Innate and Acquired Cellular Immunity in Children with Familial Hypercholesterolemia Treated with Simvastatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Methods
2.2.1. Hematological and Biochemical Measurements
2.2.2. Flow Cytometry Assessment
2.2.3. Statistical Analysis
3. Results
3.1. Blood Count
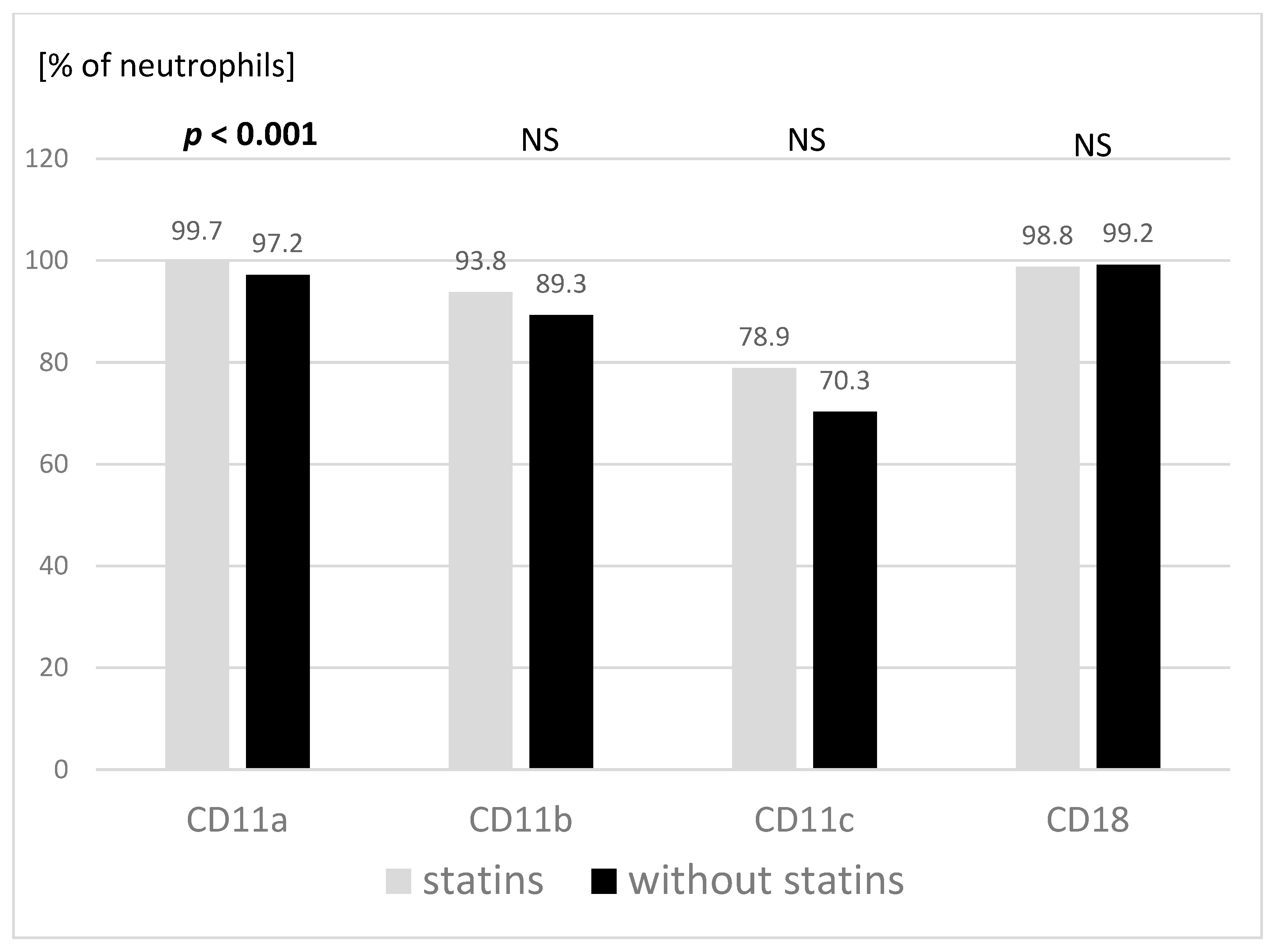
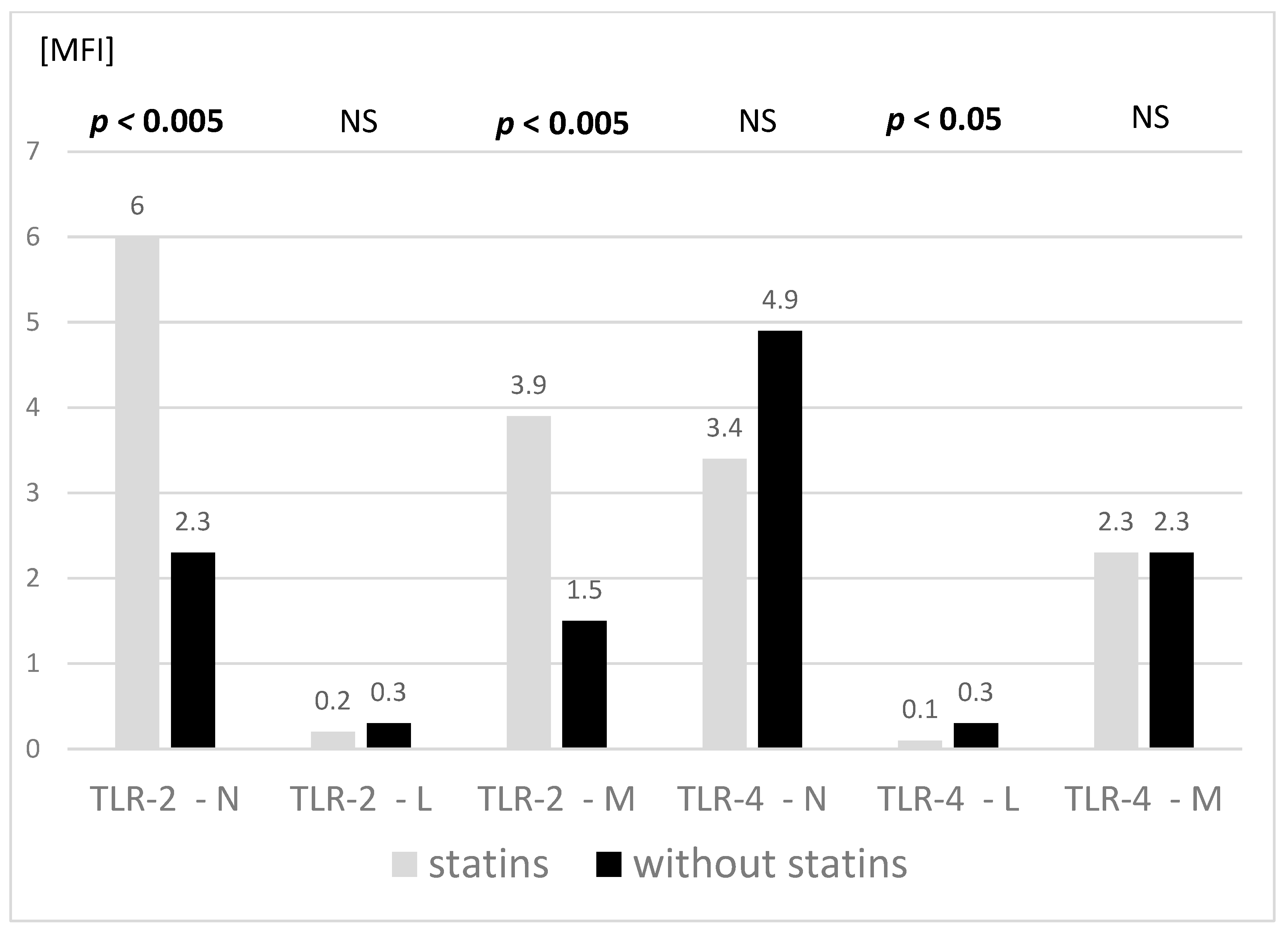
3.2. Adhesive Molecules and TLR Expression
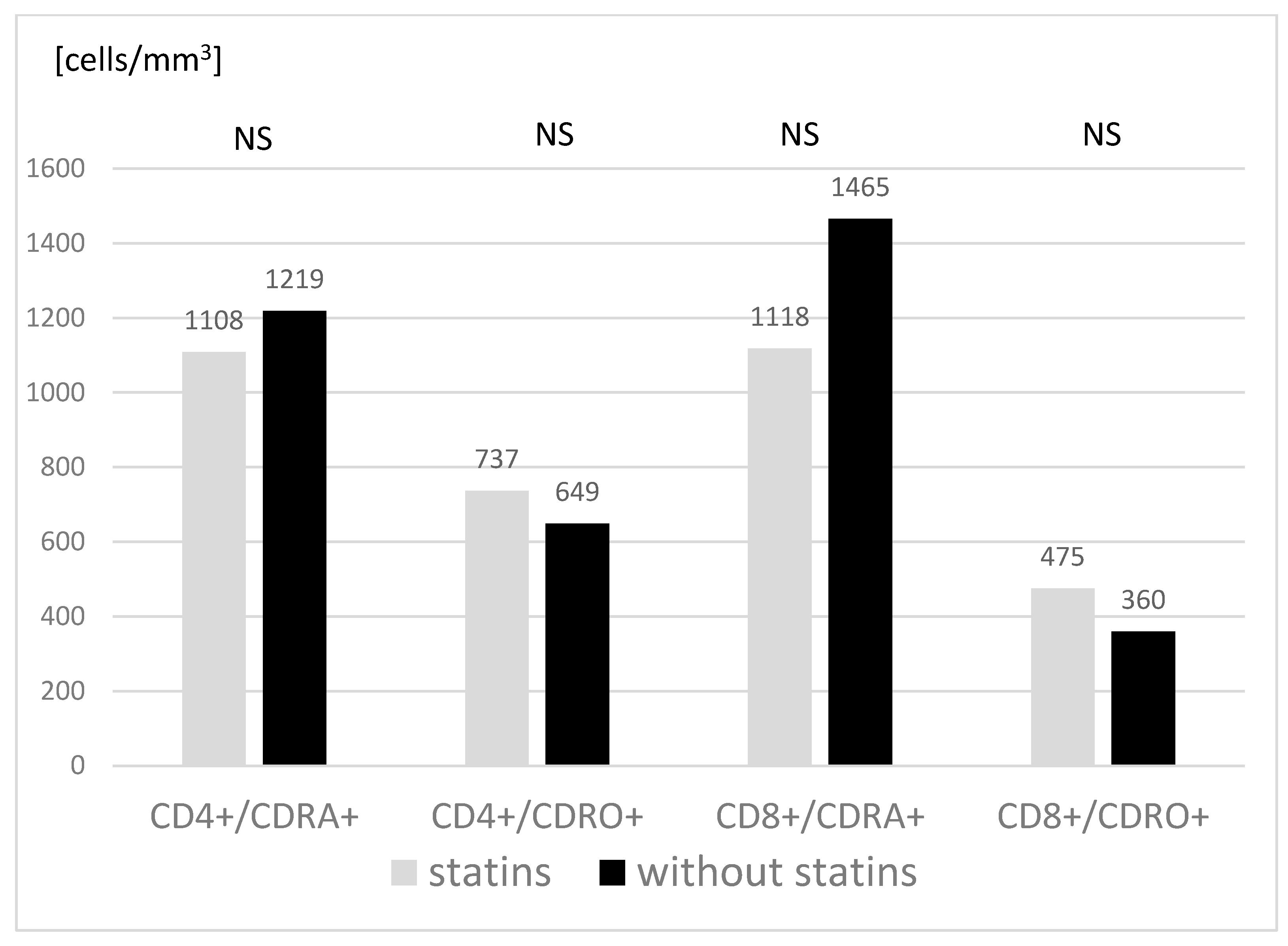
3.3. Main Subpopulations of Peripheral Blood Lymphocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordestgaard, B.G.; Benn, M. Genetic testing for familial hypercholesterolaemia is essential in individuals with high LDL cholesterol: Who does it in the world? Eur. Heart J. 2017, 38, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490a. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef]
- Mysliwiec, M.; Walczak, M.; Malecka-Tendera, E.; Dobrzanska, A.; Cybulska, B.; Filipiak, K.J.; Mazur, A.; Jarosz-Chobot, P.; Szadkowska, A.; Rynkiewicz, A.; et al. Management in familial hypercholesterolaemia in children and adolescents. Position of the Lipid Expert Forum. Kardiol. Pol. 2013, 71, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Narverud, I.; Retterstol, K.; Iversen, P.O.; Halvorsen, B.; Ueland, T.; Ulven, S.M.; Ose, L.; Aukrust, P.; Veierod, M.B.; Holven, K.B. Markers of atherosclerotic development in children with familial hypercholesterolemia: A literature review. Atherosclerosis 2014, 235, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef] [Green Version]
- Vuorio, A.; Kuoppala, J.; Kovanen, P.T.; Humphries, S.E.; Tonstad, S.; Wiegman, A.; Drogari, E.; Ramaswami, U. Statins for children with familial hypercholesterolemia. Cochrane Database Syst. Rev. 2019, CD006401. [Google Scholar] [CrossRef]
- Kounis, N.G.; Soufras, G.D.; Tsigkas, G.; Hahalis, G. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin. Appl. Thromb. Hemost. 2015, 21, 139–143. [Google Scholar] [CrossRef]
- Carlos, T.M.; Harlan, J.M. Leukocyte-endothelial adhesion molecules. Blood 1994, 84, 2068–2101. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Dalmaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors-From microbial recognition to autoimmunity: A comprehensive review. Autoimmun. Rev. 2016, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, A.M.; Hansson, G.K. Innate immune signals in atherosclerosis. Clin. Immunol. 2010, 134, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.J.; Osnes, L.T.; Halvorsen, B.; Retterstol, K.; Bogsrud, M.P.; Wium, C.; Svilaas, A.; Narverud, I.; Ulven, S.M.; Aukrust, P.; et al. Altered leukocyte distribution under hypercholesterolemia: A cross-sectional study in children with familial hypercholesterolemia. Atherosclerosis 2017, 256, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurm, H.S.; Bhatt, D.L.; Lincoff, A.M.; Tcheng, J.E.; Kereiakes, D.J.; Kleiman, N.S.; Jia, G.; Topol, E.J. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: Insights from the EPIC, EPILOG, and EPISTENT trials. Heart 2003, 89, 1200–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartaigh, B.; Bosch, J.A.; Thomas, G.N.; Lord, J.M.; Pilz, S.; Loerbroks, A.; Kleber, M.E.; Grammer, T.B.; Fischer, J.E.; Boehm, B.O.; et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis 2012, 224, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawant, A.C.; Adhikari, P.; Narra, S.R.; Srivatsa, S.S.; Mills, P.K.; Srivatsa, S.S. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol. J. 2014, 21, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Arima, H.; Bertmar, C.; Clarke, S.; Herkes, G.; Krause, M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J. Neurol. Sci. 2018, 387, 115–118. [Google Scholar] [CrossRef]
- Akin, F.; Ayca, B.; Kose, N.; Sahin, I.; Akin, M.N.; Canbek, T.D.; Gungor, O. Effect of atorvastatin on hematologic parameters in patients with hypercholesterolemia. Angiology 2013, 64, 621–625. [Google Scholar] [CrossRef]
- Tani, S.; Nagao, K.; Anazawa, T.; Kawamata, H.; Iida, K.; Matsumoto, M.; Sato, Y.; Hirayama, A. Association of circulating leukocyte count with coronary atherosclerosis regression after pravastatin treatment. Atherosclerosis 2008, 198, 360–365. [Google Scholar] [CrossRef]
- Sorensen, A.L.; Hasselbalch, H.C.; Nielsen, C.H.; Poulsen, H.E.; Ellervik, C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox. Biol. 2019, 21, 101088. [Google Scholar] [CrossRef]
- Capuano, V.; Bambacaro, A.; Darminio, T.; Mazzot, G. White blood cell count is related to arterial pressure and cholesterolemia in normal children. Ital. Heart J. 2001, 2, 685–689. [Google Scholar] [PubMed]
- Tolani, S.; Pagler, T.A.; Murphy, A.J.; Bochem, A.E.; Abramowicz, S.; Welch, C.; Nagareddy, P.R.; Holleran, S.; Hovingh, G.K.; Kuivenhoven, J.A.; et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: Studies in mice and FH children. Atherosclerosis 2013, 229, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassirer, M.; Zeltser, D.; Prochorov, V.; Schoenman, G.; Frimerman, A.; Keren, G.; Shapira, I.; Miller, H.; Roth, A.; Arber, N.; et al. Increased expression of the CD11b/CD18 antigen on the surface of peripheral white blood cells in patients with ischemic heart disease: Further evidence for smoldering inflammation in patients with atherosclerosis. Am. Heart. J. 1999, 138, 555–559. [Google Scholar] [CrossRef]
- Mazzone, A.; De Servi, S.; Mazzucchelli, I.; Fossati, G.; Gritti, D.; Canale, C.; Cusa, C.; Ricevuti, G. Increased expression of CD11b/CD18 on phagocytes in ischaemic disease: A bridge between inflammation and coagulation. Eur. J. Clin. Investig. 1997, 27, 648–652. [Google Scholar] [CrossRef]
- Rezaie-Majd, A.; Prager, G.W.; Bucek, R.A.; Schernthaner, G.H.; Maca, T.; Kress, H.G.; Valent, P.; Binder, B.R.; Minar, E.; Baghestanian, M. Simvastatin reduces the expression of adhesion molecules in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 397–403. [Google Scholar] [CrossRef] [Green Version]
- de Bont, N.; Geijtenbeek, T.B.; Netea, M.G.; Smilde, T.J.; Demacker, P.N.; Figdor, C.G.; Van Der Meer, J.W.; Stalenhoef, A.F. Integrin mediated adhesion of mononuclear cells from patients with familial hypercholesterolemia. Eur. J. Clin. Investig. 1999, 29, 749–757. [Google Scholar] [CrossRef]
- Martino, F.; Martino, E.; Iacobini, M.; Ferrara, E.; Pacifico, L.; Noto, D.; Cefalu, A.B.; Averna, M.; Chiesa, C. Down regulation of CD11b and CD18 expression in children with hypercholesterolemia: A preliminary report. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 105–109. [Google Scholar] [CrossRef]
- Rovers, C.; Netea, M.G.; de Bont, N.; Demacker, P.N.; Jacobs, C.; Kullberg, B.J.; Van der Meer, J.W.; Stalenhoef, A.F. LPS-induced cytokine production and expression of beta2-integrins and CD14 by peripheral blood mononuclear cells of patients with homozygous familial hypercholesterolemia. Atherosclerosis 1998, 141, 99–105. [Google Scholar] [CrossRef]
- Stulc, T.; Vrablik, M.; Kasalova, Z.; Marinov, I.; Svobodova, H.; Ceska, R. Leukocyte and endothelial adhesion molecules in patients with hypercholesterolemia: The effect of atorvastatin treatment. Physiol. Res. 2008, 57, 185–194. [Google Scholar] [CrossRef]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2021, 60, 175–199. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, A.; Parsamanesh, N.; Atkin, S.L.; Banach, M.; Sahebkar, A. Effect of statins on toll-like receptors: A new insight to pleiotropic effects. Pharmacol. Res. 2018, 135, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, A.H.M.; Aloor, J.J.; Fessler, M.B.; Chowdhury, S.M. Cross-linking Proteomics Indicates Effects of Simvastatin on the TLR2 Interactome and Reveals ACTR1A as a Novel Regulator of the TLR2 Signal Cascade. Mol. Cell Proteom. 2019, 18, 1732–1744. [Google Scholar] [CrossRef]
- Moutzouri, E.; Tellis, C.C.; Rousouli, K.; Liberopoulos, E.N.; Milionis, H.J.; Elisaf, M.S.; Tselepis, A.D. Effect of simvastatin or its combination with ezetimibe on Toll-like receptor expression and lipopolysaccharide-induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis 2012, 225, 381–387. [Google Scholar] [CrossRef]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, C.P.; Ye, S. Statins inhibit toll-like receptor 4-mediated lipopolysaccharide signaling and cytokine expression. Pharmacogenet. Genom. 2008, 18, 803–813. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Marsland, A.; Flory, J.D.; Rabin, B.S.; Whiteside, T.L.; Manuck, S.B. Immune system differences in men with hypo- or hypercholesterolemia. Clin. Immunol. Immunopathol. 1997, 84, 145–149. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Flory, J.D.; Marsland, A.; Manuck, S.B.; Whiteside, T.L.; Rabin, B. Effects of lovastatin on the immune system. Am. J. Cardiol. 1997, 80, 1391–1394. [Google Scholar] [CrossRef]
- Collado, A.; Marques, P.; Domingo, E.; Perello, E.; Gonzalez-Navarro, H.; Martinez-Hervas, S.; Real, J.T.; Piqueras, L.; Ascaso, J.F.; Sanz, M.J. Novel Immune Features of the Systemic Inflammation Associated with Primary Hypercholesterolemia: Changes in Cytokine/Chemokine Profile, Increased Platelet and Leukocyte Activation. J. Clin. Med. 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Moreno, L.A.; Sarria, A.; Lazaro, A.; Lasierra, M.P.; Larrad, L.; Bueno, M. Lymphocyte T subset counts in children with hypercholesterolemia receiving dietary therapy. Ann. Nutr. Metab. 1998, 42, 261–265. [Google Scholar] [CrossRef] [PubMed]
- van den Hoek, H.L.; Bos, W.J.; de Boer, A.; van de Garde, E.M. Statins and prevention of infections: Systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ 2011, 343, d7281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Statins n = 13 | Without Statins n = 13 | p | |
|---|---|---|---|
| Age [years] | 14.0 (1.9) | 13.6 (1.9) | 0.511 |
| Sex (Girls/Boys) | 9/4 | 9/4 | |
| Mutation status | |||
| –LDL-R | 7 (54%) | 5 (38%) | |
| –APO-B | 3 (23%) | 3 (23%) | |
| –neither LDL nor APO-B | 1 (8%) | 1 (8%) | |
| –not performed | 2 (15%) | 4 (31%) | |
| Weight [kg] | 54.1 (12.58) | 57.1 (13.62) | 0.488 |
| Height [m] | 1.6 (0.09) | 1.6 (0.08) | 0.840 |
| Systolic blood pressure [mmHg] | 116.2 (9.47) | 105.9 (30.44) | 0.347 |
| Diastolic blood pressure [mmHg] | 72.1 (9.79) | 71.8 (9.37) | 0.852 |
| Heart rate [bpm] | 80.4 (16.65) | 84.2 (14.5) | 0.458 |
| Glucose [mg/dL] | 87.3 (8.07) | 94.8 (9.05) | 0.014 |
| Total cholesterol [mg/dL] | 220.8 (28.81) | 257.4 (26.84) | 0.003 |
| HDL cholesterol [mg/dL] | 63.5 (18.58) | 56.4 (12.96) | 0.418 |
| LDL cholesterol [mg/dL] | 143.9 (25.68) | 186.1 (26.89) | 0.001 |
| Triglyceride [mg/dL] | 65.6 (18.35) | 74.8 (23.86) | 0.362 |
| Statins n = 13 | Without Statins n = 13 | p | |
|---|---|---|---|
| RBC [106/µL] | 4.8 (0.28) | 4.9 (0.31) | 0.657 |
| HGB [g/dL] | 13.4 (0.48) | 13.4 (0.67) | 0.909 |
| HCT [%] | 39.8 (1.48) | 40.2 (1.98) | 0.392 |
| PLT [103/µL] | 286.6 (47.72) | 256.9 (44.51) | 0.101 |
| WBC [103/µL] | 5.8 (2.00) | 6.0 (1.24) | 0.186 |
| Neutrophils [103/µL] | 3.0 (1.64) | 3.2 (0.90) | 0.376 |
| Lymphocytes [103/µL] | 1.9 (0.40) | 2.0 (0.43) | 0.979 |
| Monocytes [103/µL] | 0.5 (0.16) | 0.5 (0.11) | 0.810 |
| Eosinophils [103/µL] | 0.2 (0.18) | 0.2 (0.26) | 0.531 |
| Basophils [103/µL] | 0.1 (0.04) | 0.1 (0.06) | 0.733 |
| Statins n = 13 | Without Statins n = 12 | p | ||
|---|---|---|---|---|
| T cells (CD3+) | % | 69.8 (4.78) | 68.6 (4.26) | 0.469 |
| cells/µL | 1357.8 (338.74) | 1365.9 (367.77) | 0.909 | |
| T helper cells (CD3+/CD4+) | % | 39.8 (5.68) | 39.8 (6.14) | 0.894 |
| cells/µL | 770.0 (210.59) | 780.2 (212.85) | 0.820 | |
| T helper late activated CD3+/CD4+/HLADR+ | % | 3.5 (1.54) | 3.3 (1.34) | 0.936 |
| cells/µL | 67.9 (32.07) | 69.5 (34.06) | 0.865 | |
| T helper early activated CD3+/CD4+/CD69+ | % | 0.5 (0.61) | 0.5 (0.51) | 0.887 |
| cells/µL | 8.8 (11.37) | 11.3 (13.45) | 0.821 | |
| T cytotoxic cells (CD3+/CD8+) | % | 30.3 (5.68) | 29.3 (6.48) | 0.574 |
| cells/µL | 556.0 (191.99) | 597.7 (241.71) | 0.865 | |
| T cytotoxic late activated CD3+/CD8+/HLADR+ | % | 4.2 (2.33) | 4.4 (2.74) | 0.728 |
| cells/µL | 80.8 (49.51) | 83.6 (49.58) | 1.000 | |
| T cytotoxic early activated CD3+/CD8+/CD69+ | % | 1.1 (1.28) | 0.5 (0.66) | 0.190 |
| cells/µL | 21.4 (29.59) | 10.3 (10.88) | 0.525 | |
| B cells (CD19+) | % | 15.0 (4.84) | 18.8 (7.41) | 0.152 |
| cells/µL | 275.6 (139.33) | 348.9 (163.62) | 0.392 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motkowski, R.; Alifier, M.; Abramowicz, P.; Konstantynowicz, J.; Mikołuć, B.; Stasiak-Barmuta, A. Innate and Acquired Cellular Immunity in Children with Familial Hypercholesterolemia Treated with Simvastatin. J. Clin. Med. 2022, 11, 2924. https://doi.org/10.3390/jcm11102924
Motkowski R, Alifier M, Abramowicz P, Konstantynowicz J, Mikołuć B, Stasiak-Barmuta A. Innate and Acquired Cellular Immunity in Children with Familial Hypercholesterolemia Treated with Simvastatin. Journal of Clinical Medicine. 2022; 11(10):2924. https://doi.org/10.3390/jcm11102924
Chicago/Turabian StyleMotkowski, Radosław, Marek Alifier, Paweł Abramowicz, Jerzy Konstantynowicz, Bożena Mikołuć, and Anna Stasiak-Barmuta. 2022. "Innate and Acquired Cellular Immunity in Children with Familial Hypercholesterolemia Treated with Simvastatin" Journal of Clinical Medicine 11, no. 10: 2924. https://doi.org/10.3390/jcm11102924
APA StyleMotkowski, R., Alifier, M., Abramowicz, P., Konstantynowicz, J., Mikołuć, B., & Stasiak-Barmuta, A. (2022). Innate and Acquired Cellular Immunity in Children with Familial Hypercholesterolemia Treated with Simvastatin. Journal of Clinical Medicine, 11(10), 2924. https://doi.org/10.3390/jcm11102924






