Humanoid Robot Use in Cognitive Rehabilitation of Patients with Severe Brain Injury: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization
2.3. Outcome Measures
2.4. Robotic Rehabilitation
2.5. Traditional Rehabilitation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lineeguida del Ministro della Sanità per le Attività di Riabilitazione. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, (124). 1998. Available online: https://www.gazzettaufficiale.it/eli/id/1998/05/30/098A4518/sg (accessed on 12 November 2019).
- Lorenz, L.S.; Doonan, M. Value and Cost Savings from Access to Multi-disciplinary Rehabilitation Services After Severe Acquired Brain Injury. Front. Public Health 2021, 1, 753447. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Bramanti, A.; Garzon, M.; Celesti, A.; Russo, M.; Portaro, S.; Naro, A.; Manuli, A.; Tonin, P.; Bramanti, P. Telerehabilitation in individuals with severe acquired brain injury: Rationale, study design, and methodology. Medicine 2018, 97, e13292. [Google Scholar] [CrossRef] [PubMed]
- Castellani, G.B.; Miccoli, G.; Cava, F.C.; Salucci, P.; Colombo, V.; Maietti, E.; Palandri, G. From Shunt to Recovery: A Multidisciplinary Approach to Hydrocephalus Treatment in Severe Acquired Brain Injury Rehabilitation. Brain Sci. 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Formisano, R.; Carlesimo, G.A.; Sabbadini, M.; Loasses, A.; Penta, F.; Vinicola, V.; Caltagirone, C. Clinical predictors and neuropsychological outcome in severe traumatic brain injury patients. Acta Neurochir. 2004, 146, 457–462. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, M.; Aloisi, M.; Azicnuda, E.; Silvestro, D.; Giustini, M.; Verni, F.; Formisano, R.; Bivona, U. Changes in Caregivers Lifestyle after Severe Acquired Brain Injury: A Preliminary Investigation. Biomed. Res. Int. 2018, 2018, 2824081. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.K.; Bryant, C. Time for change in acquired brain injury. Lancet Neurol. 2019, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- De Luca, R.; Calabrò, R.S.; Bramanti, P. Cognitive rehabilitation after severe acquired brain injury: Current evidence and future directions. Neuropsychol. Rehabil. 2018, 28, 879–898. [Google Scholar] [CrossRef]
- Manuli, A.; Maggio, M.G.; Latella, D.; Cannavò, A.; Balletta, T.; De Luca, R.; Naro, A.; Calabrò, R.S. Can robotic gait rehabilitation plus Virtual Reality affect cognitive and behavioural outcomes in patients with chronic stroke? A randomized controlled trial involving three different protocols. J. Stroke Cerebrovasc. Dis. 2020, 29, 104994. [Google Scholar] [CrossRef]
- Yuan, F.; Klavon, E.; Liu, Z.; Lopez, R.P.; Zhao, X. A Systematic Review of Robotic Rehabilitation for Cognitive Training. Front. Robot. AI 2021, 8, 605715. [Google Scholar] [CrossRef]
- Yakub, F.; Khudzari, A.Z.M.; Mori, Y. Recent trends for practical rehabilitation robotics, current challenges and the future. Int. J. Rehabil. Res. 2014, 37, 9–21. [Google Scholar] [CrossRef]
- De Carolis, B.; Carofiglio, V.; Grimandli, I.; Macchiarulo, N.; Palestra, G.; Pino, O. Using the pepper robot in cognitive stimulation therapy for people with mild cognitive impairment and mild dementia. In Proceedings of the ACHI-The Thirteenth International Conference on Advances in Computer-Human Interactions, Porto, Portugal, 26–30 June 2020; pp. 452–457. [Google Scholar]
- Nocentini, O.; Fiorini, L.; Acerbi, G.; Sorrentino, A.; Mancioppi, G.; Cavallo, F. A Survey of Behavioral Models for Social Robots. Robotics 2019, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Chuah, S.H.W.; Yu, J. The future of service: The power of emotion in human-robot interaction. J. Retail. Consum. Serv. 2021, 61, 102551. [Google Scholar] [CrossRef]
- Biffi, E.; Beretta, E.; Storm, F.A.; Corbetta, C.; Strazzer, S.; Pedrocchi, A.; Ambrosini, E. The Effectiveness of Robot- vs. Virtual Reality-Based Gait Rehabilitation: A Propensity Score Matched Cohort. Life 2021, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Koutentakis, D.; Pilozzi, A.; Huang, X. Designing Socially Assistive Robots for Alzheimer’s Disease and Related Dementia Patients and Their Caregivers: Where We are and Where We are Headed. Healthcare 2020, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.S.; Mendonca, R.; Kording, K.; Avery, M.; Johnson, M.J. Towards Data-Driven Autonomous Robot-Assisted Physical Rehabilitation Therapy. IEEE Int. Conf. Rehabil. Robot 2019, 2019, 34–39. [Google Scholar]
- Gouvier, W.D.; Blanton, P.D.; LaPorte, K.K.; Nepomuceno, C. Reliability and validity of the Disability Rating Scale and the Levels of Cognitive Functioning Scale in monitoring recovery from severe head injury. Arch. Phys. Med. Rehabil. 1987, 68, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; NA, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Panisset, M.; Roudier, M.; Saxton, J.; Boiler, F. Severe impairment battery: A neuropsychological test for severely demented patients. Arch. Neurol. 1994, 51, 41–45. [Google Scholar] [CrossRef]
- Whisman, M.A.; Perez, J.E.; Ramel, W. Factor structure of the Beck Depression Inventory—Second Edition (BDI-II) in a student sample. J. Clin. Psychol. 2000, 56, 545–551. [Google Scholar] [CrossRef]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1998, 14, 61–68. [Google Scholar] [CrossRef]
- Hamilton, B.B.; Laughlin, J.A.; Fiedler, R.C. Interrater reliability of the 7-level Functional Independence Measure (FIM). Scand. J. Rehabil. Med. 1994, 26, 115–119. [Google Scholar] [PubMed]
- Balestroni, G.; Bertolotti, G. L’EuroQol-5D (EQ-5D): Uno strumento per la misura della qualità della vita [EuroQol-5D (EQ-5D): An instrument for measuring quality of life. Monaldi. Arch. Chest Dis. 2012, 78, 155–159. [Google Scholar] [PubMed]
- Manca, M.; Paternò, F.; Santoro, C.; Zedda, E.; Braschi, C.; Franco, R.; Sale, A. The impact of serious games with humanoid robots on mild cognitive impairment older adults. Int. J. Hum. Comput. Stud. 2021, 145, 102509. [Google Scholar] [CrossRef]
- Osaka, K.; Tanioka, R.; Betriana, F.; Tanioka, T.; Kai, Y.; Locsin, R.C. Robot Therapy Program for Patients with Dementia: Its Framework and Effectiveness. In Information Systems: Intelligent Information Processing Systems, Natural Language Processing, Affective Computing and Artificial Intelligence, and an Attempt to Build a Conversational Nursing Robot; IntechOpen: London, UK, 2021; p. 87. [Google Scholar]
- Wu, Y.H.; Fassert, C.; Rigaud, A.S. Designing robots for the elderly: Appearance issue and beyond. Arch. Gerontol. Geriatr. 2012, 54, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sumioka, H.; Shiomi, M.; Honda, M.; Nakazawa, A. Technical Challenges for Smooth Interaction with Seniors with Dementia: Lessons from Humanitude™. Front. Robot. AI. 2021, 8, 650906. [Google Scholar] [CrossRef] [PubMed]
- Zuschnegg, J.; Paletta, L.; Fellner, M.; Steiner, J.; Pansy-Resch, S.; Jos, A.; Koini, M.; Prodromou, D.; Halfens, R.J.G.; Lohrmann, C.; et al. Humanoid socially assistive robots in dementia care: A qualitative study about expectations of caregivers and dementia trainers. Aging Ment. Health 2021, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, C.A.; Veneman, J.F.; Rocon, E.; Rodriguez-Guerrero, C. Editorial: Interfacing Humans and Machines for Rehabilitation and Assistive Devices. Front. Robot. AI 2022, 8, 796431. [Google Scholar] [CrossRef]
- Kabacińska, K.; Prescott, T.J.; Robillard, J.M. Socially assistive robots as mental health interventions for children: A scoping review. Int. J. Soc. Robot. 2021, 13, 919–935. [Google Scholar] [CrossRef]
- La Gattuta, E.; Corallo, F.; Lo Buono, V.; De Salvo, S.; Caminiti, F.; Rifici, C.; Alagna, A.; Arcadi, F.; Bramanti, A.; Marino, S. Techniques of cognitive rehabilitation in patients with disorders of consciousness: A systematic review. Neurol. Sci. 2018, 39, 641–645. [Google Scholar] [CrossRef]

| All | EG | CG | p-value | |
|---|---|---|---|---|
| Participants | 12 | 6 | 6 | - |
| Age (years) | 46.9 ± 10.7 | 46.7 ± 10.9 | 47.2 ± 11.4 | 0.99 |
| Education (years) | 11.3 ± 2.5 | 11.3 ± 2.6 | 11.3 ± 2.6 | 0.99 |
| Males | 7 (58.3) | 3 (50.0) | 4 (66.7) | 0.99 |
| Side of the lesion—Bilateral | 8 (66.7) | 3 (50.0) | 5 (83.3) | 0.54 |
| Etiology—Traumatic | 7 (58.3) | 3 (50.0) | 4 (66.7) | 0.99 |
| SIB | 59.2 ± 6.4 | 59.8 ± 7.5 | 58.7± 5.9 | 0.04 |
| LCF | 4.1 ± 0.3 | 4.0 ± 0.0 | 4.2 ± 0.4 | 0.40 |
| MMSE | 17.8 ± 3.9 | 17.8 ± 4.1 | 17.8 ± 4.1 | 0.99 |
| HAM-A | 23.7 ± 3.4 | 23.7 ± 3.6 | 23.7 ± 3.6 | 0.99 |
| FIM | 81.7 ± 5.4 | 81.7 ± 5.7 | 81.7 ± 5.7 | 0.99 |
| EQ-5D | 13.7 ± 1.7 | 13.0 ± 2.1 | 14.5 ± 0.6 | 0.09 |
| BDI-II | 24.8 ± 4.1 | 24.8 ± 1.7 | 24.8 ± 4.4 | 0.99 |
| Outcome Measure | AIC | BIC | Deviance | Chi-Square | df | p-Value | |
|---|---|---|---|---|---|---|---|
| SIB | WG model Full model | 176.30 176.45 | 187.39 192.29 | 162.30 156.45 | 5.85 | 3 | 0.119 |
| LCF | WG model Full model | 59.36 64.46 | 70.45 80.29 | 45.36 44.46 | 0.90 | 3 | 0.825 |
| MMSE | WG model Full model | 112.96 115.36 | 124.04 131.20 | 98.96 95.36 | 3.59 | 3 | 0.309 |
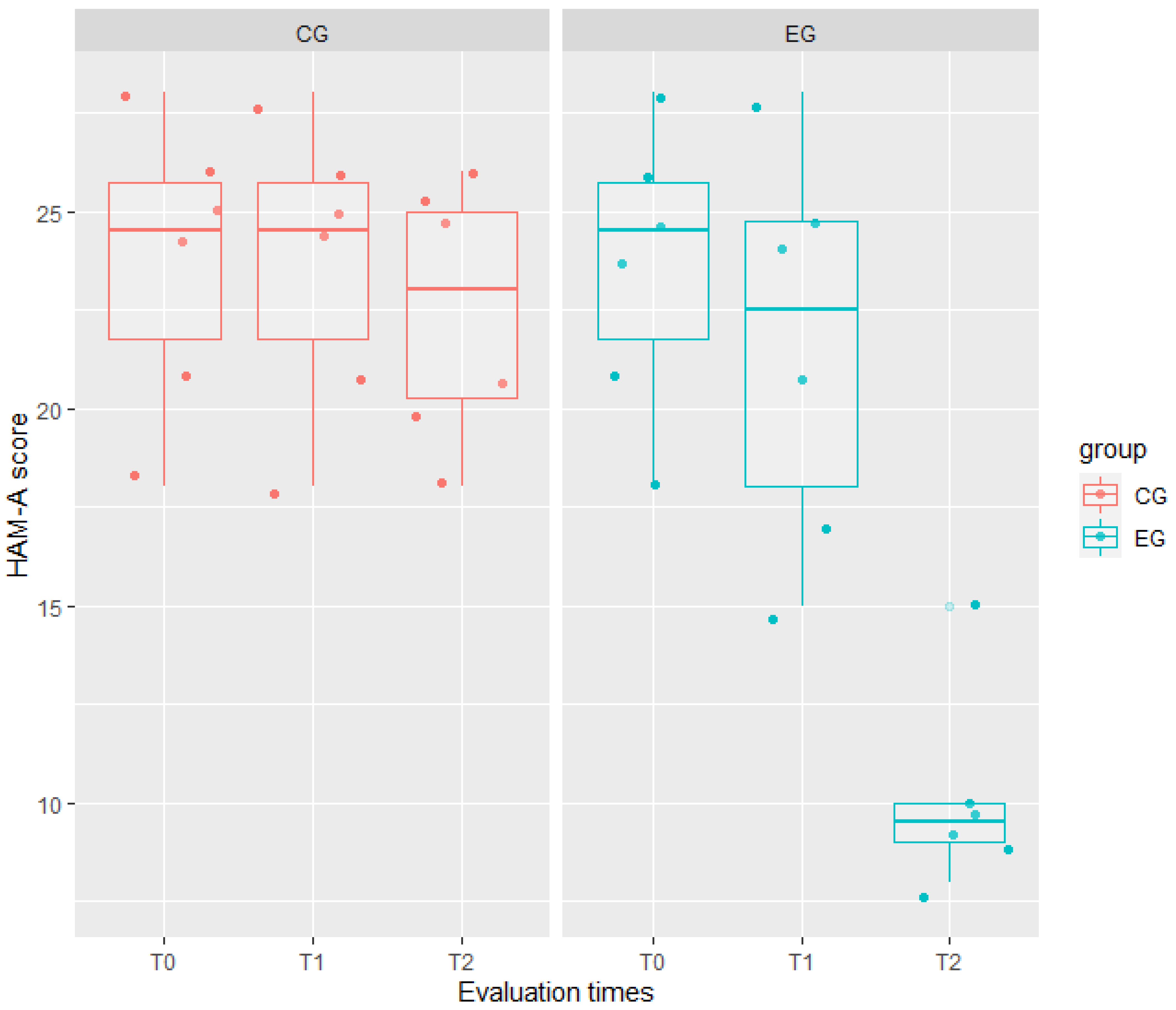
| HAM-A | WG model Full model | 224.81 177.20 | 235.90 193.03 | 210.81 157.20 | 53.62 | 3 | <0.001 |
| FIM | WG model Full model | 216.00 221.05 | 227.09 236.88 | 202.00 201.05 | 0.95 | 3 | 0.813 |
| EQ-5D | WG model Full model | 183.92 88.65 | 195.01 104.49 | 169.92 68.65 | 101.27 | 3 | <0.001 |
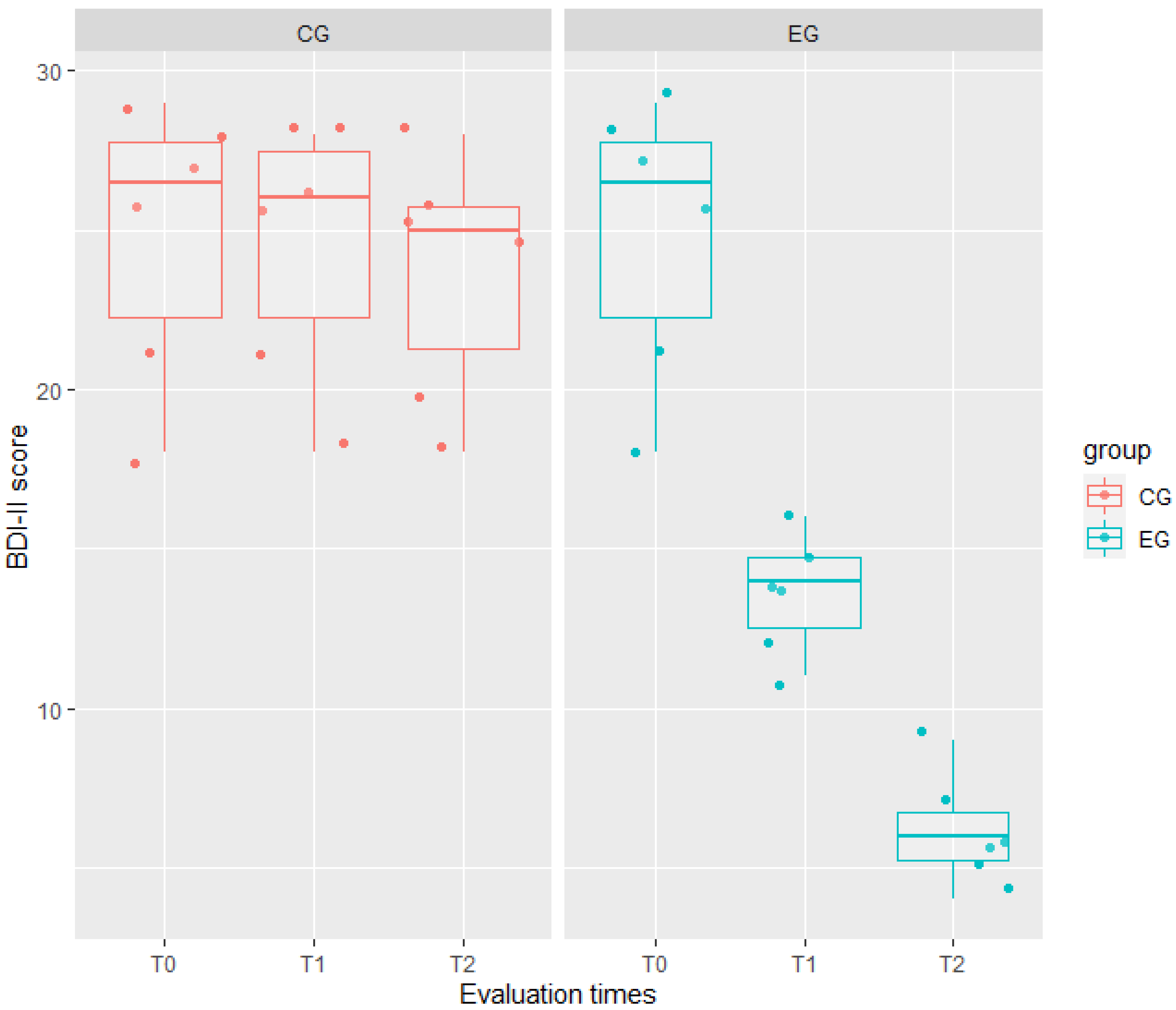
| BDI-II | WG model Full model | 239.42 187.42 | 250.51 203.26 | 225.42 167.42 | 57.99 | 3 | <0.001 |
| Outcome Measure | Coeff. Estimate | Std. Err. | t-Value | p-Value | ICC | |
|---|---|---|---|---|---|---|
| HAM-A | EG | <0.001 | 0.97 | 0.00 | 1.000 | 0.76 |
| T1 | <0.001 | 0.97 | 0.00 | 1.000 | ||
| T2 | −1.17 | 0.97 | 1.20 | 0.239 | ||
| EG × T1 | −2.00 | 1.37 | 1.45 | 0.156 | ||
| EG × T2 | −12.33 | 1.37 | 8.98 | <0.001 | ||
| EQ-5D | EG | −1.50 | 0.73 | 2.04 | 0.041 | 0.87 |
| T1 | <0.001 | 0.23 | 0.00 | 1.000 | ||
| T2 | <0.001 | 0.23 | 0.00 | 1.000 | ||
| EG × T1 | −3.50 | 0.33 | 10.65 | <0.001 | ||
| EG × T2 | −8.67 | 0.33 | 26.37 | <0.001 | ||
| BDI-II | EG | <−0.001 | 1.72 | 0.00 | 1.000 | 0.84 |
| T1 | −0.33 | 1.18 | 0.28 | 0.779 | ||
| T2 | −1.17 | 1.18 | 0.99 | 0.330 | ||
| EG × T1 | −10.83 | 1.67 | 6.50 | <0.001 | ||
| EG × T2 | −17.50 | 1.67 | 10.50 | <0.001 | ||
| (a) | ||||||
| Outcome measure | Variance | Std. Dev. | Correlation | |||
| HAM-A | Subj (Intercept) | 8.85 | 2.97 | −1.00 | ||
| Subj (EG) | 0.03 | 0.16 | ||||
| Residual | 2.83 | 1.68 | ||||
| EQ-5D | Subj (Intercept) | 0.19 | 0.44 | −0.44 | ||
| Subj (EG) | 2.92 | 1.71 | ||||
| Residual | 0.16 | 0.40 | ||||
| BDI-II | Subj (Intercept) | 12.48 | 3.53 | −1.00 | ||
| Subj (EG) | 9.33 | 3.05 | ||||
| Residual | 4.16 | 2.04 | ||||
| (b) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corallo, F.; Maresca, G.; Formica, C.; Bonanno, L.; Bramanti, A.; Parasporo, N.; Giambò, F.M.; De Cola, M.C.; Lo Buono, V. Humanoid Robot Use in Cognitive Rehabilitation of Patients with Severe Brain Injury: A Pilot Study. J. Clin. Med. 2022, 11, 2940. https://doi.org/10.3390/jcm11102940
Corallo F, Maresca G, Formica C, Bonanno L, Bramanti A, Parasporo N, Giambò FM, De Cola MC, Lo Buono V. Humanoid Robot Use in Cognitive Rehabilitation of Patients with Severe Brain Injury: A Pilot Study. Journal of Clinical Medicine. 2022; 11(10):2940. https://doi.org/10.3390/jcm11102940
Chicago/Turabian StyleCorallo, Francesco, Giuseppa Maresca, Caterina Formica, Lilla Bonanno, Alessia Bramanti, Nicholas Parasporo, Fabio Mauro Giambò, Maria Cristina De Cola, and Viviana Lo Buono. 2022. "Humanoid Robot Use in Cognitive Rehabilitation of Patients with Severe Brain Injury: A Pilot Study" Journal of Clinical Medicine 11, no. 10: 2940. https://doi.org/10.3390/jcm11102940
APA StyleCorallo, F., Maresca, G., Formica, C., Bonanno, L., Bramanti, A., Parasporo, N., Giambò, F. M., De Cola, M. C., & Lo Buono, V. (2022). Humanoid Robot Use in Cognitive Rehabilitation of Patients with Severe Brain Injury: A Pilot Study. Journal of Clinical Medicine, 11(10), 2940. https://doi.org/10.3390/jcm11102940









