1235–1239. [Google Scholar]
- Kang, K.Y.; Kwok, S.-K.; Ju, J.H.; Hong, Y.S.; Park, S.-H. Assessment of fracture risk in patients with axial spondyloarthritis: A case-control study using the fifth Korean National Health and Nutrition Examination Survey (KNHANES V). Scand. J. Rheumatol. 2015, 45, 23–31. [Google Scholar] [CrossRef]
- Cohen, A.; Shane, E. Treatment of premenopausal women with low bone mineral density. Curr. Osteoporos. Rep. 2008, 6, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepe, J.; Body, J.-J.; Hadji, P.; McCloskey, E.; Meier, C.; Obermayer-Pietsch, B.; Palermo, A.; Tsourdi, E.; Zillikens, M.C.; Langdahl, B.; et al. Osteoporosis in premenopausal women: A clinical narrative review by the ECTS and the IOF. J. Clin. Endocrinol. Metab. 2020, 105, e1931. [Google Scholar] [CrossRef] [PubMed]
- Maillefert, J.F.; Aho, L.S.; El Maghraoui, A.; Dougados, M.; Roux, C. Changes in bone density in patients with ankylosing spondylitis: A two-year follow-up study. Osteoporos. Int. 2001, 12, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ortego, J.; Vestergaard, P.; Rubio, J.B.; Wordsworth, P.; Judge, A.; Javaid, M.; Arden, N.K.; Cooper, C.; Diez-Perez, A.; Prieto-Alhambra, D. Ankylosing spondylitis is associated with an increased risk of vertebral and nonvertebral clinical fractures: A population-based cohort study. J. Bone Miner. Res. 2014, 29, 1770–1776. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Inflammation, bone loss and fracture risk in spondyloarthritis. RMD Open 2015, 1, e000052. [Google Scholar] [CrossRef]
- Magray, M.N.; Khan, M.A. The paradox of bone formation and bone loss in ankylosing spondylitis: Evolving new concepts of bone formation and future trends in management. Curr. Rheumatol. Rep. 2017, 19, 17. [Google Scholar] [CrossRef]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef]
- Spoorenberg, A.; van Tubergen, A.; Landewé, R.; Dougados, M.; van der Linden, S.; Mielants, H.; van de Tempel, H.; van der Heijde, D. Measuring disease activity in ankylosing spondylitis: Patient and physician have different perspectives. Rheumatology 2005, 44, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.Y.; Kim, I.J.; Jung, S.M.; Kwok, S.-K.; Ju, J.H.; Park, K.-S.; Hong, Y.S.; Park, S.-H. Incidence and predictors of morphometric vertebral fractures in patients with ankylosing spondylitis. Arthritis Res. Ther. 2014, 16, R124. [Google Scholar] [CrossRef] [Green Version]
- Muntean, L.; Rojas-Vargas, M.; Font, P.; Simon, S.-P.; Rednic, S.; Schiotis, R.; Stefan, S.; Tamas, M.M.; Bolosiu, H.D.; Estévez, E.C. Relative value of the lumbar spine and hip bone mineral density and bone turnover markers in men with ankylosing spondylitis. Clin. Rheumatol. 2011, 30, 691–695. [Google Scholar] [CrossRef]
- Başkan, B.M.; Doğan, Y.P.; Sivas, F.; Bodur, H.; Özoran, K. The relation between osteoporosis and vitamin D levels and disease activity in ankylosing spondylitis. Rheumatol. Int. 2009, 30, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, V.; Bhakuni, D.; Garg, M.K.; Narayanan, K.; Jain, R.; Chadha, D. Bone mineral density in young males with ankylosing spondylitis. Int. J. Rheum. Dis. 2010, 14, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Leufkens HG, M.; Abenhaim, L.; Zhang, B.; Cooper, C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000, 15, 993–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, M.B.; Madden, D.M.; Murray, A.M.; Heaney, R.P.; Kerzner, L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am. J. Med. 2005, 118, 778–781. [Google Scholar] [CrossRef]
- Romdhane, H.; Ayadi, S.; Elleuch, N.; AbdelGhani, K. Effect of long-term proton pump inhibitors on bone mineral density. Tunis. Med. 2018, 96, 193–197. [Google Scholar]
- Arévalo, M.; López-Medina, C.; Martinez-Losa, M.M.; Moltó, A.; Font, P.; Collantes-Estevez, E.; Gratacós, J. Role of HLA-B27 in the comorbidities observed in Axial Spondyloarthritis: Data from COMOSPA. Jt. Bone Spine 2020, 87, 445–448. [Google Scholar] [CrossRef]
- Rauner, M.; Stupphann, D.; Haas, M.; Fert, I.; Glatigny, S.; Sipos, W.; Breban, M.; Pietschmann, P. The HLA-B27 transgenic rat, a model of spondyloarthritis, has decreased bone mineral density and increased RANKL to osteoprotegerin mRNA ratio. J. Rheumatol. 2009, 36, 120–126. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Srivastava, A.K.E.; Wergedal, J.; Zwerina, J.; Klaushofer, K.; Paschalis, E.P.; Tatakis, D.N. Altered bone material properties in HLA-B27 rats include reduced mineral to matrix ratio and altered collagen cross-links. J. Bone Miner. Res. 2014, 29, 2382–2391. [Google Scholar] [CrossRef]
- Johansson, H.; Azizieh, F.; al Ali, N.; Alessa, T.; Harvey, N.C.; McCloskey, E.; Kanis, J.A. FRAX- vs. T-score-based intervention thresholds for osteoporosis. Osteoporos. Int. 2017, 28, 3099–3105. [Google Scholar] [CrossRef] [Green Version]
- Siris, E.S.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.J.; Harris, S.T.; de Beur, S.M.J.; Khosla, S.; Lane, N.E.; et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Fauny, M.; Verhoeven, F.; Allado, E.; Albuisson, E.; Pinzano, A.; Morizot, C.; Chary-Valckenaere, I.; Loeuille, D. Relationship between spinal structural damage on radiography and bone fragility on CT in ankylosing spondylitis patients. Sci. Rep. 2021, 11, 9342. [Google Scholar] [CrossRef] [PubMed]
| Variable | AS Patients (n = 219) | Healthy Controls (n = 219) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), years | 47.6 (13.8) | 47.5 ± 13.7 | 0.972 |
| Sex, N (%) | |||
| Men | 144 (65.8) | 144 (65.8) | |
| Women | 75 (34.2) | 75 (34.2) | |
| Menopause, N (%) | 43 (57.3) | 40 (53.3) | 0.628 |
| Bodyweight, mean (SD), kg | 64.3 (12.2) | 66.4 (11.3) | 0.71 |
| Height, mean (SD), cm | 163.9 (10.1) | 165.6 (8.41) | 0.06 |
| Body mass index, mean (SD), kg/m2 | 23.9 (3.7) | 24.3 (3.09) | 0.227 |
| Smoking, N (%) | 53 (24.2) | 41 (18.7) | 0.082 |
| Alcohol, N (%) | 53 (24.2) | 68 (31.1) | 0.225 |
| Fractures, N (%) | 25 (11.4) | 3 (1.7) | <0.001 * |
| Vertebral fractures, N (%) | 20 (9.1) | 3 (1.7) | <0.001 * |
| Non-vertebral fractures, N (%) | 5 (2.3) | 0 (0) | 0.025 * |
| Disease-related | |||
| Disease duration, mean (SD), months | 48.6 (46.6) | ||
| HLA-B27-positive, N (%) | 183 (83.6) | ||
| ESR, mm/h, median (IQR) | 31 (13–59) | ||
| CRP, mg/dL, median (IQR) | 1.57 (0.39–3.02) | ||
| Peripheral arthritis, N (%) | 84 (38.4) | ||
| Enthesopathy, N (%) | 25 (11.4) | ||
| Uveitis, N (%) | 34 (15.5) | ||
| Syndesmophyte, N (%) | 88 (40.2) | ||
| mSASSS score, median (IQR) | 13 (5–32) | ||
| Medications | |||
| NSAIDs, N (%) | 190 (86.8) | ||
| Methotrexate, N (%) | 59 (26.9) | ||
| Sulfasalazine, N (%) | 137 (62.6) | ||
| TNF inhibitor, N (%) | 98 (44.7) | ||
| GC, N (%) | 69 (31.5) | ||
| GC lifetime use, mean (SD), g (prednisone-equivalent dose) | 10.7 (2.04) | ||
| GC current dose, mean (SD), mg/d | 1.69 (3.13) | ||
| Vitamin D intake, N (%) | 105 (47.9) | ||
| Calcium intake, N (%) | 89 (40.6) | ||
| Proton pump inhibitor, N (%) | 51 (23.3) | ||
| Treatment for osteoporosis, N (%) | 33 (15.1) | ||
| Bisphosphonate, N (%) | 24 (11) | ||
| SERM, N (%) | 6 (2.7) | ||
| Denosumab, N (%) | 3 (1.4) |
| High Risk of Fracture in FRAX with BMD | High Risk of Fracture in FRAX without BMD | Osteoporosis by the WHO Criteria | p Value 1 | p Value 2 | p Value 3 | |
|---|---|---|---|---|---|---|
| Overall | 20/152 (13.2%) | 23/152 (15.1%) | 44/219 (20.1%) | 0.678 | <0.001 * | 0.01 * |
| Men | 11/88 (12.5%) | 6/88 (6.8%) | 27/144 (18.8%) | <0.001 * | 0.006 * | <0.001 * |
| Women | 9/64 (14.1%) | 17/64 (26.6%) | 17/75 (22.7%) | <0.001 * | 0.029 * | 0.007 * |
| FRAX with BMD | FRAX without BMD | Osteoporosis of the WHO | |
|---|---|---|---|
| Candidates for pharmacological treatment | 20/152 (13.2%) | 23/152 (15.1%) | 44/219 (20.1%) |
| Current treatment | |||
| Overall | 10/20 (50%) | 9/23 (39.1%) | 33/44 (75%) |
| Men | 5/11 (45.5%) | 2/6 (33.3%) | 18/27 (66.7%) |
| Women | 5/9 (55.6%) | 7/17 (41.2%) | 15/17 (88.2%) |
| FRAX with BMD | FRAX without BMD | Osteoporosis (WHO) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 1.12 (0.99, 1.26) | 0.062 | 1.94 (0.98, 3.86) | 0.058 | 1.05 (0.98, 1.12) | 0.156 |
| Sex (Men) | 0.64 (0.28, 1.47) | 0.293 | 0.87 (0.79, 3.1) | 0.102 | 0.31 (0.01, 1.66) | 0.117 |
| Menopause | 4.36 (1.68, 13.5) | 0.005 * | 5.66 (2.3, 20.5) | <0.001 * | 10.2 (1.53, 196) | 0.025 * |
| BMI < 25 kg/m2 | 0.99 (0.09, 3.89) | 0.59 | 0.22 (0.02, 2.75) | 0.24 | 2.4 (0.62, 9.21) | 0.203 |
| HLA-B27 positivity | 4.22 (0.81, 77.8) | 0.171 | 2.89 (0.74, 5.32) | 0.089 | 5.3 (1.07, 15.3) | 0.046 * |
| ESR | 1.2 (0.46, 27.5) | 0.139 | 2.78 (1.01, 4.68) | 0.045 * | 1.27 (0.13, 1.81) | 0.278 |
| CRP | 1.25 (0.89, 1.78) | 0.203 | 3.78 (0.29, 93) | 0.028 * | 1.67 (0.13, 0.96) | 0.041 * |
| Syndesmophyte | 0.22 (0.21, 2.35) | 0.210 | 1.28 (0.17, 4.84) | 0.465 | 1.09 (0.25, 4.63) | 0.913 |
| mSASSS | 0.97 (0.9, 1.04) | 0.344 | 1.14 (0.96, 1.36) | 0.136 | 0.98 (0.94, 1.02) | 0.245 |
| Glucocorticoid use | 4.32 (0.59, 31.4) | 0.149 | 1.54 (1.46, 7.5) | 0.02 * | 6.02 (1.36, 26.6) | 0.018 * |
| Biologics use | 1.01 (0.99, 1.12) | 0.059 | 1.34 (0.55, 3.32) | 0.515 | 1.43 (0.33, 6.18) | 0.09 |
| PPI use | 1.3 (0.46, 3.41) | 0.603 | 1.68 (1.07, 4.71) | 0.048 * | 0.35 (0.08, 1.55) | 0.167 |
| Vitamin D use | 0.61 (0.05, 7.17) | 0.692 | 0.3 (0.01,1.02) | 0.051 | 0.45 (0.06, 3.5) | 0.447 |
| Calcium use | 1 (0.12, 8.56) | 0.997 | 0.07 (0, 17.5) | 0.349 | 0.62 (0.1, 3.78) | 0.604 |
| Osteoporotic Fracture | ||||
|---|---|---|---|---|
| Univariable Model | Multivariable Model | |||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 1.04 (1.01, 1.07) | 0.015 | 0.98 (0.91, 1.06) | 0.683 |
| Sex (Men) | 0.92 (0.38, 2.19) | 0.844 | ||
| Menopause | 1.19 (0.44, 3.23) | 0.734 | ||
| Disease duration | 0.99 (0.98, 1.00) | 0.097 | 0.98 (0.96, 1.01) | 0.12 |
| BMI | 1.03 (0.92, 1.15) | 0.648 | ||
| HLA-B27 positivity | 1.42 (0.31, 6.47) | 0.652 | ||
| ESR | 1.01 (0.99, 1.02) | 0.862 | ||
| CRP | 0.98 (0.85, 1.14) | 0.822 | ||
| Syndesmophyte | 2.03 (0.85, 4.86) | 0.113 | ||
| mSASSS | 1.03 (1.01, 1.05) | 0.007 | 1.03 (1.01, 1.06) | 0.043 * |
| Glucocorticoid cumulative dose | 1.05 (0.88, 1.26) | 0.571 | ||
| Biologics use | 1.14 (0.49, 2.61) | 0.766 | ||
| PPI use | 1.9 (0.77, 4.66) | 0.164 | ||
| FRAX with BMD | 6.35 (2.07, 19.5) | 0.001 | 8.56 (2.32, 31.5) | 0.001 * |
| FRAX without BMD | 6.19 (2.11, 18.1) | 0.001 | 2.67 (0.47, 15.3) | 0.271 |
| BMD by DXA | 1.01 (0.36, 2.85) | 0.99 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Park, S.; Jung, J.-Y.; Kim, H.-A.; Kwon, S.-R.; Choi, S.T.; Kim, S.-S.; Kim, S.-H.; Suh, C.-H. Prevalence and Factors of Osteoporosis and High Risk of Osteoporotic Fracture in Patients with Ankylosing Spondylitis: A Multicenter Comparative Study of Bone Mineral Density and the Fracture Risk Assessment Tool. J. Clin. Med. 2022, 11, 2830. https://doi.org/10.3390/jcm11102830
Kim J-W, Park S, Jung J-Y, Kim H-A, Kwon S-R, Choi ST, Kim S-S, Kim S-H, Suh C-H. Prevalence and Factors of Osteoporosis and High Risk of Osteoporotic Fracture in Patients with Ankylosing Spondylitis: A Multicenter Comparative Study of Bone Mineral Density and the Fracture Risk Assessment Tool. Journal of Clinical Medicine. 2022; 11(10):2830. https://doi.org/10.3390/jcm11102830
Chicago/Turabian StyleKim, Ji-Won, Sunghoon Park, Ju-Yang Jung, Hyoun-Ah Kim, Seong-Ryul Kwon, Sang Tae Choi, Sung-Soo Kim, Sang-Hyeon Kim, and Chang-Hee Suh. 2022. "Prevalence and Factors of Osteoporosis and High Risk of Osteoporotic Fracture in Patients with Ankylosing Spondylitis: A Multicenter Comparative Study of Bone Mineral Density and the Fracture Risk Assessment Tool" Journal of Clinical Medicine 11, no. 10: 2830. https://doi.org/10.3390/jcm11102830
APA StyleKim, J.-W., Park, S., Jung, J.-Y., Kim, H.-A., Kwon, S.-R., Choi, S. T., Kim, S.-S., Kim, S.-H., & Suh, C.-H. (2022). Prevalence and Factors of Osteoporosis and High Risk of Osteoporotic Fracture in Patients with Ankylosing Spondylitis: A Multicenter Comparative Study of Bone Mineral Density and the Fracture Risk Assessment Tool. Journal of Clinical Medicine, 11(10), 2830. https://doi.org/10.3390/jcm11102830







