Rubus occidentalis and Ellagic Acid Affect the Contractility of Penile Corpus Cavernosum Smooth Muscle through the Nitric Oxide-Cyclic Guanosine Monophosphate and Cyclic Adenosine 3′,5′-Monophosphate Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Crude Material
2.3. Animals and Experimental Protocol
2.4. Organ Bath Study Protocol
2.5. Measurement of cAMp and cGMp Concentrations via a Radioimmunoassay
2.6. Western Blot Analysis
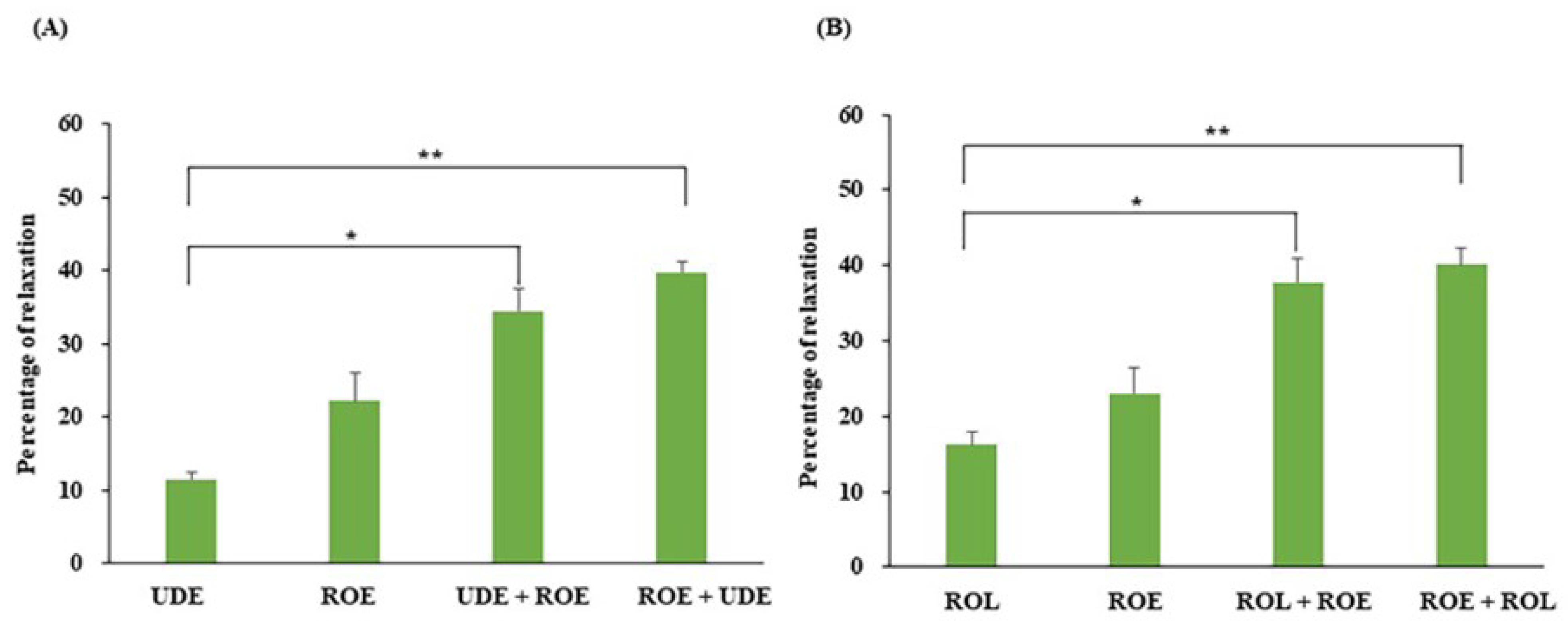
2.7. Assessment of Interactive Effects between ROE or EA with UDE or ROL on PCCSM Tension
2.8. Statistical Analysis
3. Results
3.1. Determination of the EA Content in the Crude Extract
3.2. Cumulative Effects of ROE and EA on PCCSM Tissue
3.3. Effects of ROE and EA on cAMp and cGMp Concentrations in Perfusate
3.4. Expression of eNOS and nNOS in PCCSM Tissue
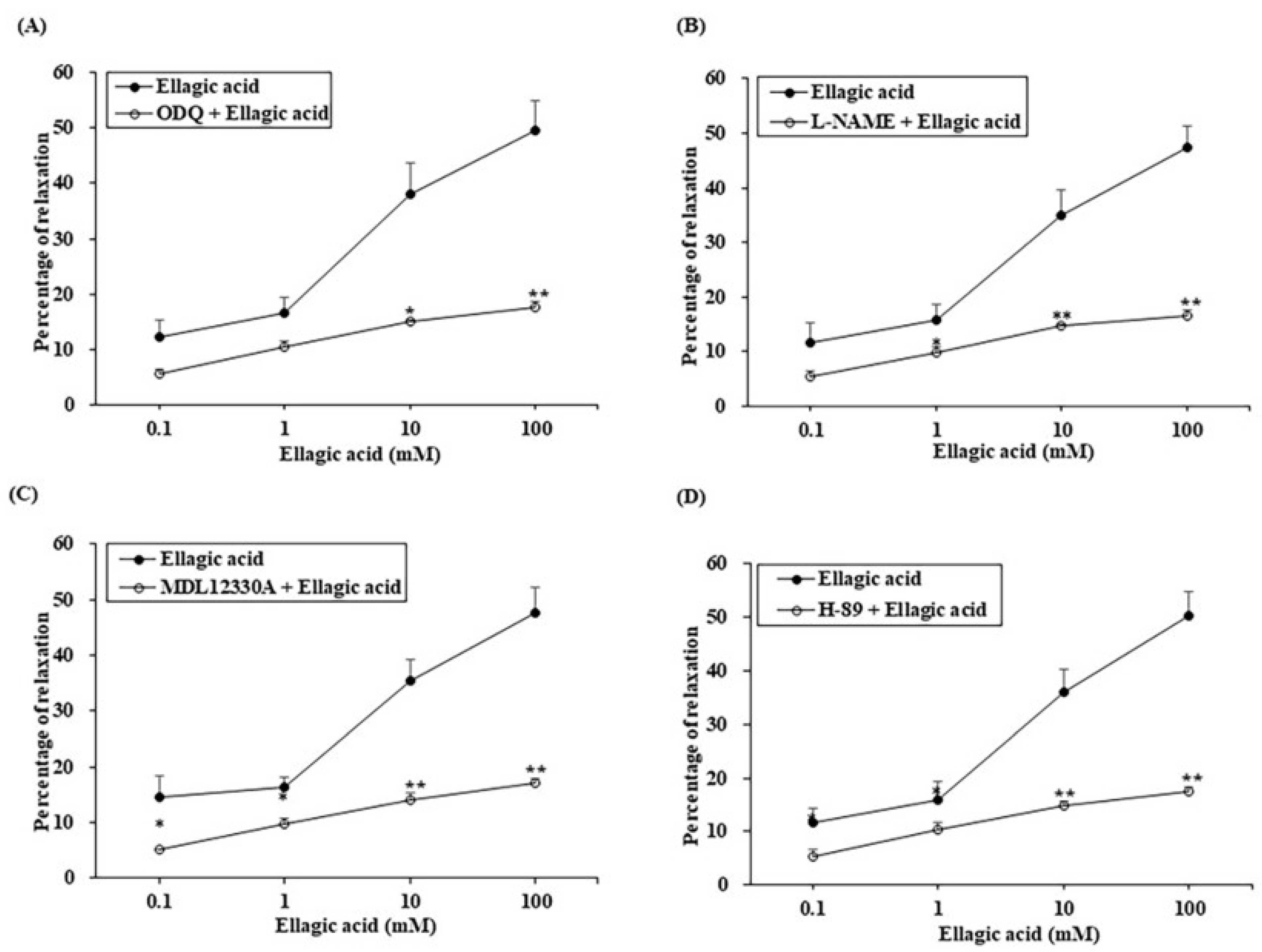
3.5. Effect of ROE on PCCSM Preincubated with a PDE4 or -5 Inhibitor
3.6. Effect of EA on PCCSM Preincubated with a PDE4 or -5 Inhibitor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Matz, E.L.; Terlecki, R.; Zhang, Y.; Jackson, J.; Atala, A. Stem Cell Therapy for Erectile Dysfunction. Sex. Med. Rev. 2019, 7, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Bak, Y.O.; Choi, B.R.; Zhao, C.; Lee, H.J.; Kim, C.Y.; Lee, S.W.; Jeon, J.H.; Park, J.K. The role of the lignan constituents in the effect of Schisandra chinensis fruit extract on penile erection. Phytother. Res. 2011, 25, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; Schwedhelm, E.; Albsmeier, J.; Böger, R.H. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc. Med. 2002, 7, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L. The role of nitric oxide in erectile dysfunction: Implications for medical therapy. J. Clin. Hypertens. 2006, 8 (Suppl. S4), 53–62. [Google Scholar] [CrossRef]
- Aversa, A.; Pili, M.; Fabbri, A.; Spera, E.; Spera, G. Erectile dysfunction: Expectations beyond phosphodiesterase type 5 inhibition. J. Endocrinol. Investig. 2004, 27, 192–206. [Google Scholar] [CrossRef]
- La Fuente, J.M.; Pepe-Cardoso, A.J.; Martínez-Salamanca, J.I.; Louro, N.; Angulo, J. L-cysteine/hydrogen sulfide pathway induces cGMP-dependent relaxation of corpus cavernosum and penile arteries from patients with erectile dysfunction and improves arterial vasodilation induced by PDE5 inhibition. Eur. J. Pharmacol. 2019, 863, 172675. [Google Scholar] [CrossRef]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef]
- Kuthe, A. Phosphodiesterase 5 inhibitors in male sexual dysfunction. Curr. Opin. Urol. 2003, 13, 405–410. [Google Scholar] [CrossRef]
- Anand Ganapathy, A.; Priya, V.M.H.; Kumaran, A. Medicinal plants as a potential source of Phosphodiesterase-5 inhibitors: A review. J. Ethnopharmacol. 2021, 267, 113536. [Google Scholar] [CrossRef]
- McMahon, C.G. Current diagnosis and management of erectile dysfunction. Med. J. Aust. 2019, 210, 469–476. [Google Scholar] [CrossRef]
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunol. Immunother. 2014, 63, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Cho, J.Y.; Lee, T.-B.; Kwon, J.-W.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.-S. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Shin, S.Y.; Kim, S.; Lee, K.H.; Shin, J.C.; Park, K.M. Comparison of antiaging, anti-melanogenesis effects, and active components of Raspberry (Rubus occidentalis L.) extracts according to maturity. J. Food Biochem. 2020, 44, e13464. [Google Scholar] [CrossRef] [PubMed]
- Eskra, J.N.; Schlicht, M.J.; Bosland, M.C. Effects of Black Raspberries and Their Ellagic Acid and Anthocyanin Constituents on Taxane Chemotherapy of Castration-Resistant Prostate Cancer Cells. Sci. Rep. 2019, 9, 4367. [Google Scholar] [CrossRef] [PubMed]
- Jordão, J.B.R.; Porto, H.K.P.; Lopes, F.M.; Batista, A.C.; Rocha, M.L. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017, 83, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chae, H.J.; Kim, S.H.; Cui, W.S.; Lee, S.W.; Jeon, J.-H.; Park, J.K. A new perfusion model for studying erectile function. J. Sex. Med. 2010, 7 Pt 1, 1419–1428. [Google Scholar] [CrossRef]
- Choi, B.R.; Kumar, S.K.; Zhao, C.; Zhang, L.T.; Kim, C.Y.; Lee, S.W.; Jeon, J.-H.; So, I.; Kim, S.H.; Park, N.C.; et al. Additive effects of Artemisia capillaris extract and scopoletin on the relaxation of penile corpus cavernosum smooth muscle. Int. J. Impot. Res. 2015, 27, 225–232. [Google Scholar] [CrossRef][Green Version]
- Choi, B.R.; Kim, H.K.; Park, J.K. Effects of Schisandra chinensis fruit extract and gomisin A on the contractility of penile corpus cavernosum smooth muscle: A potential mechanism through the nitric oxide-cyclic guanosine monophosphate pathway. Nutr. Res. Pract. 2018, 12, 291–297. [Google Scholar] [CrossRef]
- Sun, K.; Zhao, C.; Chen, X.-F.; Kim, H.-K.; Choi, B.-R.; Huang, Y.-R.; Park, J.-K. Ex vivo relaxation effect of Cuscuta chinensis extract on rabbit corpus cavernosum. Asian J. Androl. 2013, 15, 134–137. [Google Scholar] [CrossRef]
- Chiu, J.-H.; Chen, K.-K.; Chien, T.-M.; Chiou, W.-F.; Chen, C.-C.; Wang, J.-Y.; Lui, W.-Y.; Wu, C.-W. Epimedium brevicornum Maxim extract relaxes rabbit corpus cavernosum through multitargets on nitric oxide/cyclic guanosine monophosphate signaling pathway. Int. J. Impot. Res. 2006, 18, 335–342. [Google Scholar] [CrossRef]
- Li, X.; Oh, H.C.; Son, S.B.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Effect of an Ethanol Extract of Scutellaria baicalensis on Relaxation in Corpus Cavernosum Smooth Muscle. Evid. Based Complement. Alternat. Med. 2012, 2012, 148929. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Vishwanath, M.; Gangadarappa, S.K.; Razdan, R.; Inamdar, M.N. Efficacy of ellagic acid and sildenafil in diabetes-induced sexual dysfunction. Pharmacogn. Mag. 2014, 10 (Suppl. S3), S581–S587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kim, H.K.; Kim, S.Z.; Chae, H.J.; Cui, W.S.; Lee, S.W.; Jeon, J.H.; Park, J.K. What is the role of unripe Rubus coreanus extract on penile erection? Phytother. Res. 2011, 25, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Uckert, S.; Hedlund, P.; Waldkirch, E.; Sohn, M.; Jonas, U.; Andersson, K.E.; Stief, C.G. Interactions between cGMP- and cAMP-pathways are involved in the regulation of penile smooth muscle tone. World J. Urol. 2004, 22, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.D.; Xin, Z.C.; Choi, H.K. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int. J. Impot. Res. 1998, 10, 37–43. [Google Scholar] [CrossRef]
- Sohn, D.W.; Kim, H.Y.; Kim, S.D.; Lee, E.J.; Kim, H.S.; Kim, J.K.; Hwang, S.Y.; Cho, Y.-H.; Kim, S.W. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of spontaneous hypertensive male rats. J. Ethnopharmacol. 2008, 120, 176–180. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Liu, B.; Li, D.; Yuan, Z.; Zhang, H. A Chinese herbal formula, Shuganyiyang capsule, improves erectile function in male rats by modulating Nos-CGMP mediators. Urology 2012, 79, 241.e1–241.e6. [Google Scholar] [CrossRef]
- Hurt, K.J.; Sezen, S.F.; Lagoda, G.F.; Musicki, B.; Rameau, G.A.; Snyder, S.H.; Burnett, A.L. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. USA 2012, 109, 16624–16629. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, H.K.; Park, J.K. Penile Erection Induced by Scoparone from Artemisia capillaris through the Nitric Oxide-Cyclic Guanosine Monophosphate Signaling Pathway. World J. Mens. Health 2017, 35, 196–204. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, H.K.; Park, J.K. Quercetin Relaxed the Smooth Muscle of Rabbit Penile Corpus Cavernosum by Activating the NO-cGMP Signaling Pathway. Nat. Prod. Sci. 2017, 23, 169–174. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, B.R.; Bak, Y.O.; Zhao, C.; Lee, S.W.; Jeon, J.H.; So, I.; Park, J.K. The role of capillarisin from Artemisia capillaris on penile erection. Phytother. Res. 2012, 26, 800–805. [Google Scholar] [CrossRef] [PubMed]





| Samples | Concentrations (mg/mL) | Cyclic Nucleotides | |
|---|---|---|---|
| cAMp (fmol/mg) | cGMp (fmol/mg) | ||
| ROE (mg/mL) | Control | 957.25 ± 46.34 | 121.86 ± 4.51 |
| 1 | 1032.09 ± 13.44 | 154.36 ± 7.87 * | |
| 2 | 1036.84 ± 26.25 | 157.35 ± 7.06 ** | |
| 3 | 1069.60 ± 40.58 * | 162.86 ± 4.91 ** | |
| 4 | 1135.82 ± 78.94 * | 173.76 ± 5.77 ** | |
| Samples | Concentrations (µM) | Cyclic Nucleotides | |
|---|---|---|---|
| cAMp (fmol/mg) | cGMp (fmol/mg) | ||
| EA (µM) | Control | 947.09 ± 38.33 | 114.45 ± 0.86 |
| 0.1 | 1034.10 ± 14.93 * | 152.27 ± 6.95 * | |
| 1 | 1037.60 ± 26.41 * | 156.68 ± 10.35 * | |
| 10 | 1065.52 ± 42.43 * | 163.26 ± 5.70 ** | |
| 100 | 1209.52 ± 27.53 ** | 172.32 ± 2.91 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karna, K.K.; Choi, B.-R.; Kim, C.-Y.; Kim, H.-K.; Park, J.-K. Rubus occidentalis and Ellagic Acid Affect the Contractility of Penile Corpus Cavernosum Smooth Muscle through the Nitric Oxide-Cyclic Guanosine Monophosphate and Cyclic Adenosine 3′,5′-Monophosphate Signaling Pathway. J. Clin. Med. 2022, 11, 2947. https://doi.org/10.3390/jcm11102947
Karna KK, Choi B-R, Kim C-Y, Kim H-K, Park J-K. Rubus occidentalis and Ellagic Acid Affect the Contractility of Penile Corpus Cavernosum Smooth Muscle through the Nitric Oxide-Cyclic Guanosine Monophosphate and Cyclic Adenosine 3′,5′-Monophosphate Signaling Pathway. Journal of Clinical Medicine. 2022; 11(10):2947. https://doi.org/10.3390/jcm11102947
Chicago/Turabian StyleKarna, Keshab Kumar, Bo-Ram Choi, Chul-Young Kim, Hye-Kyung Kim, and Jong-Kwan Park. 2022. "Rubus occidentalis and Ellagic Acid Affect the Contractility of Penile Corpus Cavernosum Smooth Muscle through the Nitric Oxide-Cyclic Guanosine Monophosphate and Cyclic Adenosine 3′,5′-Monophosphate Signaling Pathway" Journal of Clinical Medicine 11, no. 10: 2947. https://doi.org/10.3390/jcm11102947
APA StyleKarna, K. K., Choi, B.-R., Kim, C.-Y., Kim, H.-K., & Park, J.-K. (2022). Rubus occidentalis and Ellagic Acid Affect the Contractility of Penile Corpus Cavernosum Smooth Muscle through the Nitric Oxide-Cyclic Guanosine Monophosphate and Cyclic Adenosine 3′,5′-Monophosphate Signaling Pathway. Journal of Clinical Medicine, 11(10), 2947. https://doi.org/10.3390/jcm11102947






