Working Memory Phenotypes in Early Multiple Sclerosis: Appraisal of Phenotype Frequency, Progression and Test Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
Clinical Tests: Modifying Factors
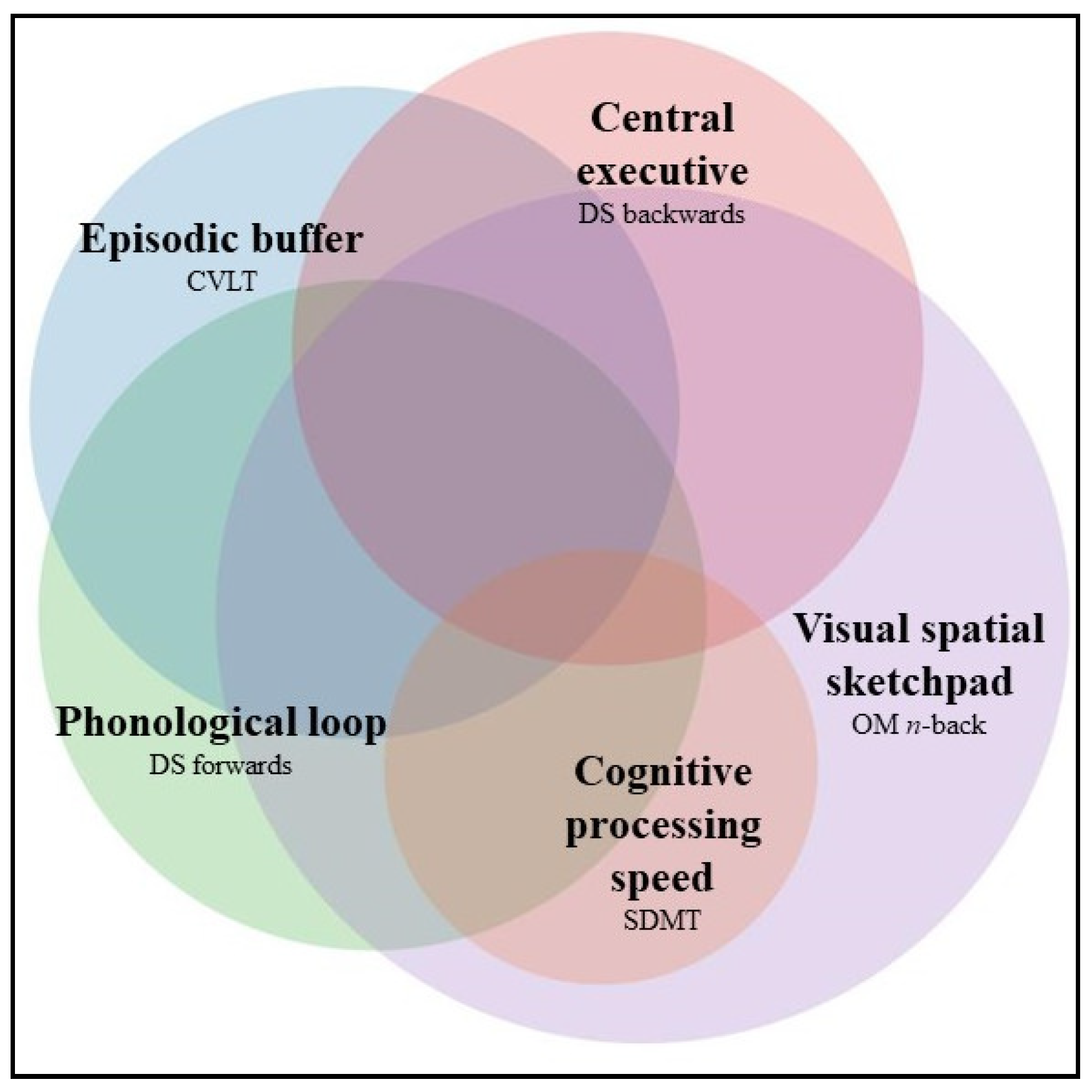
2.3. Tests of WM
2.3.1. Episodic Buffer: California Verbal Learning Test (CVLT-II)
2.3.2. Phonological Loop: Digit Span Forwards
2.3.3. Central Executive: Digit Span Backwards
2.3.4. Visual–Spatial Sketchpad: Ocular–Motor n-Back Test
2.3.5. Cognitive Processing Speed: Symbol Digit Modalities Test (SDMT)
2.4. Data Analysis
3. Results
3.1. Descriptive Information for Early RRMS and Healthy Controls
3.2. Diagnostic Accuracy of WM Tests for Identifying WM Impairment
3.3. Frequency of WM Test Failure
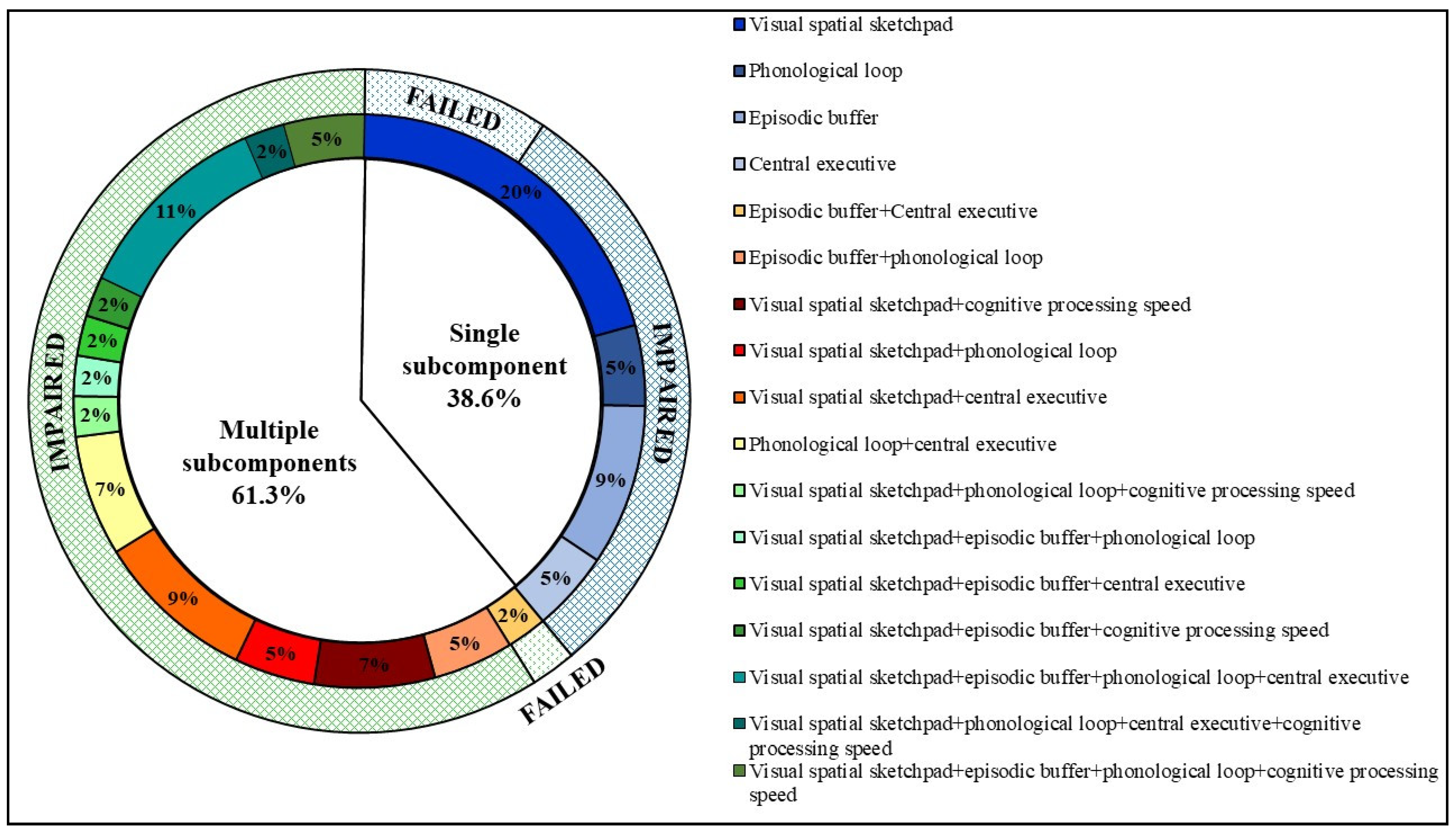
3.4. Frequency of Single and Multi-Subcomponent WM Impairment
3.5. Diagnostic Accuracy for Combination of WM Subcomponent Tests for Identifying WM Impairment
3.6. Change in WM Subcomponent Performance over Two Years (Baseline, +1 Year, +2 Years)
3.6.1. Visual–Spatial Sketchpad: OM n-Back Test
Error
Response Time
3.6.2. Episodic: CVLT
3.6.3. Phonological Loop: DS (Forwards)
3.6.4. Central Executive: DS (Backwards)
3.6.5. Cognitive Processing Speed: SDMT
4. Discussion
4.1. Frequency of WM Subcomponent Test Failure and WM Phenotypes in Early RRMS
4.2. Diagnostic Accuracy of WM Subcomponent Tests: Recommendation for Test Selection in Early RRMS
4.3. Progression in WM Subcomponent over Two Years
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brochet, B.; Ruet, A. Cognitive Impairment in Multiple Sclerosis With Regards to Disease Duration and Clinical Phenotypes. Front. Neurol. 2019, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Fuso, S.F.; Callegaro, D.; Pompeia, S.; Bueno, O.F. Working memory impairment in multiple sclerosis relapsing-remitting patients with episodic memory deficits. Arq. Neuro-Psiquiatr. 2010, 68, 205–211. [Google Scholar] [CrossRef]
- DeLuca, J.; Barbieri-Berger, S.; Johnson, S.K. The nature of memory impairments in multiple sclerosis: Acquisition versus retrieval. J. Clin. Exp. Neuropsychol. 1994, 16, 183–189. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.; Gaudino, E.A.; Diamond, B.J.; Christodoulou, C.; Engel, R.A. Acquisition and storage deficits in multiple sclerosis. J. Clin. Exp. Neuropsyc. 1998, 20, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, P.L.; Beier, M.E.; Boyle, M.O. Working memory and intelligence: The same or different constructs? Psychol. Bull. 2005, 131, 30–60. [Google Scholar] [CrossRef]
- MacMahon, H.; El Refaie, A. The audiovestibular manifestations as early symptoms of multiple sclerosis: A scoping review of the literature. Ir. J. Med. Sci. 2022, 191, 391–400. [Google Scholar] [CrossRef]
- Leocani, L.; Guerrieri, S.; Comi, G. Visual Evoked Potentials as a Biomarker in Multiple Sclerosis and Associated Optic Neuritis. J. Neuro-Ophthalmol. 2018, 38, 350–357. [Google Scholar] [CrossRef]
- DeLuca, J.; Chiaravalloti, N.D.; Sandroff, B.M. Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 319–332. [Google Scholar] [CrossRef]
- Schwaighofer, M.; Fischer, F.; Bühner, M. Does Working Memory Training Transfer? A Meta-Analysis Including Training Conditions as Moderators. Educ. Psychol. 2015, 50, 138–166. [Google Scholar] [CrossRef]
- Schoonheim, M.M.; Geurts, J.J.; Barkhof, F. The limits of functional reorganization in multiple sclerosis. Neurology 2010, 74, 1246–1247. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working Memory. In Psychology of Learning and Motivation; Bower, G.H., Ed.; Academic Press: Cambridge, MA, USA, 1974; Volume 8, pp. 47–89. [Google Scholar]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef]
- D’Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. Chapter 20 What are the differences between long-term, short-term, and working memory? In Progress in Brain Research; Sossin, W.S., Lacaille, J.-C., Castellucci, V.F., Belleville, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 169, pp. 323–338. [Google Scholar]
- Chai, W.J.; Abd Hamid, A.I.; Abdullah, J.M. Working Memory From the Psychological and Neurosciences Perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Blasiman, R.N.; Was, C.A. Why Is Working Memory Performance Unstable? A Review of 21 Factors. Eur. J. Psychol. 2018, 14, 188–231. [Google Scholar] [CrossRef] [PubMed]
- Ruano, L.; Portaccio, E.; Goretti, B.; Niccolai, C.; Severo, M.; Patti, F.; Cilia, S.; Gallo, P.; Grossi, P.; Ghezzi, A.; et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult. Scler. 2017, 23, 1258–1267. [Google Scholar] [CrossRef]
- Grzegorski, T.; Losy, J. Cognitive impairment in multiple sclerosis—A review of current knowledge and recent research. Rev. Neurosci. 2017, 28, 845–860. [Google Scholar] [CrossRef]
- Kouvatsou, Z.; Masoura, E.; Kiosseoglou, G.; Kimiskidis, V.K. Working memory profiles of patients with multiple sclerosis: Where does the impairment lie? J. Clin. Exp. Neuropsyc. 2019, 41, 832–844. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef]
- Clough, M.; Mitchell, L.; Millist, L.; Lizak, N.; Beh, S.; Frohman, T.C.; Frohman, E.M.; White, O.B.; Fielding, J. Ocular motor measures of cognitive dysfunction in multiple sclerosis II: Working memory. J. Neurol. 2015, 262, 1138–1147. [Google Scholar] [CrossRef]
- Panou, T.; Mastorodemos, V.; Papadaki, E.; Simos, P.G.; Plaitakis, A. Early signs of memory impairment among multiple sclerosis patients with clinically isolated syndrome. Behav. Neurol. 2012, 25, 311–326. [Google Scholar] [CrossRef]
- Reuter, F.; Zaaraoui, W.; Crespy, L.; Faivre, A.; Rico, A.; Malikova, I.; Confort-Gouny, S.; Cozzone, P.J.; Ranjeva, J.-P.; Pelletier, J.; et al. Cognitive impairment at the onset of multiple sclerosis: Relationship to lesion location. Mult. Scler. J. 2011, 17, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, R.G.; Iaffaldano, P.; Trojano, M. Verbal fluency deficits in clinically isolated syndrome suggestive of multiple sclerosis. J. Neurol. Sci. 2013, 330, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.d.P.; Rodrigues, J.d.C.; Sbicigo, J.B.; Piccolo, L.d.R.; Zortea, M.; Duarte Junior, S.; Salles, J.F.d. Tasks for assessment of the episodic buffer: A systematic review. Psychol. Neurosci. 2013, 6, 331–343. [Google Scholar] [CrossRef][Green Version]
- Swanson, H.L.; Mink, J.; Bocian, K.M. Cognitive processing deficits in poor readers with symptoms of reading disabilities and ADHD: More alike than different? J. Educ. Psychol. 1999, 91, 321–333. [Google Scholar] [CrossRef]
- Jeter, C.B.; Patei, S.S.; Sereno, A.B. Novel n-back spatial working memory task using eye movement response. Behav. Res. Methods 2011, 43, 879–887. [Google Scholar] [CrossRef][Green Version]
- Strober, L.; DeLuca, J.; Benedict, R.H.; Jacobs, A.; Cohen, J.A.; Chiaravalloti, N.; Hudson, L.D.; Rudick, R.A.; LaRocca, N.G. Symbol Digit Modalities Test: A valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult. Scler. J. 2019, 25, 1781–1790. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef]
- Patti, F.; Amato, M.P.; Trojano, M.; Bastianello, S.; Tola, M.R.; Goretti, B.; Caniatti, L.; Di Monte, E.; Ferrazza, P.; Brescia Morra, V.; et al. Cognitive impairment and its relation with disease measures in mildly disabled patients with relapsing-remitting multiple sclerosis: Baseline results from the Cognitive Impairment in Multiple Sclerosis (COGIMUS) study. Mult. Scler. J. 2009, 15, 779–788. [Google Scholar] [CrossRef]
- Achiron, A.; Chapman, J.; Magalashvili, D.; Dolev, M.; Lavie, M.; Bercovich, E.; Polliack, M.; Doniger, G.M.; Stern, Y.; Khilkevich, O.; et al. Modeling of cognitive impairment by disease duration in multiple sclerosis: A cross-sectional study. PLoS ONE 2013, 8, e71058. [Google Scholar] [CrossRef]
- Nocentini, U.; Pasqualetti, P.; Bonavita, S.; Buccafusca, M.; De Caro, M.F.; Farina, D.; Girlanda, P.; Le Pira, F.; Lugaresi, A.; Quattrone, A.; et al. Cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Mult. Scler. J. 2006, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Valadbeigi, A.; Weisi, F.; Rohbakhsh, N.; Rezaei, M.; Heidari, A.; Rasa, A.R. Central auditory processing and word discrimination in patients with multiple sclerosis. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Backner, Y.; Petrou, P.; Glick-Shames, H.; Raz, N.; Zimmermann, H.; Jost, R.; Scheel, M.; Paul, F.; Karussis, D.; Levin, N. Vision and Vision-Related Measures in Progressive Multiple Sclerosis. Front. Neurol. 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Balcer, L.J.; Miller, D.H.; Reingold, S.C.; Cohen, J.A. Vision and vision-related outcome measures in multiple sclerosis. Brain A J. Neurol. 2015, 138, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Jasse, L.; Vukusic, S.; Durand-Dubief, F.; Vartin, C.; Piras, C.; Bernard, M.; Pélisson, D.; Confavreux, C.; Vighetto, A.; Tilikete, C. Persistent visual impairment in multiple sclerosis: Prevalence, mechanisms and resulting disability. Mult. Scler. J. 2013, 19, 1618–1626. [Google Scholar] [CrossRef]
- Iva, P.; Fielding, J.; Clough, M.; White, O.; Godic, B.; Martin, R.; Rajan, R. Speech Discrimination Tasks: A Sensitive Sensory and Cognitive Measure in Early and Mild Multiple Sclerosis. Front. Neurosci. 2020, 14, 1363. [Google Scholar] [CrossRef]
- Iva, P.; Fielding, J.; Clough, M.; White, O.; Noffs, G.; Godic, B.; Martin, R.; Van Der Walt, A.; Rajan, R. Speech discrimination impairments as a marker of disease severity in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 47, 102608. [Google Scholar] [CrossRef]
- Fielding, J.; Kilpatrick, T.; Millist, L.; White, O. Control of visually guided saccades in multiple sclerosis: Disruption to higher-order processes. Neuropsychologia 2009, 47, 1647–1653. [Google Scholar] [CrossRef]
- Lopes Costa, S.; Goncalves, O.F.; DeLuca, J.; Chiaravalloti, N.; Chakravarthi, R.; Almeida, J. The Temporal Dynamics of Visual Processing in Multiple Sclerosis. Appl. Neuropsychol. Adult 2016, 23, 133–140. [Google Scholar] [CrossRef][Green Version]
- Ball, K.; Pearson, D.G.; Smith, D.T. Oculomotor involvement in spatial working memory is task-specific. Cognition 2013, 129, 439–446. [Google Scholar] [CrossRef]
- Belopolsky, A.V.; Theeuwes, J. No functional role of attention-based rehearsal in maintenance of spatial working memory representations. Acta Psychol. 2009, 132, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Polliack, M.; Rao, S.M.; Barak, Y.; Lavie, M.; Appelboim, N.; Harel, Y. Cognitive patterns and progression in multiple sclerosis: Construction and validation of percentile curves. J. Neurol. Neurosurg. Psychiatry 2005, 76, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Dobbing, J.; Stankovich, J.; Ternes, A.; Kolbe, S.; White, O.B.; Fielding, J. Cognitive processing speed deficits in multiple sclerosis: Dissociating sensorial and motor processing changes from cognitive processing speed. Mult. Scler. Relat. Disord. 2020, 38, 101522. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Foletta, P.; Frohman, A.N.; Sears, D.; Ternes, A.; White, O.B.; Fielding, J. Multiple sclerosis: Executive dysfunction, task switching and the role of attention. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318771781. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.; Millist, L.; Lizak, N.; Beh, S.; Frohman, T.C.; Frohman, E.M.; White, O.B.; Fielding, J. Ocular motor measures of cognitive dysfunction in multiple sclerosis I: Inhibitory control. J. Neurol. 2015, 262, 1130–1137. [Google Scholar] [CrossRef]
- Fielding, J.; Kilpatrick, T.; Millist, L.; Clough, M.; White, O. Longitudinal assessment of antisaccades in patients with multiple sclerosis. PLoS ONE 2012, 7, e30475. [Google Scholar] [CrossRef]
- Gajamange, S.; Shelton, A.; Clough, M.; White, O.; Fielding, J.; Kolbe, S. Functional correlates of cognitive dysfunction in clinically isolated syndromes. PLoS ONE 2019, 14, e0219590. [Google Scholar] [CrossRef]
- Huijbregts, S.C.J.; Kalkers, N.F.; de Sonneville, L.M.J.; de Groot, V.; Polman, C.H. Cognitive impairment and decline in different MS subtypes. J. Neurol. Sci. 2006, 245, 187–194. [Google Scholar] [CrossRef]
- López-Góngora, M.; Querol, L.; Escartín, A. A one-year follow-up study of the Symbol Digit Modalities Test (SDMT) and the Paced Auditory Serial Addition Test (PASAT) in relapsing-remitting multiple sclerosis: An appraisal of comparative longitudinal sensitivity. BMC Neurol. 2015, 15, 40. [Google Scholar] [CrossRef]
- Pavisian, B.; Patel, V.P.; Feinstein, A. Cognitive mediated eye movements during the SDMT reveal the challenges with processing speed faced by people with MS. BMC Neurol. 2019, 19, 340. [Google Scholar] [CrossRef]
- Patel, V.P.; Walker, L.A.S.; Feinstein, A. Deconstructing the symbol digit modalities test in multiple sclerosis: The role of memory. Mult. Scler. Relat. Disord. 2017, 17, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Camp, S.J.; Stevenson, V.L.; Thompson, A.J.; Ingle, G.T.; Miller, D.H.; Borras, C.; Brochet, B.; Dousset, V.; Falautano, M.; Filippi, M.; et al. A longitudinal study of cognition in primary progressive multiple sclerosis. Brain 2005, 128, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rodgers, J.; Drake, A.S.; Zivadinov, R.; Weinstock-Guttman, B.; Benedict, R.H.B. Stable neuropsychiatric status in multiple sclerosis: A 3-year study. Mult. Scler. J. 2016, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Wojcik, C.; Wilding, G.E.; Pol, J.; Dwyer, M.G.; Weinstock-Guttman, B.; Zivadinov, R.; Benedict, R.H.B. Trait Conscientiousness predicts rate of longitudinal SDMT decline in multiple sclerosis. Mult. Scler. J. 2020, 26, 245–252. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, R.J.; Westervelt, H.J. Issues associated with repeated neuropsychological assessments. Neuropsychol. Rev. 1995, 5, 203–221. [Google Scholar] [CrossRef]
- Jonides, J. How does practice makes perfect? Nat. Neurosci. 2004, 7, 10–11. [Google Scholar] [CrossRef]
- Alioto, A.G.; Kramer, J.H.; Borish, S.; Neuhaus, J.; Saloner, R.; Wynn, M.; Foley, J.M. Long-term test-retest reliability of the California Verbal Learning Test—Second edition. Clin. Neuropsychol. 2017, 31, 1449–1458. [Google Scholar] [CrossRef]
- Roar, M.; Illes, Z.; Sejbaek, T. Practice effect in Symbol Digit Modalities Test in multiple sclerosis patients treated with natalizumab. Mult. Scler. Relat. Disord. 2016, 10, 116–122. [Google Scholar] [CrossRef]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–114. [Google Scholar] [CrossRef]
- Delis, D.C.; Freeland, J.; Kramer, J.H.; Kaplan, E. Integrating clinical assessment with cognitive neuroscience: Construct validation of the California Verbal Learning Test. J. Consult. Clin. Psychol. 1988, 56, 123–130. [Google Scholar] [CrossRef]
- Mathy, F.; Chekaf, M.; Cowan, N. Simple and Complex Working Memory Tasks Allow Similar Benefits of Information Compression. J. Cogn. 2018, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Lizak, N.; Clough, M.; Millist, L.; Kalincik, T.; White, O.B.; Fielding, J. Impairment of Smooth Pursuit as a Marker of Early Multiple Sclerosis. Front. Neurol. 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Ternes, A.M.; Clough, M.; Foletta, P.; White, O.; Fielding, J. Characterization of inhibitory failure in Multiple Sclerosis: Evidence of impaired conflict resolution. J. Clin. Exp. Neuropsychol. 2019, 41, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Blekher, T.; Weaver, M.R.; Cai, X.; Hui, S.; Marshall, J.; Jackson, J.G.; Wojcieszek, J.; Yee, R.D.; Foroud, T.M. Test–Retest Reliability of Saccadic Measures in Subjects at Risk for Huntington Disease. Investig. Ophth. Vis. Sci. 2009, 50, 5707–5711. [Google Scholar] [CrossRef]
- Gooding, D.C.; Mohapatra, L.; Shea, H.B. Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychol. Med. 2004, 34, 921–932. [Google Scholar] [CrossRef]
- Klein, C.; Berg, P. Four-weeks test-retest stability of the saccadic CNV, two measures of saccade task performance and selected neuropsychological tests. Int. J. Psychophysiol. 2001, 41, 219. [Google Scholar]
- Fozard, J.L.; Vercryssen, M.; Reynolds, S.L.; Hancock, P.A.; Quilter, R.E. Age differences and changes in reaction time: The Baltimore Longitudinal Study of Aging. J. Gerontol. 1994, 49, P179–P189. [Google Scholar] [CrossRef]
- Jerković, A.; Matijaca, M.; Proroković, A.; Šikić, A.; Košta, V.; Ćurković Katić, A.; Dolić, K.; Duka Glavor, K.; Šoda, J.; Đogaš, Z.; et al. Information Processing Speed Assessed with Letter Digit Substitution Test in Croatian Sample of Multiple Sclerosis Patients. Diagnostics 2022, 12, 111. [Google Scholar] [CrossRef]
| Healthy Controls (n = 25) | Early RRMS | |||||||
|---|---|---|---|---|---|---|---|---|
| CIS (n = 14) | CDMS (n = 49) | Total Early RRMS (n = 63) | ||||||
| M (SD) | Range | M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Age (years) | 38.63 (11.07) | 21–65 | 33.00 * (8.38) | 20–46 | 42.18 * (11.64) | 19–66 | 40.14 (11.60) | 19–66 |
| Gender F (M) | 21 (3) | - | 12 (2) | - | 45 (4) | - | 57 (6) | - |
| NART | 117.00 (4.23) | 110–124 | 115.29 (5.14) | 105–123 | 115.92 (4.56) | 106–125 | 115.78 (4.65) | 105–125 |
| BDI | 3.95 (3.29) | 0–14 | 6.85 (5.36) | 1–18 | 6.67 (8.19) | 0–38 | 6.71 (7.61) | 0–38 |
| EDSS | 0.00 (0.00) | 0–2 | 0.00 (1.00) | 0–3.5 | 0.00 (1.00) | 0–3.5 | ||
| Disease duration (months) | - | - | 12.85 ** (11.72) | 2–37 | 104.54 ** (110.75) | 4–513 | 83.43 (104.54) | 2–513 |
| WM Test | AUC (95% CI) | Cut-Off Scores | Youden J | Sensitivity (Specificity) (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| CVLT Episodic buffer | 0.66 (0.53–0.79) * | 34.49 | 39.24 | 43.6 (95.7) | 61.1 | 50 | 62.9 |
| SDMT Cognitive processing speed | 0.53 (0.39–0.68) | 51.51 | 20.51 | 20.5 (100) | 100 | 56.6 | 50.8 |
| Digit span-Forwards Phonological loop | 0.71 (0.59–0.83) * | 9.50 | 48.72 | 48.7 (100) | 100 | 46.5 | 67.7 |
| Digit span-Backwards Central executive | 0.69 (0.56–0.82) * | 5.50 | 29.77 | 38.5 (91.3) | 88.2 | 53.3 | 58 |
| OM n-back Visual–spatial sketchpad | |||||||
| Response time (ms) | 0.52 (0.38–0.66) | 674.46 | 25.00 | 33.3 (91.2) | 86.6 | 54.2 | 55.5 |
| Error rate (%) | 0.73 (0.60–0.85) * | 26.10 | 51.68 + | 53.8 (95.8) | 95.5 | 43.9 | 70.1 |
| AUC | Cut-Off Probability | Youden J | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Two WM tests | |||||
| DS backwards + DS forwards | 0.754 * | 0.7 | 52.84 | 61 | 91 |
| CVLT + DS backwards | 0.755 * | 0.71 | 47.71 | 56 | 91 |
| CVLT + DS forwards | 0.760 * | 0.72 | 57.19 | 61 | 95 |
| DS backwards + OM n-back | 0.762 * | 0.76 | 53.62 | 66 | 87 |
| CVLT + OM n-back | 0.775 ** | 0.49 | 48.72 | 48 | 100 |
| DS forwards + OM n-back | 0.784 ** | 0.72 | 54.63 | 59 | 85 |
| Three WM tests | |||||
| CVLT + DS forwards + DS backwards | 0.786 ** | 0.71 | 62.32 | 66 | 95 |
| DS backwards + DS forwards + OM n-back | 0.789 ** | 0.69 | 57.19 | 61 | 95 |
| CVLT + DS backwards + OM n-back | 0.798 ** | 0.65 | 57.97 | 66 | 91 |
| CVLT + DS forwards + OM n-back | 0.802 ** | 0.77 | 58.97 | 59 | 100 |
| Four WM tests | |||||
| CVLT + DS forwards + DS backwards + OM n-back | 0.810 ** | 0.75 | 58.97 | 59 | 100 |
| Early RRMS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CIS M (SE) | CDMS M (SE) | Total Early RRMS M (SE) | |||||||
| WM Tests | Baseline n = 14 | +1 Year n = 14 | +2 Year n = 12 | Baseline n = 49 | +1 Year n = 45 | +2 Year n = 42 | Baseline n = 63 | +1 Year n = 59 | +2 Year n = 54 |
| CVLT Episodic buffer | 89.20 (15.75) | 79.72 (14.28) | 80.63 (12.25) | 40.12 (1.88) | 42.38 (2.46) | 46.97 (1.91) | 64.66 (7.93) | 61.05 (7.24) | 63.80 (6.21) |
| SDMT Cognitive processing speed | 61.64 (14.63) | 67.25 (12.84) | 71.93 (12.09) | 64.04 (1.71) | 66.91 (1.90) | 67.59 (2.81) | 62.84 (7.37) | 67.08 (6.49) | 69.76 (6.21) |
| Digit span-Forwards Phonological loop | 15.65 (2.84) | 15.60 (2.50) | 16.90 (2.10) | 11.19 (0.34) | 11.17 (0.41) | 11.36 (0.36) | 13.42 (1.43) | 13.39 (1.27) | 14.13 (1.07) |
| DS-Backwards Central executive | 10.21 (2.67) | 9.57 (2.33) | 10.44 (1.97) | 6.97 (0.34) | 7.59 (0.36) | 7.67 (0.33) | 8.59 (1.34) | 8.58 (1.18) | 9.05 (1.00) |
| OM n-back Visual–spatial sketchpad | |||||||||
| Response time (ms) | 539.80 (111.44) | 671.56 (119.25) | 703.00 (147.70) | 600.40 (152.97) | 667.31 (120.19) | 672.46 (117.91) | 586.94 (146.17) | 668.26 (118.63) | 677.55 (122.52) |
| Error rate (%) | 17.15 (10.70) | 15.04 (11.06) | 16.48 (25.12) | 25.26 (20.98) | 19.06 (20.53) | 18.96 (14.42) | 23.46 (20.18) | 19.61 (20.53) | 18.55 (16.65) |
| Clinical covariates | |||||||||
| BDI | 4.8 (1.23) | 3.00 (1.22) | 4.4 (1.08) | 6.29 (1.79) | 6.19 (1.32) | 4.48 (0.85) | 6.71 (7.61) | 6.95 (7.73) | 5.88 (6.64) |
| EDSS Mdn | 0 (0) | 0.3 (0.20) | 0.3 (0.20) | 0.57 (0.27) | 0.67 (0.28) | 0.67 (0.28) | 0.29 (0.14) | 0.48 (0.24) | 0.48 (0.24) |
| Disease duration (m) | 15.4 (6.64) | 27.00 (6.52) | 39.80 (5.88) | 125.90 (30.99) | 137.43 (31.01) | 149.28 (30.99) | 83.43 (104.54) | 95.70 (106.17) | 114.98 (107.41) |
| Age | 34.8 (3.73) | 35.80 (3.73) | 37.00 (3.73) | 42.48 (2.30) | 43.43 (2.70) | 44.38 (2.70) | 40.14 (11.60) | 41.64 (11.34) | 42.94 (11.34) |
| Sex F(M) | 12 (2) | 12 (2) | 10 (2) | 45 (4) | 43 (4) | 38 (4) | 57 (6) | 53 (6) | 48 (6) |
| NART+ | 115.29 (5.14) | - | - | 115.92 (4.56) | - | - | 115.78 (4.65) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clough, M.; Bartholomew, J.; White, O.B.; Fielding, J. Working Memory Phenotypes in Early Multiple Sclerosis: Appraisal of Phenotype Frequency, Progression and Test Sensitivity. J. Clin. Med. 2022, 11, 2936. https://doi.org/10.3390/jcm11102936
Clough M, Bartholomew J, White OB, Fielding J. Working Memory Phenotypes in Early Multiple Sclerosis: Appraisal of Phenotype Frequency, Progression and Test Sensitivity. Journal of Clinical Medicine. 2022; 11(10):2936. https://doi.org/10.3390/jcm11102936
Chicago/Turabian StyleClough, Meaghan, Jade Bartholomew, Owen B. White, and Joanne Fielding. 2022. "Working Memory Phenotypes in Early Multiple Sclerosis: Appraisal of Phenotype Frequency, Progression and Test Sensitivity" Journal of Clinical Medicine 11, no. 10: 2936. https://doi.org/10.3390/jcm11102936
APA StyleClough, M., Bartholomew, J., White, O. B., & Fielding, J. (2022). Working Memory Phenotypes in Early Multiple Sclerosis: Appraisal of Phenotype Frequency, Progression and Test Sensitivity. Journal of Clinical Medicine, 11(10), 2936. https://doi.org/10.3390/jcm11102936





