Nutrition Assessment and Management in Patients with Cirrhosis and Cognitive Impairment: A Comprehensive Review of Literature
Abstract
1. Introduction: Hepatic Encephalopathy
- -
- Laxative effect: by creating a hyperosmolar intestinal environment, lactulose accelerates intestinal transit and prevents ammonium absorption in the colon;
- -
- Ammonium ionization: acidification of intestinal contents results in ionization of ammonium, which in this form can no longer diffuse freely across cell membranes;
- -
- Bacterial uptake of ammonium and the beneficial effect on intestinal microbiota: volatile fatty acids released because of lactulose metabolism, are used by bacteria as an energy substrate for proliferation, while ammonium trapped in the colon is used as a source of nitrogen for protein synthesis;
- -
- Reduction of the intestinal production of ammonium: lactulose inhibits the activity of the enzyme glutaminase and the intestinal uptake of glutamine, blocking the subsequent conversion into ammonium;
2. Metabolic Alterations in Liver Cirrhosis
3. Protein-Calorie Malnutrition in Liver Cirrhosis
4. Beyond BMI: Sarcopenia, Myosteatosis and Sarcopenic Obesity in Liver Cirrhosis
5. Malnutrition in Patients with Hepatic Encephalopathy
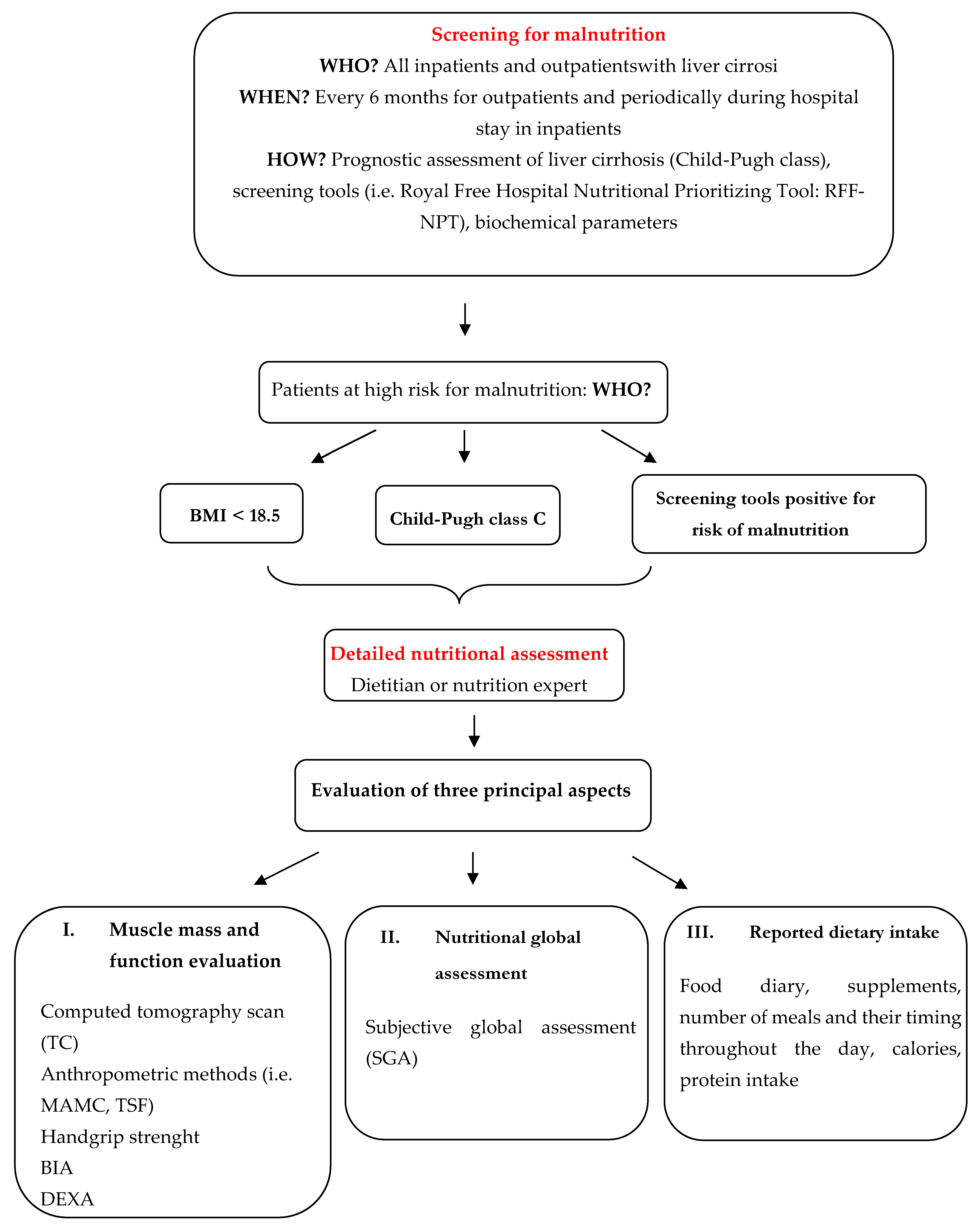
6. Assessment of Nutritional Status
- -
- There are gender differences in body composition and tissue loss characteristics that limit the usefulness of instruments measuring muscle mass and function in women. In fact, the study by Riggio et al. showed that body composition is different between men and women. In particular, in women, fat reserves were more deficient with maintenance of muscle mass. In contrast, in men, the loss of muscle tissue was more evident as observed under stress conditions [28].
- -
- There is no standardized approach to diagnosing and classifying malnutrition;
- -
- Prevalence is affected by aetiology of cirrhosis, being very high in hospitalized patients with alcoholic aetiology;
- -
- Hydrosaline retention makes body weight and body mass index unreliable;
- -
- The value of biochemical markers, such as albumin, are affected by plasma dilution and altered hepatic synthesis;
- -
- More accurate measurements, such as DEXA and dilution techniques, have high costs, are not always available and require specialized personnel.
6.1. Non-Instrumental Methods
- -
- Food diary: represents a simple tool to obtain information regarding daily food intake [8];
- -
- Objective examination: allows recognition of signs of nutritional deficiency such as loss of muscle mass, loss of subcutaneous fat, dry skin, hair loss and signs of vitamin and micronutrient deficiencies;
- -
- Biochemical parameters: parameters such as albumin, pre-albumin and retinol-binding protein are influenced by the residual capacity of hepatic synthesis, so they are not reliable for the assessment of nutritional status in cirrhotic patients; therefore, the level of total plasma proteins correlates more with severity of hepatopathy than with nutritional status [8].
- -
- Micronutrient dosage: zinc deficiency is extremely common in cirrhotic patients and has a prevalence of 84–96%; it may be due to reduced intestinal absorption or excessive diuretic use; symptoms of deficiency include anorexia, immune system dysfunction and dysgeusia [8]; in addition, because zinc participates in urea detoxification, it may increase the risk of HE.
- -
- Plasma vitamin dosage: among possible vitamin deficiencies, thiamine deficiency is often encountered, especially in alcoholic aetiology, because of reduced intake, reduced storage and reduced intestinal absorption [7]. Its deficiency causes Wernicke’s encephalopathy, which must be placed in differential diagnosis with HE, so it must always be supplemented.
- -
- Creatinine-to-weight ratio: if patient’s renal function is normal, this ratio can be used to estimate muscle mass. In fact, creatinine is almost entirely contained in skeletal and smooth muscle and reduced urinary excretion may be due either to impaired renal function or to reduced muscle mass, but not to impaired hepatic function [33];
- -
- Anthropometric measurements: these are objective methods for assessing patient’s nutritional status. They are rapid, non-invasive and low-cost techniques specifically designed to assess somatic characteristics. However, even these have limitations when applied to cirrhotic patients, especially with HE [25].
- -
- Subjective global assessment questionnaire (SGA): this is one of the most widely used methods for hospitalized patients. It is a questionnaire that uses several components of nutritional assessment including objective examination, dietary and clinical history (weight, dietary intake, gastrointestinal symptoms, functional capacity, nutritional demands and metabolic demands), to classify patients according to their degree of malnutrition. It is quick and easy to administer and takes approximately 15 min; it has been validated for nutritional assessment in cirrhotic patient and may provide prognostic information [8].
6.2. Instrumental Methods
- -
- Bioimpedance testing: it is a tricompartmental model technique as it identifies the muscle body mass and the non-fat body mass, which is divided into extracellular body mass and cellular body mass or metabolically active tissue.
- -
- Handgrip strength: this is an instrument used to assess muscle strength. It is one of the most sensitive methods for measuring nutritional status and has been shown to predict prognosis in patients with advanced liver disease [6].
- -
- DEXA: this examination has received particular attention because it is widely used to validate body composition results obtained with other methods. The procedure is based on measuring body composition according to a model that divides body elements into bone, fat, muscle mass and body free mass based on the passage of photon. In addition, compared to other techniques, radiation exposure is minimal [25].
- -
- Indirect calorimetry: is used to define REE by measuring oxygen consumption and carbon dioxide production. The patient is considered hypermetabolic if the REE is 10–20% higher than the reference value. However, this method is expensive, available only in some centres and may be affected by the presence of ascites [8].
- -
- Computed tomography scan: recently there has been a growing interest in the use of CT scan for the assessment of muscle mass loss and the presence of porto-systemic shunts, that may favour the development of HE in cirrhotic patients.
- -
- Assessment of global physical performance: up-and-go test, six minutes’ walk test.
7. Optimization of Nutritional Status
7.1. Caloric Requirement
7.2. Protein
- -
- The lower ratio of sulphur amino acids, such as methionine and cysteine, to BCAAs [59];
- -
- The reduced formation of mercaptans from sulphur amino acids fermentation, which appear to be involved in the genesis of HE along with ammonium [13];
- -
- The significant increase in fermentable fibre. In fact, levels of protein fermentation end products, such as ammonium and phenols, are significantly reduced when dietary fibre intake is increased. This occurs because increased fermentation of carbohydrates by colonic bacteria results in increased nitrogen utilization by bacteria and in pH reduction, which favours excretion of ammonium over its absorption [61].
- -
- The increased clearance and reduced intestinal absorption of nitrogen products as a result of reduced intestinal transit time due to the mass-forming effect of fibres [17].
- -
7.3. Protein Supplementation
7.4. Carbohydrates and Lipids
7.5. Fibres, Vitamins and Micronutrients
7.6. Parenteral Nutrition
7.7. Physical Exercise
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, B.C.; Puri, V.; Sachdeva, S.; Srivastava, S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab. Brain Dis. 2017, 32, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Formentin, C.; Garrido, M.; Montagnese, S. Assessment and Management of Sleep Disturbance in Cirrhosis. Curr. Hepatol. Rep. 2018, 17, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Ridola, L.; Riggio, O.; Gioia, S.; Faccioli, J.; Nardelli, S. Clinical management of type C hepatic encephalopathy. United Eur. Gastroenterol. J. 2020, 8, 536–543. [Google Scholar] [CrossRef]
- Yao, C.K.; Fung, J.; Chu, N.H.S.; Tan, V.P.Y. Dietary Interventions in Liver Cirrhosis. J. Clin. Gastroenterol. 2018, 52, 663–673. [Google Scholar] [CrossRef]
- Fallahzadeh, M.A.; Rahimi, R.S. Hepatic Encephalopathy and Nutrition Influences: A Narrative Review. Nutr. Clin. Pract. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Silva, M.; Gomes, S.; Peixoto, A.; Torres-Ramalho, P.; Cardoso, H.; Azevedo, R.; Cunha, C.; Macedo, G. Nutrition in Chronic Liver Disease. GE Port J. Gastroenterol. 2015, 22, 268–276. [Google Scholar] [CrossRef]
- Mizock, B.A. Nutritional support in hepatic encephalopathy. Nutrition 1999, 15, 220–228. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Verboeket-van de Venne, W.P.; Westerterp, K.R.; van Hoek, B.; Swart, G.R. Energy expenditure and substrate metabolism in patients with cirrhosis of the liver: Effects of the pattern of food intake. Gut 1995, 36, 110–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amodio, P.; Canesso, F.; Montagnese, S. Dietary management of hepatic encephalopathy revisited. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, G.G. Diets in Encephalopathy. Clin. Liver Dis. 2015, 19, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Böttcher, J.; Selberg, O.; Weselmann, S.; Böker, K.H.; Schwarze, M.; von zur Mühlen, A.; Manns, M.P. Hypermetabolism in clinically stable patients with liver cirrhosis. Am. J. Clin. Nutr. 1999, 69, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Irigoin, R.R.; Abilés, J. Nutritional support in patients with liver cirrhosis. Gastroenterol. Hepatol. 2012, 35, 594–601. [Google Scholar]
- Ferreira Figueiredo, F.A.; De Mello Perez, R.; Kondo, M. Effect of liver cirrhosis on body composition: Evidence of significant depletion even in mild disease. J. Gastroenterol. Hepatol. 2005, 20, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Riggio, O. Dietary and nutritional indications in hepatic encephalopathy. Metab. Brain Dis. 2009, 24, 211–221. [Google Scholar] [CrossRef]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef]
- Ebadi, M.; Tsien, C.; Bhanji, R.A.; Dunichand-Hoedl, A.R.; Rider, E.; Motamedrad, M.; Mazurak, V.C.; Baracos, V.; Montano-Loza, A.J. Skeletal Muscle Pathological Fat Infiltration (Myosteatosis) Is Associated with Higher Mortality in Patients with Cirrhosis. Cells 2022, 11, 1345. [Google Scholar] [CrossRef]
- Bhanji, R.A.; Moctezuma-Velazquez, C.; Duarte-Rojo, A.; Ebadi, M.; Ghosh, S.; Rose, C.; Montano-Loza, A.J. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol. Int. 2018, 12, 377–386. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia Sarcopenia Muscle 2016, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Kramp, W.; Lurje, I.; Miller, H.; Bednarsch, J.; Lang, S.A.; Ulmer, T.M.; Bruners, P.; Strnad, P.; Trautwein, C.; et al. The role of recipient myosteatosis in graft and patient survival after deceased donor liver transplantation. J. Cachexia Sarcopenia Muscle 2021, 12, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Nishiguchi, S.; Iijima, H. Sarcopenic Obesity in Liver Cirrhosis: Possible Mechanism and Clinical Impact. Int. J. Mol. Sci. 2021, 22, 1917. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Shirai, H.; Yao, S.; Kamo, N.; Yagi, S.; Taura, K.; Okajima, H.; et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2019, 269, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Gomes Romeiro, F.; Augusti, L. Nutritional assessment in cirrhotic patients with hepatic encephalopathy. World J. Hepatol. 2015, 7, 2940–2954. [Google Scholar] [CrossRef]
- Ridola, L.; Gioia, S.; Faccioli, J.; Riggio, O.; Nardelli, S. Gut liver muscle brain axis: A comprehensive viewpoint on prognosis in cirrhosis. J. Hepatol. 2022, 25, 168–278. [Google Scholar] [CrossRef]
- Amodio, P.; Bemeur, C.; Butterworth, R.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013, 58, 325–336. [Google Scholar] [CrossRef]
- Riggio, O.; Andreoli, A.; Diana, F.; Fiore, P.; Meddi, P.; Lionetti, R.; Montagnese, F.; Merli, M.; Capocaccia, L.; De Lorenzo, A. Whole body and regional body composition analysis by dual-energy X-ray absorptiometry in cirrhotic patients. Eur. J. Clin. Nutr. 1997, 51, 810–814. [Google Scholar] [CrossRef]
- Savio, J.; Thuluvath, P.J. Hyponatremia in cirrhosis: Pathophysiology and management. World J. Gastroenterol. 2015, 21, 3197–3205. [Google Scholar]
- Vidot, H.; Potter, A.; Cheng, R.; Allman-Farinelli, M.; Shackel, N. Serum 25-hydroxyvitamin D deficiency and hepatic encephalopathy in chronic liver disease. World J. Hepatol. 2017, 9, 510–518. [Google Scholar] [CrossRef]
- Pazgan-Simon, M.; Zuwała-Jagiełło, J.; Serafińska, S.; Simon, K. Nutrition principles and recommendations in different types of hepatic encephalopathy. Clin. Exp. Hepatol. 2016, 1, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, A.; Berne, B.; Nordlinder, H.; Busch, C.; Eriksson, U.; Lööf, L.; Vahlquist, A. Impaired release of vitamin A from liver in primary biliary cirrhosis. Hepatology 1988, 8, 136–141. [Google Scholar] [CrossRef]
- Pirlich, M.; Selberg, O.; Böker, K.; Schwarze, M.; Müller, M.J. The creatinine approach to estimate skeletal muscle mass in patients with cirrhosis. Hepatology 1996, 24, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Fiore, P.; Merli, M.; Andreoli, A.; De Lorenzo, A.; Masini, A.; Ciuffa, L.; Valeriano, V.; Balotta, M.T.; Riggio, O. A comparison of skinfold anthropometry and dual-energy X-ray absorptiometry for the evaluation of body fat in cirrhotic patients. Clin. Nutr. 1999, 18, 349–351. [Google Scholar] [CrossRef]
- Alberino, F.; Gatta, A.; Amodio, P.; Merkel, C.; Di Pascoli, L.; Boffo, G.; Caregaro, L. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001, 17, 445–450. [Google Scholar] [CrossRef]
- Merli, M.; Giusto, M.; Lucidi, C.; Giannelli, V.; Pentassuglio, I.; Di Gregorio, V.; Lattanzi, B.; Riggio, O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: Results of a prospective study. Metab. Brain Dis. 2013, 28, 281–284. [Google Scholar] [CrossRef]
- Alvares-da-Silva, M.R.; Reverbel da Silveira, T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005, 21, 113–117. [Google Scholar]
- Kalaitzakis, E.; Josefsson, A.; Castedal, M.; Henfridsson, P.; Bengtsson, M.; Andersson, B.; Björnsson, E. Hepatic encephalopathy is related to anemia and fat-free mass depletion in liver transplant candidates with cirrhosis. Scand. J. Gastroenterol. 2013, 48, 577–584. [Google Scholar] [CrossRef]
- Plauth, M.; Cabré, E.; Riggio, O.; Assis-Camilo, M.; Pirlich, M.; Kondrup, J.; DGEM (German Society for Nutritional Medicine); Ferenci, P.; Holm, E.; Vom Dahl, S.; et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin. Nutr. 2006, 25, 285–294. [Google Scholar] [CrossRef]
- Huynh, D.K.; Selvanderan, S.P.; Harley, H.A.J.; Holloway, R.H.; Nguyen, N.Q. Nutritional care in hospitalized patients with chronic liver disease. World J. Gastroenterol. 2015, 21, 12835–12842. [Google Scholar] [CrossRef]
- Iwasa, M.; Iwata, K.; Hara, N.; Hattori, A.; Ishidome, M.; Sekoguchi-Fujikawa, N.; Mifuji-Moroka, R.; Sugimoto, R.; Fujita, N.; Kobayashi, Y.; et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition 2013, 29, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Swart, G.R.; Zillikens, M.C.; van Vuure, J.K.; van den Berg, J.K. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ 1989, 299, 1202–1203. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Okita, K.; Suzuki, K.; Moriwaki, H.; Kato, A.; Miwa, Y.; Shiraishi, K.; Okuda, H.; Onji, M.; Kanazawa, H.; et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 2007, 23, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Manguso, F.; D’Ambra, G.; Menchise, A.; Sollazzo, R.; D’Agostino, L. Effects of an appropriate oral diet on the nutritional status of patients with HCV-related liver cirrhosis: A prospective study. Clin. Nutr. 2005, 24, 751–759. [Google Scholar] [CrossRef]
- Maharshi, S.; Sharma, B.C.; Sachdeva, S.; Srivastava, S.; Sharma, P. Efficacy of Nutritional Therapy for Patients With Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2016, 14, 454–460.e3. [Google Scholar] [CrossRef]
- Kato, A.; Tanaka, H.; Kawaguchi, T.; Kanazawa, H.; Iwasa, M.; Sakaida, I.; Moriwaki, H.; Murawaki, Y.; Suzuki, K.; Okita, K. Nutritional management contributes to improvement in minimal hepatic encephalopathy and quality of life in patients with liver cirrhosis: A preliminary, prospective, open-label study. Hepatol. Res. 2013, 43, 452–458. [Google Scholar] [CrossRef]
- Gheorghe, L.; Iacob, R.; Vădan, R.; Iacob, S.; Gheorghe, C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom. J. Gastroenterol. 2005, 14, 231–238. [Google Scholar]
- Hirsch, S.; Bunout, D.; de la Maza, P.; Iturriaga, H.; Petermann, M.; Icazar, G.; Gattas, V.; Ugarte, G. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. J. Parenter. Enteral. Nutr. 1993, 17, 119–124. [Google Scholar] [CrossRef]
- Córdoba, J.; López-Hellín, J.; Planas, M.; Sabín, P.; Sanpedro, F.; Castro, F.; Esteban, R.; Guardia, J. Normal protein diet for episodic hepatic encephalopathy: Results of a randomized study. J. Hepatol. 2004, 41, 38–43. [Google Scholar] [CrossRef]
- Bianchi, G.; Marchesini, G.; Fabbri, A.; Rondelli, A.; Bugianesi, E.; Zoli, M.; Pisi, E. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J. Intern. Med. 1993, 233, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Margáin, A.; Macías-Rodríguez, R.U.; Ríos-Torres, S.L.; Román-Calleja, B.M.; Méndez-Guerrero, O.; Rodríguez-Córdova, P.; Torre, A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev. Gastroenterol. Mex. 2018, 83, 9–15. [Google Scholar] [CrossRef]
- Horst, D.; Grace, N.D.; Conn, H.O.; Schiff, E.; Schenker, S.; Viteri, A.; Law, D.; Atterbury, C.E. Comparison of dietary protein with an oral, branched chain-enriched amino acid supplement in chronic portal-systemic encephalopathy: A randomized controlled trial. Hepatology 1984, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.; Márquez, M.A.; Garcia Ramos, G.; Ramos-Uribe, M.H.; Vargas, F.; Villalobos, A.; Ramos, C. Treatment of chronic portal--systemic encephalopathy with vegetable and animal protein diets. A controlled crossover study. Dig. Dis. Sci. 1982, 27, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, K.M.; Blendis, L.M.; Zilm, D.H.; Carlen, P.L.; Anderson, G.H. Effect of dietary protein manipulation in subclinical portal-systemic encephalopathy. Gut 1983, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Meek, J.; Sutton, C.; Emery, V.M.; Hughes, E.A.; Hodgson, H.J. Dietary protein supplementation from vegetable sources in the management of chronic portal systemic encephalopathy. Am. J. Gastroenterol. 1984, 79, 945–949. [Google Scholar] [PubMed]
- Zenith, L.; Meena, N.; Ramadi, A.; Yavari, M.; Harvey, A.; Carbonneau, M.; Juan, M.; Abraldes, G.; Paterson, I.; Haykowsky, M.J.; et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1920–1926.e2. [Google Scholar] [CrossRef]
- Henkel, A.S.; Buchman, A.L. Nutritional support in patients with chronic liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 202–209. [Google Scholar] [CrossRef]
- Amodio, P.; Caregaro, L.; Pattenò, E.; Marcon, M.; Del Piccolo, F.; Gatta, A. Vegetarian diets in hepatic encephalopathy: Facts or fantasies? Dig. Liver Dis. 2001, 33, 492–500. [Google Scholar] [CrossRef]
- Merli, M.; Iebba, V.; Giusto, M. What is new about diet in hepatic encephalopathy. Metab. Brain Dis. 2016, 31, 1289–1294. [Google Scholar] [CrossRef]
- Birkett, A.; Muir, J.; Phillips, J.; Jones, G.; O’Dea, K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 1996, 63, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Russo, F.P.; Amodio, P.; Burra, P.; Gasbarrini, A.; Loguercio, C.; Marchesini, G.; Merli, M.; Ponziani, F.R.; Riggio, O.; et al. Hepatic encephalopathy 2018: A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Dig. Liver Dis. 2019, 51, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Dam, G.; Borre, M.; Les, I.; Cordoba, J.; Marchesini, G.; Aagaard, N.K.; Risum, N.; Vilstrup, H. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J. Nutr. 2013, 143, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition 2010, 26, 482–490. [Google Scholar] [CrossRef]
- Schulte-Frohlinde, E.; Wagenpfeil, S.; Willis, J.; Lersch, C.; Eckel, F.; Schmid, R.; Schusdziarra, V. Role of meal carbohydrate content for the imbalance of plasma amino acids in patients with liver cirrhosis. J. Gastroenterol. Hepatol. 2007, 22, 1241–1248. [Google Scholar] [CrossRef]
| Normal | Moderate Malnutrition | Severe Malnutrition | ||||
|---|---|---|---|---|---|---|
| BMI | <30 | >30 | <30 | >30 | <30 | >30 |
| Caloric intake (kcal/die) | 35–40 | 20–35 | 35–40 | 20–35 | 35–40 | 20–35 |
| Carbohydrate intake (%) | 50–60% | |||||
| Protein intake (g/die) | 1.2–1.5 | 1–1.5 | 1.2–1.5 | |||
| Number of meals/die | 4–6 meals | |||||
| Bedtime snacks | High in calories (at least 50 g of complex carbohydrate) | |||||
| Protein source | Vegetables and dairy products | |||||
| Fibre (g/die) | 25–45 g | |||||
| Vitamin and micronutrients | Correction of deficiency as good clinical practice | |||||
| Target | Author/Year | Study Design | N. Patients | Intervention | Comparison | Duration | Main Results |
|---|---|---|---|---|---|---|---|
| Number of meals/late snack | Swart et al., 1989 [42] | Randomised crossover | n = 9 cirrhotic patients | 4–6 meals/die | Three meals/die | 2 periods of consecutive five days. | 4–6 meals/die resulted in more positive nitrogen balances than three meals/die. |
| Plank et al., 2008 [43] | Randomized | n = 103 cirrhotic patients | Night-time supplementary nutrition (n = 52) | Daytime supplementary nutrition (n = 51) | 12 months | A night-time snack resulted in a total body protein accretion sustained over 12 months (equivalent to about 2 kg of lean tissue). | |
| Verboeket-van de Venne, 1995 [11] | Randomized crossover | n = 8 cirrhotic patients and 23 healthy subjects (controls) | 4–7 meals/die (“nibbling pattern”) | 2 large meals (“gorging pattern”) | Two periods of 2 consecutive days. | The “gorging pattern” had greater fluctuations in respiratory quotient and higher nocturnal protein oxidation than in the daytime in both groups, reflecting a higher oxidation ratio of fat to carbohydrate compatible with a more catabolic state. | |
| Nakaya et al., 2007 [44] | Randomized | n = 48 cirrhotic patients | Late-evening supplementation with BCAA-enriched nutrient mixture (= 25) | Late-evening supplementation with ordinary food (n = 23) | 3 months | BCAA supplementation significantly improved serum albumin level, nitrogen balance and respiratory quotient than ordinary food. | |
| Caloric and protein intake | Manguso et al., 2005 [45] | Randomized | n = 90 cirrhotic patients | Controlled diet (n = 45) | Spontaneous diet (n = 45) | 3 months | The controlled diet caused an increase in MAMC, serum albumin and creatinine-height index |
| Maharshi et al., 2016 [46] | Randomized | n = 120 cirrhotic patients with MHE | Nutritional therapy (30–35 kcal/kg/die and 1.0–1.5 g vegetable protein/kg/die) | No nutritional therapy | 6 months | A higher proportion of patients in the nutritional therapy group reversed MHE; nutritional therapy increased PHES and HRQOL and reduced OHE incidence. | |
| Kato et al., 2013 [47] | Prospective | n = 19 cirrhotic patients with MHE | Nutritional consultation (30–35 Kcal/Kg/die and 1–1.5 g/Kg/die of protein) | - | 8 weeks | The MHE scores significantly improved at 8 weeks. | |
| Gheorghe et al., 2005 [48] | Prospective | n = 153 cirrhotic patients with OHE | High caloric and high protein diet (vegetable and dairy products) | - | 1 year | Almost 80% of patients improved their mental status; high protein diet significantly reduced ammonia level. | |
| Hirsch et al., 1993 [49] | Randomized | n = 51 patients with decompensated alcoholic cirrhosis | Oral nutrition support (1000 Kcal, 34 g protein) (n = 26) | One placebo capsule (n = 25) | Oral nutrition support significantly improved nutritional status, MAMC, serum albumin and handgrip strength than placebo. | ||
| Cordoba et al., 2004 [50] | Randomized | n = 30 cirrhotic patients with acute HE | Normal protein diet (n = 10) | Low protein diet (n = 10) | 14 days | The outcome of HE was not significantly different between both groups. | |
| Protein source/ type of protein | Bianchi et al., 1993 [51] | Crossover randomized | n = 8 cirrhotic patients with chronic HE in therapy with lactulose | Diet containing vegetal proteins (50 g) | Isocaloric, isonitrogenous diets containing animal protein (50 g) | 2 consecutive periods of 7 days | The vegetable protein diet improved ammonia level, insulin and nitrogen balance, and clinical grading of HE. Psycometric tests improved significantly but remained abnormal |
| Ruiz-Margáin et al., 2017 [52] | Randomized | n = 72 Cirrhotic patients | High protein and high-fibre diet with BCAA (protein: 1.2 g/Kg/die, fibre 30 g, BCAA 110 g) (n = 37) | High protein, high-fibre diet and no BCAA (n = 35) | 6 months | BCAA supplementation increased muscle mass. No significant changes in PHES or CFF score resulted in both groups (no development of HE). | |
| Horst et al., 1984 [53] | Randomized | n = 37 cirrhotic patients with recurrent HE | 20 g of dietary protein for 1 week, after which BCAA were added weekly to obtain a protein intake of 80 g/die (n = 14) | 20 g of dietary protein for 1 week, after which 20 g of proteins were added weekly to obtain a protein intake of 80 g/die (n = 12) | BCAA supplementation significantly reduced HE recurrence and improved mental status grade and asterixis. | ||
| Uribe et al., 1982 [54] | Crossover randomized single blind | n = 10 cirrhotic patients with chronic HE | 40 g/die of vegetable protein (high fibre diet, low methionine and low aromatic amino acids) and 80 g/die of vegetable protein (rich in BCAA and fibre, with same amount of sulfurated amino acids). | 40 g/die of meat protein plus neomycin-milk of magnesia | 3 consecutive periods of 2 weeks | After 2 weeks, patients on vegetarian diets performed the NCT more quickly than meat diet. Patients treated with the 80 g/day vegetable diet improved EEG. | |
| De Brujin et al., 1983 [55] | Randomized crossover | n = 8 cirrhotic patients with MHE | 60 g/die of vegetable diet (second and fourth week) | 60 g/die of a mix diet with 1:1 ratio of vegetable and meat diet (first, third and fifth week) | 5 consecutive periods of 1 week. | During the vegetable diet, the nitrogen balance tended to be more positive, but without changes in neurological status or ammonia level. | |
| Keshavarzian et al., 1984 [56] | Crossover randomized | n = 6 cirrhotic patients with chronic HE on lactulose therapy | 80 g vegetable-supplemented diet (3:5 ratio of animal and vegetable protein) | 40 g protein conventional diet (3:1 ratio of animal and vegetable protein) | 2 consecutive periods of 5 days. | After 10 days, patients treated with a vegetable diet showed clinical improvement and amelioration of EEG. | |
| Physical exercise | Zenith et al., 2014 [57] | Randomized | n = 20 | exercise training (n = 10) | usual care (n = 10) | 8 weeks | Aerobic exercise increased peak VO2 and muscle mass and reduced fatigue in cirrhotic patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccioli, J.; Nardelli, S.; Gioia, S.; Riggio, O.; Ridola, L. Nutrition Assessment and Management in Patients with Cirrhosis and Cognitive Impairment: A Comprehensive Review of Literature. J. Clin. Med. 2022, 11, 2842. https://doi.org/10.3390/jcm11102842
Faccioli J, Nardelli S, Gioia S, Riggio O, Ridola L. Nutrition Assessment and Management in Patients with Cirrhosis and Cognitive Impairment: A Comprehensive Review of Literature. Journal of Clinical Medicine. 2022; 11(10):2842. https://doi.org/10.3390/jcm11102842
Chicago/Turabian StyleFaccioli, Jessica, Silvia Nardelli, Stefania Gioia, Oliviero Riggio, and Lorenzo Ridola. 2022. "Nutrition Assessment and Management in Patients with Cirrhosis and Cognitive Impairment: A Comprehensive Review of Literature" Journal of Clinical Medicine 11, no. 10: 2842. https://doi.org/10.3390/jcm11102842
APA StyleFaccioli, J., Nardelli, S., Gioia, S., Riggio, O., & Ridola, L. (2022). Nutrition Assessment and Management in Patients with Cirrhosis and Cognitive Impairment: A Comprehensive Review of Literature. Journal of Clinical Medicine, 11(10), 2842. https://doi.org/10.3390/jcm11102842






