Efficacy of Continuous Glucose Monitoring on Glycaemic Control in Pregnant Women with Gestational Diabetes Mellitus—A Systematic Review
Abstract
:1. Introduction
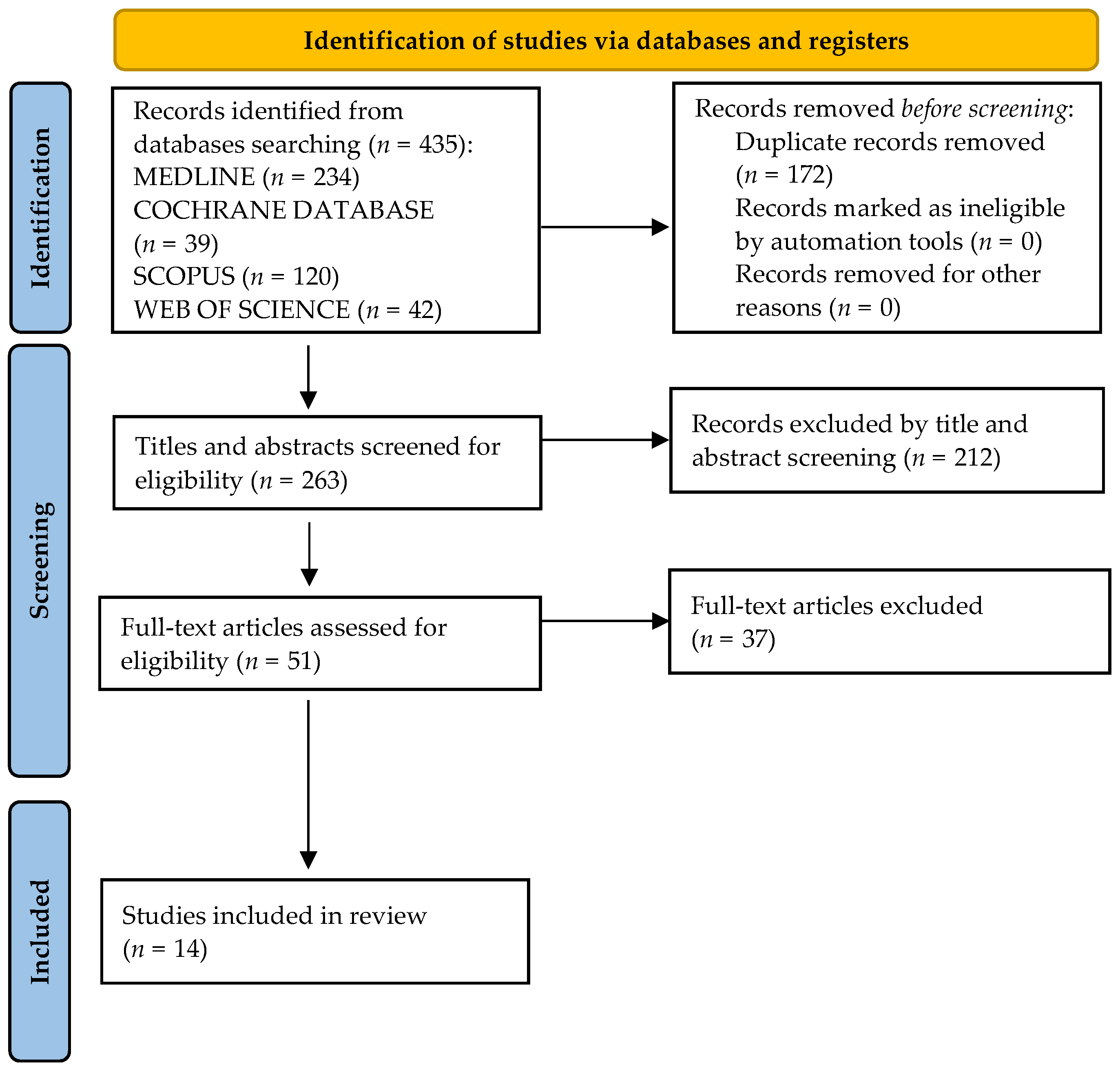
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Analysis
2.3. Outcomes
3. Results
3.1. Glycaemic Control
3.1.1. Hyperglycaemia
3.1.2. Hypoglycaemia
3.2. Insulin Therapy
3.3. HBA1c
3.4. Gestational Weight Gain
3.5. Neonatal Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Association of Diabetes; Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.P.; Kim, S.Y.; Conrey, E.J.; Bullard, K.M. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes Among Women Who Had a Live Birth—United States, 2012–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramasivam, S.S.; Chinna, K.; Singh, A.K.K.; Ratnasingam, J.; Ibrahim, L.; Lim, L.L.; Tan, A.T.B.; Chan, S.P.; Tan, P.C.; Omar, S.Z.; et al. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: A randomized controlled trial. Diabet. Med. 2018, 35, 1118–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afandi, B.; Hassanein, M.; Roubi, S.; Nagelkerke, N. The value of Continuous Glucose Monitoring and Self-Monitoring of Blood Glucose in patients with Gestational Diabetes Mellitus during Ramadan fasting. Diabetes Res. Clin. Pract. 2019, 151, 260–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez-Pardo, R.; Torres-Barea, I.; Cordoba-Dona, J.A.; Cruzado-Begines, C.; Garcia-Garcia-Doncel, L.; Aguilar-Diosdado, M.; Baena-Nieto, M.G. Continuous Glucose Monitoring and Glycemic Patterns in Pregnant Women with Gestational Diabetes Mellitus. Diabetes Technol. Ther. 2020, 22, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yogev, Y.; Ben-Haroush, A.; Jovanovic, L.; Hod, M.; Phillip, M. Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J. Matern. Fetal Neonatal. Med. 2003, 14, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Rayman, G.; Lewis, K.; Kelly, S.; Johal, B.; Duffield, K.; Fowler, D.; Campbell, P.J.; Temple, R.C. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: Randomised clinical trial. BMJ 2008, 337, a1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, A.S.; Mlynarczyk, M.A.; de Veciana, M.; Green, L.M.; Baraki, D.I.; Abuhamad, A.Z. Real-Time Continuous Glucose Monitoring in Gestational Diabetes: A Randomized Controlled Trial. Am. J. Perinatol. 2019, 36, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lv, L.; Liang, Z.; Wang, Y.; Wen, J.; Lin, X.; Zhou, Y.; Mai, C.; Niu, J. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: A prospective cohort study. J. Clin. Endocrinol. Metab. 2014, 99, 4674–4682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cypryk, K.; Pertynska-Marczewska, M.; Szymczak, W.; Wilcynski, J.; Lewinski, A. Evaluation of metabolic control in women with gestational diabetes mellitus by the continuous glucose monitoring system: A pilot study. Endocr. Pract. 2006, 12, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, D.; Wang, X. The effects of the instantaneous scanning glucose monitoring system on hypoglycemia, weight gain, and health behaviors in patients with gestational diabetes: A randomised trial. Ann. Palliat. Med. 2021, 10, 5714–5720. [Google Scholar] [CrossRef] [PubMed]
- Buhling, K.J.; Kurzidim, B.; Wolf, C.; Wohlfarth, K.; Mahmoudi, M.; Wascher, C.; Siebert, G.; Dudenhausen, J.W. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycemia by comparing the self-monitoring of blood glucose (SMBG) in non-pregnant women and in pregnant women with impaired glucose tolerance and gestational diabetes. Exp. Clin. Endocrinol. Diabetes 2004, 112, 556–560. [Google Scholar] [PubMed]
- Zaharieva, D.P.; Teng, J.H.; Ong, M.L.; Lee, M.H.; Paldus, B.; Jackson, L.; Houlihan, C.; Shub, A.; Tipnis, S.; Cohen, O.; et al. Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose to Assess Glycemia in Gestational Diabetes. Diabetes Technol. Ther. 2020, 22, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Alfadhli, E.; Osman, E.; Basri, T. Use of a real time continuous glucose monitoring system as an educational tool for patients with gestational diabetes. Diabetol. Metab. Syndr. 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kestila, K.K.; Ekblad, U.U.; Ronnemaa, T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2007, 77, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Ben-Haroush, A.; Chen, R.; Kaplan, B.; Phillip, M.; Hod, M. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies—A pilot study. Diabet. Med. 2003, 20, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Sun, Z.; Yang, Y.; Yu, H.; Ding, H.; Wang, S. Effect of a CGMS and SMBG on Maternal and Neonatal Outcomes in Gestational Diabetes Mellitus: A Randomized Controlled Trial. Sci. Rep. 2016, 6, 19920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomized controlled trials and observational studies | Case reports, review articles, editorial comments |
| Human studies Studies in English | Animal studies Studies in different languages than English |
| Study ID | Study Design | Study Population | Type of CGM | Duration of CGM Usage | Outcome | Results |
|---|---|---|---|---|---|---|
| Paramasivam S et al. [6] | RCT * | 57 GDM patients | iPro™ 2 Medtronic | 6 days | Incidence of hypoglycaemia, insulin therapy, maternal and neonatal outcomes | Higher detection of hypoglycaemia in CGM group; no difference in other outcomes |
| Afandi B et al. [7] | Prospective observational study | 25 GDM patients | iPro™ 2 Medtronic | 5 days | Incidence of hyper- and hypoglycaemia, HbA1c level, qualification to insulin therapy | Lower incidence of hyperglycaemia and higher detection of hypoglycaemia in CGM group |
| Márquez-Pardo S et al. [8] | Prospective observational study | 77 GDM patients | iPro™ 2 Medtronic | 6 days | Incidence of hyperglycaemia, qualification to insulin therapy | Higher detection of hyperglycaemia, more qualification to insulin therapy in CGM group |
| Chen R et al. [9] | Prospective observational study | 57 GDM patients | Medtronic MiniMed | 72 h | Incidence of postprandial hyperglycaemia and nocturnal hypoglycaemia; HbA1c level | Higher detection of nocturnal hypoglycaemia and postprandial hyperglycaemia in CGM group, no difference in HbA1c level between the groups |
| Lane AF et al. [11] | RCT | 40 GDM patients | Medtronic MiniMed/iPro™ 2 Medtronic | 28 days | Incidence of hyper- and hypoglycaemia, time in range, HbA1c level, maternal and neonatal outcomes | No difference between the groups |
| Yu F et al. [12] | Prospective cohort study | 340 GDM patients | Medtronic MiniMed | 72 h a week for 5 weeks | Glycaemia control, insulin therapy, maternal and neonatal outcomes | Shorter durations of hyper- and hypoglycaemia, more patients qualified to insulin therapy in CGM group; less incidence of LGA *, neonatal hypoglycaemia and hyperbilirubinemia in CGM group |
| Cypryk K et al. [13] | Prospective observational study | 12 GDM patients, 7 patients non-GDM | Medtronic MiniMed | 72 h | Glycaemia control | No difference between the groups |
| Zhang X et al. [14] | RCT | 110 GDM patients | ISGMS * (Abbott Diabetes Care) | 14 days | Incidence of hypoglycaemia, gestational weight gain, health behaviour patterns | Lower gestational weight gain, better health behaviour patterns and lower incidence of hypoglycaemia in CGM group |
| Buhling KJ et al. [15] | Prospective observational study | 63 GDM, 17 IGT, 24 non-GDM, 9 non-pregnant patients | Medtronic MiniMed | 72 h | Glycaemia control, neonatal outcomes | Higher detection of hyperglycaemia in CGM group, no difference in other outcomes between the groups |
| Zaharieva D et al. [16] | Prospective Observational Study | 90 GDM patients | iPRO Medtronic | 7 days | Incidence of hyperglycaemia | Higher detection of hyperglycaemia in CGM group |
| Alfadhli E et al. [17] | RCT | 130 GDM patients | Guardian® RT-CGMS MiniMed | 3–7 days | Fasting and postprandial glycaemia, HbA1c level, insulin therapy, maternal and neonatal outcomes | No difference between the groups |
| Kestila K et al. [18] | RCT | 73 GDM patients | Medtronic MiniMed | Mean 47.4 h | Insulin therapy, maternal and neonatal outcomes | Higher number of patients qualified for insulin therapy in CGM group; no difference in maternal and neonatal outcomes between the groups |
| Yogev Y et al. [19] | Prospective observational study | 6 PGDM, 2 GDM patients, | Medtronic MiniMed | 72 h | Glycaemia, HbA1c level, insulin therapy, maternal and neonatal outcomes | Higher detection of nocturnal hypoglycaemia and postprandial hyperglycaemia, better modification of insulin therapy in CGM group; no difference in other outcomes between the groups |
| Wei Q et al. [20] | RCT | 106 GDM patients | Medtronic MiniMed | 48–72 h | Glycaemia, HbA1c level, insulin therapy, maternal and neonatal outcomes | Higher number of patients qualified to insulin therapy, better detection of nocturnal hypoglycaemia and postprandial hyperglycaemia, less gestational weight gain in CGM group; No difference in other outcomes between the groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, A.; Stanirowski, P.J.; Wielgoś, M.; Bomba-Opoń, D. Efficacy of Continuous Glucose Monitoring on Glycaemic Control in Pregnant Women with Gestational Diabetes Mellitus—A Systematic Review. J. Clin. Med. 2022, 11, 2932. https://doi.org/10.3390/jcm11102932
Majewska A, Stanirowski PJ, Wielgoś M, Bomba-Opoń D. Efficacy of Continuous Glucose Monitoring on Glycaemic Control in Pregnant Women with Gestational Diabetes Mellitus—A Systematic Review. Journal of Clinical Medicine. 2022; 11(10):2932. https://doi.org/10.3390/jcm11102932
Chicago/Turabian StyleMajewska, Agata, Paweł Jan Stanirowski, Mirosław Wielgoś, and Dorota Bomba-Opoń. 2022. "Efficacy of Continuous Glucose Monitoring on Glycaemic Control in Pregnant Women with Gestational Diabetes Mellitus—A Systematic Review" Journal of Clinical Medicine 11, no. 10: 2932. https://doi.org/10.3390/jcm11102932
APA StyleMajewska, A., Stanirowski, P. J., Wielgoś, M., & Bomba-Opoń, D. (2022). Efficacy of Continuous Glucose Monitoring on Glycaemic Control in Pregnant Women with Gestational Diabetes Mellitus—A Systematic Review. Journal of Clinical Medicine, 11(10), 2932. https://doi.org/10.3390/jcm11102932







