Transcranial Direct Current Stimulation as an Approach to Mitigate Neurodevelopmental Disorders Affecting Excitation/Inhibition Balance: Focus on Autism Spectrum Disorder, Schizophrenia, and Attention Deficit/Hyperactivity Disorder
Abstract
:1. Introduction
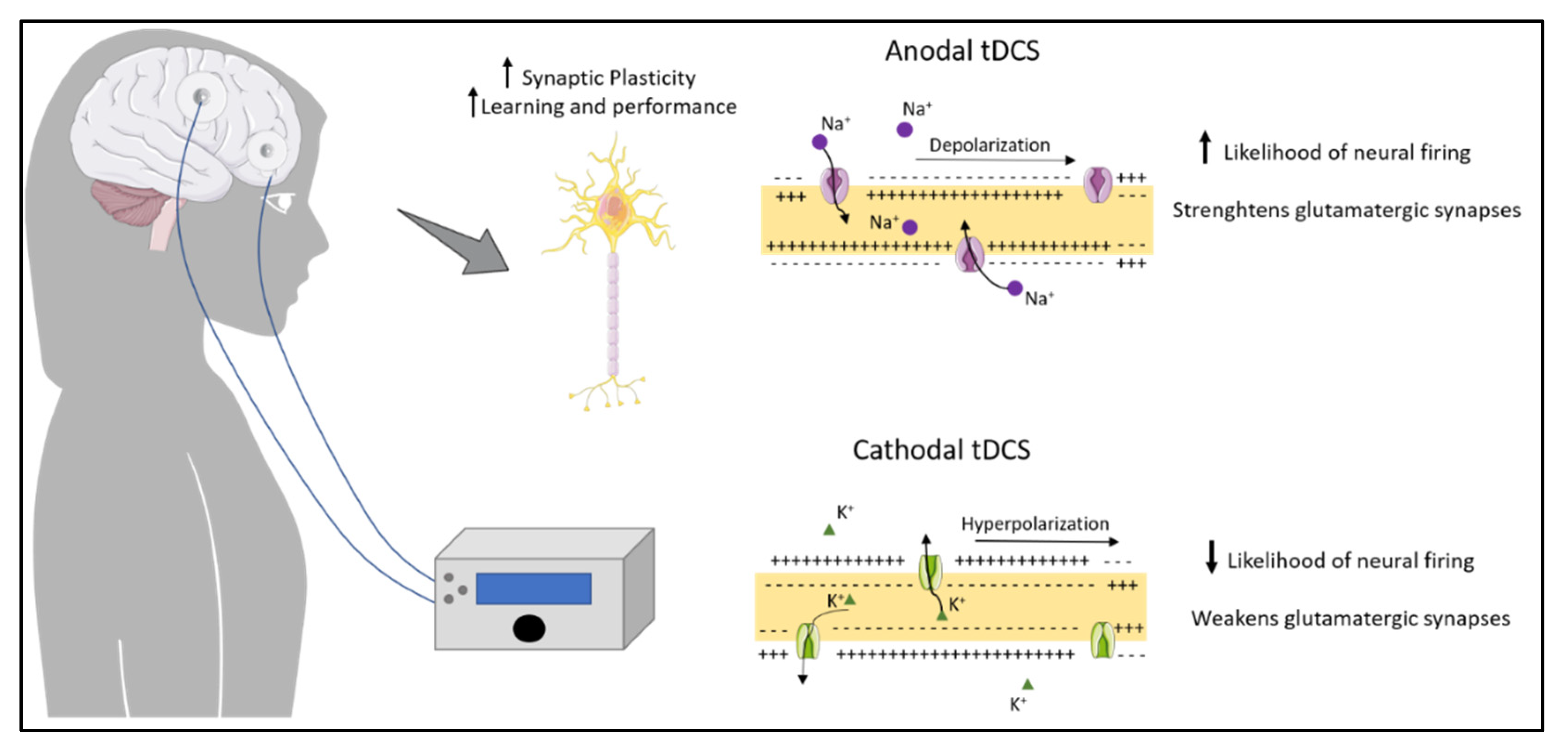
2. Transcranial Direct Current Stimulation
2.1. TDCS as Treatment for Neurodevelopmental Disorders
2.1.1. Effect of tDCS on Autistic-like Behavior
2.1.2. Effect of tDCS on Schizophrenia
2.1.3. Effect of tDCS on Attention Deficit/Hyperactivity Disorder (ADHD)
| Subjects | tDCS Intervention | Brain Region | Conclusion | Ref. |
|---|---|---|---|---|
| SHR rats | Anodal; 0.5 mA; 20 min; 8 consecutive days | Pre-frontal cortex | Restore long-term memory deficits, modulate neuroinflammatory molecules, and increase oxidative stress | [61] |
| Teenagers with a diagnosis of ADHD | Anodal; 1 mA; 20 min; 5 consecutive days | Dorsolateral prefrontal cortex | Reduction of inattention, and a reduction of hyperactivity | [62] |
| Adult ADHD patients | Anodal; 2 mA; 20 min | Dorsolateral pre-frontal cortex | Improve of performance on a measure of impulsivity | [63] |
| Teenagers with ADHD | Anodal; 1 mA; 20 min | Dorsolateral pre-frontal cortex | Improve of working memory performance. | [64] |
| ADHD patients (10–18 years old) | Anodal bifrontal; 1 mA; 20 min combined with cognitive training | Dorsolateral frontal cortex | No significant improvement of ADHD symptoms or cognitive performance | [65] |
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Mullin, A.P.; Gokhale, A.; Moreno-De-Luca, A.; Sanyal, S.; Waddington, J.L.; Faundez, V. Neurodevelopmental disorders: Mechanisms and boundary definitions from genomes, interactomes and proteomes. Transl. Psychiatry 2013, 3, e329. [Google Scholar] [CrossRef] [PubMed]
- Selten, M.; Van Bokhoven, H.; Kasri, N.N. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Research 2018, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Lopatina, O.L.; Malinovskaya, N.A.; Komleva, Y.K.; Gorina, Y.V.; Shuvaev, A.N.; Olovyannikova, R.Y.; Belozor, O.; Belova, O.A.; Higashida, H.; Salmina, A.B. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev. Neurosci. 2019, 30, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Lin, Y. The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol. Med. 2011, 17, 452–462. [Google Scholar] [CrossRef]
- Wehr, M.; Zador, A.M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 2003, 426, 442–446. [Google Scholar] [CrossRef]
- Peerboom, C.; Wierenga, C.J. The postnatal GABA shift: A developmental perspective. Neurosci. Biobehav. Rev. 2021, 124, 179–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, Y.-Y.; Sun, Q.-Q. Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neuroscience 2011, 174, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Rahimi-Balaei, M.; Bergen, H.; Kong, J.; Marzban, H. Neuronal Migration During Development of the Cerebellum. Front. Cell. Neurosci. 2018, 12, 484. [Google Scholar] [CrossRef]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Finisguerra, A.; Borgatti, R.; Urgesi, C. Non-invasive Brain Stimulation for the Rehabilitation of Children and Adolescents With Neurodevelopmental Disorders: A Systematic Review. Front. Psychol. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellaro, R.; Nitsche, M.A.; Colzato, L. The stimulated social brain: Effects of transcranial direct current stimulation on social cognition. Ann. N. Y. Acad. Sci. 2016, 1369, 218–239. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.; Bikson, M.; Kappenman, E.S.; Clark, V.P.; Coslett, H.B.; Hamblin, M.R.; Hamilton, R.; Jankord, R.; Kozumbo, W.J.; McKinley, R.A.; et al. Mechanisms and Effects of Transcranial Direct Current Stimulation. Dose-Response 2017, 15, 1559325816685467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.F.; De Souza, I.C.C.; Vidor, L.P.; De Souza, A.; Deitos, A.; Volz, M.S.; Fregni, F.; Caumo, W.; Torres, I.L.S. Neurobiological Effects of Transcranial Direct Current Stimulation: A Review. Front. Psychiatry 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Cai, E.; Han, J.; Tong, Z.; Li, X.; Sokhadze, E.M.; Casanova, M.F.; Ouyang, G.; Li, X. Transcranial Direct Current Stimulation (tDCS) Can Modulate EEG Complexity of Children With Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 201. [Google Scholar] [CrossRef]
- Krause, B.; Márquez-Ruiz, J.; Kadosh, R.C. The effect of transcranial direct current stimulation: A role for cortical excitation/inhibition balance? Front. Hum. Neurosci. 2013, 7, 602. [Google Scholar] [CrossRef] [Green Version]
- Bikson, M.; Rahman, A. Origins of specificity during tDCS: Anatomical, activity-selective, and input-bias mechanisms. Front. Hum. Neurosci. 2013, 7, 688. [Google Scholar] [CrossRef] [Green Version]
- Katz, B.; Au, J.; Buschkuehl, M.; Abagis, T.; Zabel, C.; Jaeggi, S.M.; Jonides, J. Individual Differences and Long-term Consequences of tDCS-augmented Cognitive Training. J. Cogn. Neurosci. 2017, 29, 1498–1508. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Haker, H.; Schneebeli, M.; Stephan, K.E. Can Bayesian Theories of Autism Spectrum Disorder Help Improve Clinical Practice? Front. Psychiatry 2016, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, J.; Kim, E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 2017, 81, 838–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Man, H.-Y. Fundamental Elements in Autism: From Neurogenesis and Neurite Growth to Synaptic Plasticity. Front. Cell. Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Casanova, M.F.; Buxhoeveden, D.; Gomez, J. Disruption in the Inhibitory Architecture of the Cell Minicolumn: Implications for Autisim. Neuroscientist 2003, 9, 496–507. [Google Scholar] [CrossRef]
- Casanova, M.F.; Van Kooten, I.A.J.; Switala, A.; Van Engeland, H.; Heinsen, H.; Steinbusch, H.W.M.; Hof, P.R.; Trippe, J.; Stone, J.; Schmitz, C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006, 112, 287–303. [Google Scholar] [CrossRef]
- Gonçalves, J.; Violante, I.R.; Sereno, J.; Leitão, R.A.; Cai, Y.; Abrunhosa, A.; Silva, A.P.; Silva, A.J.; Castelo-Branco, M. Testing the excitation/inhibition imbalance hypothesis in a mouse model of the autism spectrum disorder: In Vivo neurospectroscopy and molecular evidence for regional phenotypes. Mol. Autism 2017, 8, 47. [Google Scholar] [CrossRef]
- Kubas, B.; Kułak, W.; Sobaniec, W.; Tarasow, E.; Łebkowska, U.M.; Walecki, J. Metabolite alterations in autistic children: A 1H MR spectroscopy study. Adv. Med Sci. 2012, 57, 152–156. [Google Scholar] [CrossRef]
- Puts, N.A.; Wodka, E.L.; Harris, A.D.; Crocetti, D.; Tommerdahl, M.; Mostofsky, S.H.; Edden, R.A. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2016, 10, 608–619. [Google Scholar] [CrossRef] [Green Version]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D. Glutamate and GABA in autism spectrum disorder—a translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.E.; Trumbo, M.C.; Tesche, C.D. Transcranial direct current stimulation (tDCS) over right temporoparietal junction (rTPJ) for social cognition and social skills in adults with autism spectrum disorder (ASD). J. Neural Transm. 2018, 125, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.D.; Hopp, J.P. The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin. Linguist. Phon. 2011, 25, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.; Vidal, B.; Maragoto, C.; Morales, L.M.; Berrillo, S.; Cuesta, H.V.; Baez, M.; Denis, M.; Marín, T.; Cabrera, Y.; et al. Non-Invasive Brain Stimulation for Children with Autism Spectrum Disorders: A Short-Term Outcome Study. Behav. Sci. 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Amatachaya, A.; Jensen, M.P.; Patjanasoontorn, N.; Auvichayapat, N.; Suphakunpinyo, C.; Janjarasjitt, S.; Ngernyam, N.; Aree-Uea, B.; Auvichayapat, P. The Short-Term Effects of Transcranial Direct Current Stimulation on Electroencephalography in Children with Autism: A Randomized Crossover Controlled Trial. Behav. Neurol. 2015, 2015, 928631. [Google Scholar] [CrossRef] [PubMed]
- Van Steenburgh, J.J.; Varvaris, M.; Schretlen, D.J.; Vannorsdall, T.D.; Gordon, B. Balanced bifrontal transcranial direct current stimulation enhances working memory in adults with high-functioning autism: A sham-controlled crossover study. Mol. Autism 2017, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Luckhardt, C.; Schütz, M.; Mühlherr, A.; Mössinger, H.; Boxhoorn, S.; Dempfle, A.; Salvador, R.; Ruffini, G.; Pereira, H.C.; Castelo-Branco, M.; et al. Phase-IIa randomized, double-blind, sham-controlled, parallel group trial on anodal transcranial direct current stimulation (tDCS) over the left and right tempo-parietal junction in autism spectrum disorder—StimAT: Study protocol for a clinical trial. Trials 2021, 22, 248. [Google Scholar] [CrossRef]
- Han, Y.M.; Chan, M.M.; Shea, C.K.; Lai, O.L.-H.; Krishnamurthy, K.; Cheung, M.-C.; Chan, A.S. Neurophysiological and behavioral effects of multisession prefrontal tDCS and concurrent cognitive remediation training in patients with autism spectrum disorder (ASD): A double-blind, randomized controlled fNIRS study. Brain Stimul. 2022, 15, 414–425. [Google Scholar] [CrossRef]
- Lewis, D.A.; Curley, A.A.; Glausier, J.R.; Volk, D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Gejman, P.V.; Sanders, A.R.; Duan, J. The Role of Genetics in the Etiology of Schizophrenia. Psychiatr. Clin. N. Am. 2010, 33, 35–66. [Google Scholar] [CrossRef] [Green Version]
- Van Os, J.; Kenis, G.; Rutten, B.P.F. The environment and schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef]
- Shi, J.; Gershon, E.S.; Liu, C. Genetic associations with schizophrenia: Meta-analyses of 12 candidate genes. Schizophr. Res. 2008, 104, 96–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Penzes, P. Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Peer Rev. J. Formul. Manag. 2014, 39, 638–645. [Google Scholar]
- Boksa, P. On the neurobiology of hallucinations. J. Psychiatry Neurosci. 2009, 34, 260–262. [Google Scholar] [PubMed]
- Marsman, A.; Mandl, R.C.; Klomp, D.W.; Bohlken, M.M.; Boer, V.; Andreychenko, A.; Cahn, W.; Kahn, R.S.; Luijten, P.R.; Pol, H.H. GABA and glutamate in schizophrenia: A 7 T 1H-MRS study. NeuroImage: Clin. 2014, 6, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Jauhar, S.; McCutcheon, R.; Borgan, F.; Veronese, M.; Nour, M.; Pepper, F.; Rogdaki, M.; Stone, J.; Egerton, A.; Turkheimer, F.; et al. The relationship between cortical glutamate and striatal dopamine in first-episode psychosis: A cross-sectional multimodal PET and magnetic resonance spectroscopy imaging study. Lancet Psychiatry 2018, 5, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Hadar, R.; Winter, R.; Edemann-Callesen, H.; Wieske, F.; Habelt, B.; Khadka, N.; Felgel-Farnholz, V.; Barroeta-Hlusicka, E.; Reis, J.; Tatarau, C.A.; et al. Prevention of schizophrenia deficits via non-invasive adolescent frontal cortex stimulation in rats. Mol. Psychiatry 2020, 25, 896–905. [Google Scholar] [CrossRef]
- Brunelin, J.; Mondino, M.; Gassab, L.; Haesebaert, F.; Gaha, L.; Suaud-Chagny, M.-F.; Saoud, M.; Mechri, A.; Poulet, E. Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia. Am. J. Psychiatry 2012, 169, 719–724. [Google Scholar] [CrossRef]
- Valiengo, L.; Goerigk, S.; Gordon, P.C.; Padberg, F.; Serpa, M.H.; Koebe, S.; Dos Santos, L.A.; Lovera, R.A.M.; De Carvalho, J.B.; Van De Bilt, M.; et al. Efficacy and Safety of Transcranial Direct Current Stimulation for Treating Negative Symptoms in Schizophrenia. JAMA Psychiatry 2020, 77, 121–129. [Google Scholar] [CrossRef]
- Orlov, N.D.; Tracy, D.K.; Joyce, D.; Patel, S.; Rodzinka-Pasko, J.; Dolan, H.; Hodsoll, J.; Collier, T.; Rothwell, J.; Shergill, S. Stimulating cognition in schizophrenia: A controlled pilot study of the effects of prefrontal transcranial direct current stimulation upon memory and learning. Brain Stimul. 2016, 10, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Naaijen, J.; Bralten, J.; Poelmans, G.; Glennon, J.C.; Franke, B.; Buitelaar, J.K. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: Association to overlapping traits in ADHD and autism. Transl. Psychiatry 2017, 7, e999. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, B.; Sandersleben, H.U.-V.; Gevensleben, H.; Rothenberger, A. Pathophysiology of ADHD and associated problems—starting points for NF interventions? Front. Hum. Neurosci. 2015, 9, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Neto, P.; Lou, H.C.; Cumming, P.; Pryds, O.; Karrebaek, H.; Lunding, J.; Gjedde, A. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. NeuroImage 2005, 25, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Pretus, C.; Ramos-Quiroga, J.A.; Richarte, V.; Corrales, M.; Picado, M.; Carmona, S.; Vilarroya, Ó. Time and psychostimulants: Opposing long-term structural effects in the adult ADHD brain. A longitudinal MR study. Eur. Neuropsychopharmacol. 2017, 27, 1238–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveri, M.M.; Sneider, J.T.; Crowley, D.; Covell, M.J.; Acharya, D.; Rosso, I.M.; Jensen, J.E. Frontal Lobe γ-Aminobutyric Acid Levels During Adolescence: Associations with Impulsivity and Response Inhibition. Biol. Psychiatry 2013, 74, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.C.; Gleich, T.; Buchert, R.; Schlagenhauf, F.; Kühn, S.; Gallinat, J. Interactions between glutamate, dopamine, and the neuronal signature of response inhibition in the human striatum. Hum. Brain Mapp. 2015, 36, 4031–4040. [Google Scholar] [CrossRef] [Green Version]
- Endres, D.; Van Elst, L.T.; Maier, S.J.; Feige, B.; Goll, P.; Meyer, S.A.; Matthies, S.; Domschke, K.; Lange, T.; Sobanski, E.; et al. Neurochemical sex differences in adult ADHD patients: An MRS study. Biol. Sex Differ. 2019, 10, 50. [Google Scholar] [CrossRef]
- Maltezos, S.; Horder, J.; Coghlan, S.; Skirrow, C.; O’Gorman, R.; Lavender, T.J.; Mendez, M.A.; Mehta, M.A.; Daly, E.; Xenitidis, K.; et al. Glutamate/glutamine and neuronal integrity in adults with ADHD: A proton MRS study. Transl. Psychiatry 2014, 4, e373. [Google Scholar] [CrossRef]
- Edden, R.A.E.; Crocetti, D.; Zhu, H.; Gilbert, D.; Mostofsky, S.H. Reduced GABA Concentration in Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2012, 69, 750–753. [Google Scholar] [CrossRef]
- Leffa, D.T.; Bellaver, B.; Salvi, A.A.; de Oliveira, C.; Caumo, W.; Grevet, E.H.; Fregni, F.; Quincozes-Santos, A.; Rohde, L.A.; Torres, I.L. Transcranial direct current stimulation improves long-term memory deficits in an animal model of attention-deficit/hyperactivity disorder and modulates oxidative and inflammatory parameters. Brain Stimul. 2018, 11, 743–751. [Google Scholar] [CrossRef]
- Soff, C.; Sotnikova, A.; Christiansen, H.; Becker, K.; Siniatchkin, M. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J. Neural Transm. 2017, 124, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Allenby, C.; Falcone, M.; Bernardo, L.; Wileyto, E.P.; Rostain, A.; Ramsay, J.; Lerman, C.; Loughead, J. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 2018, 11, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, A.; Soff, C.; Tagliazucchi, E.; Becker, K.; Siniatchkin, M. Transcranial Direct Current Stimulation Modulates Neuronal Networks in Attention Deficit Hyperactivity Disorder. Brain Topogr. 2017, 30, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Westwood, S.J.; Criaud, M.; Lam, S.-L.; Lukito, S.; Wallace-Hanlon, S.; Kowalczyk, O.S.; Kostara, A.; Mathew, J.; Agbedjro, D.; Wexler, B.E.; et al. Transcranial direct current stimulation (tDCS) combined with cognitive training in adolescent boys with ADHD: A double-blind, randomised, sham-controlled trial. Psychol. Med. 2021, 1–16. [Google Scholar] [CrossRef]

| Subjects | tDCS Intervention | Brain Region | Conclusion | Ref. |
|---|---|---|---|---|
| Adults with high functioning ASD | Anodal; 2.0 mA; 30 min | Temporoparietal junction | Improvement of emotional processing | [32] |
| ASD children with Immature syntax | Anodal; 0.08 mA/cm2; 30 min | Dorsolateral prefrontal cortex | Improvement of syntax acquisition | [33] |
| ASD patients < 11 years of age | Cathodal; 1 mA; 20 min | Dorsolateral pre-frontal cortex | Significant decrease in the total score of three ASD clinical scales, accompanied by an improvement in autistic behavior up to six months after stimulation; increase in brain functional connectivity. | [34] |
| Male autism patients (5–8 years old) with mild to moderate autistic symptoms | Anodal; 1 mA; 20 min | Dorsolateral pre-frontal cortex | Improvements social/behavioral and health problems subscale | [35] |
| Adults with high functioning ASD | Anodal bifrontal; 1.5 mA; 40 min | Dorsolateral pre-frontal cortex | Improve working memoryperformance | [36] |
| ASD patients (10–18 years old) | Multi-channel anodal; 2 mA, 20 min | Temporoparietal junction | Ongoing | [37] |
| Male ASD patients (14–21 years old) | Cathodal and anodal; 1.5 mA, 20 min, 2 weeks with cognitive training | Left dorsolateral prefrontal cortex and and right supraorbital region | Promote social functioning | [38] |
| Subjects | tDCS Intervention | Brain Region | Conclusion | Ref. |
|---|---|---|---|---|
| Adolescent MIS rats | Anodal or cathodal; 50 µA; 20 min × 2/day | Prefrontal cortex | Prevent positive neurological and behavior symptoms of schizophrenia | [48] |
| Patients with refractory auditory verbal hallucinations | Anodal and cathodal; 2 mA; 20 min × 2/day | Dorsolateral prefrontal cortex (anodal) and temporoparietal junction (cathodal) | Significant improvement on AHRS for up to 3 months. | [33,49] |
| SCZ patients18–25 years of age | Anodal and cathodal; 2 mA; 20 min × 2/day | Dorsolateral pre-frontal cortex (an-odal) and temporo-parietal junction (cathodal) | Amelioration of negative symptoms, except passive/apathetic withdrawal and stereotyped thinking, that lasted up to 6 weeks after the end of the trial. | [50] |
| SCZ patients | Eight cognitive training sessions (two session/day) combined with anodal, 2 mA, 30 min | Dorsolateral pre-frontal cortex | tDCS therapy leads to improvements in working memory, and a positive effect on retention of learning | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, B.; Martins, J.; Castelo-Branco, M.; Gonçalves, J. Transcranial Direct Current Stimulation as an Approach to Mitigate Neurodevelopmental Disorders Affecting Excitation/Inhibition Balance: Focus on Autism Spectrum Disorder, Schizophrenia, and Attention Deficit/Hyperactivity Disorder. J. Clin. Med. 2022, 11, 2839. https://doi.org/10.3390/jcm11102839
Sousa B, Martins J, Castelo-Branco M, Gonçalves J. Transcranial Direct Current Stimulation as an Approach to Mitigate Neurodevelopmental Disorders Affecting Excitation/Inhibition Balance: Focus on Autism Spectrum Disorder, Schizophrenia, and Attention Deficit/Hyperactivity Disorder. Journal of Clinical Medicine. 2022; 11(10):2839. https://doi.org/10.3390/jcm11102839
Chicago/Turabian StyleSousa, Beatriz, João Martins, Miguel Castelo-Branco, and Joana Gonçalves. 2022. "Transcranial Direct Current Stimulation as an Approach to Mitigate Neurodevelopmental Disorders Affecting Excitation/Inhibition Balance: Focus on Autism Spectrum Disorder, Schizophrenia, and Attention Deficit/Hyperactivity Disorder" Journal of Clinical Medicine 11, no. 10: 2839. https://doi.org/10.3390/jcm11102839
APA StyleSousa, B., Martins, J., Castelo-Branco, M., & Gonçalves, J. (2022). Transcranial Direct Current Stimulation as an Approach to Mitigate Neurodevelopmental Disorders Affecting Excitation/Inhibition Balance: Focus on Autism Spectrum Disorder, Schizophrenia, and Attention Deficit/Hyperactivity Disorder. Journal of Clinical Medicine, 11(10), 2839. https://doi.org/10.3390/jcm11102839








