Acute Coronary Syndrome in the COVID-19 Era—Differences and Dilemmas Compared to the Pre-COVID-19 Era
Abstract
:1. Introduction
2. The Role of CVD in the Course of COVID-19 Infection
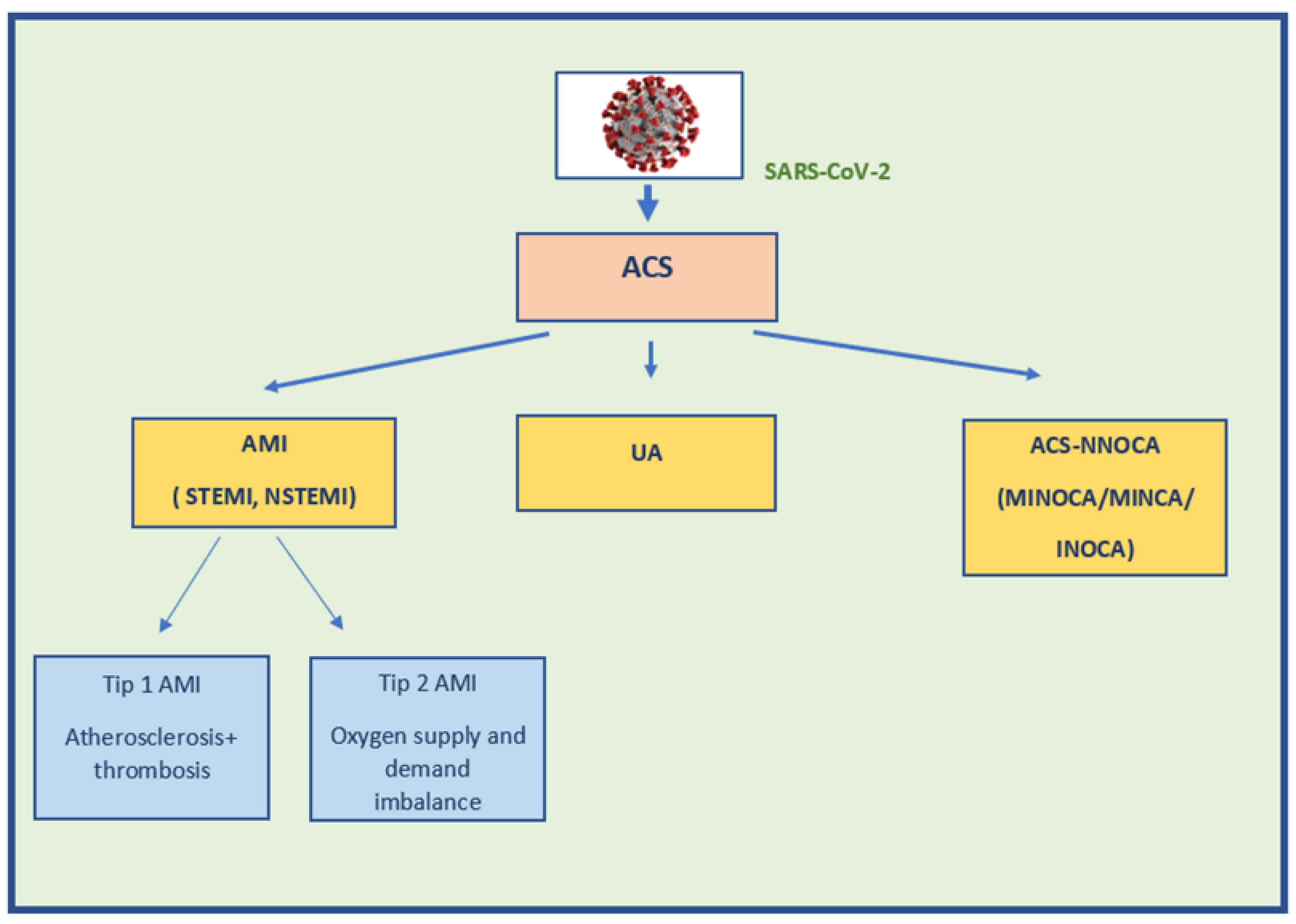
3. Epidemiology and Etiology of ACS in COVID-19
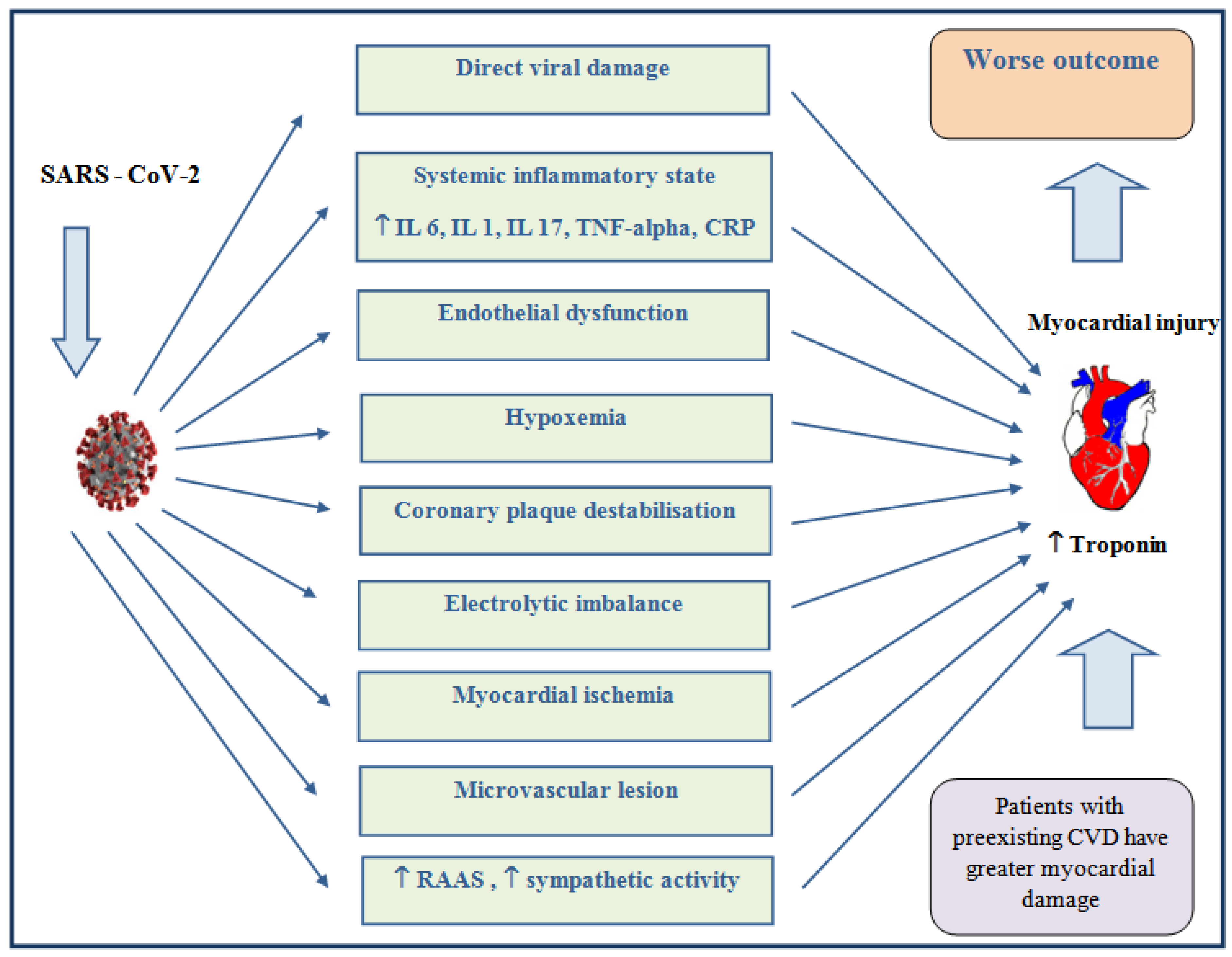
4. Pathogenesis of ACS in COVID-19
5. Clinical Picture of ACS in COVID-19
6. Diagnostic of ACS in COVID-19
7. Treatment of ACS in COVID-19
8. Impact of COVID-19 on Mortality from AMI
9. The Impact of the COVID-19 Pandemic on the Outcome of Patients with AMI
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://COVID19.who.int/ (accessed on 1 April 2022).
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Henkens, M.T.H.M.; Raafs, A.G.; Verdonschot, J.A.J.; Linschoten, M.; van Smeden, M.; Wang, P.; van der Hooft, B.H.M.; Tieleman, R.; Janssen, M.L.F.; Ter Bekke, R.M.A.; et al. CAPACITY-COVID collaborative consortium. Age is the main determinant of COVID-19 related in-hospital mortality with minimal impact of pre-existing comorbidities, a retrospective cohort study. BMC Geriatr. 2022, 22, 184. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. STOP-COVID Investigators. Factors Associated With Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020, 180, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; The Northwell COVID-19 Research Consortium; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. ISARIC4C investigators. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, 1985. [Google Scholar]
- Fried, M.W.; Crawford, J.M.; Mospan, A.R.; Watkins, S.E.; Munoz, B.; Zink, R.C.; Elliott, S.; Burleson, K.; Landis, C.; Reddy, K.R.; et al. Patient Characteristics and Outcomes of 11 721 Patients With Coronavirus Disease 2019 (COVID-19) Hospitalized Across the United States. Clin. Infect. Dis. 2021, 72, e558–e565. [Google Scholar] [CrossRef]
- Cordero, A.; Santos García-Gallego, C.; Bertomeu-González, V.; Fácila, L.; Rodríguez-Mañero, M.; Escribano, D.; Castellano, J.M.; Zuazola, P.; Núñez, J.; Badimón, J.J.; et al. Mortality associated with cardiovascular disease in patients with COVID-19. Rec. Cardioclinics 2021, 56, 30–38. [Google Scholar] [CrossRef]
- Mishra, P.; Parveen, R.; Bajpai, R.; Samim, M.; Agarwal, N.B. Impact of cardiovascular diseases on severity of COVID-19 patients: A systematic review. Ann. Acad. Med. Singap. 2021, 50, 52–60. [Google Scholar] [CrossRef]
- The CAPACITY-COVID Collaborative Consortium and LEOSS Study Group. Clinical presentation, disease course, and outcome of COVID-19 in hospitalized patients with and without pre-existing cardiac disease: A cohort study across 18 countries. Eur. Heart J. 2022, 43, 1104–1120. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar]
- Kumar, A.; Cannon, C.P. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Mount Sinai COVID Informatics Center. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [Green Version]
- de Cortina Camarero, C.; Gómez Mariscal, E.; Espejo Bares, V.; Núñez Garcia, A.; Muñoz Aguilera, R.; Botas Rodriguez, J. SARS-CoV-2 infection: A predisposing factor for acute coronary syndrome. Med. Clin. 2021, 157, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Lindmark, K.; Fors Connolly, A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet 2021, 398, 599–607. [Google Scholar] [CrossRef]
- Schiavone, M.; Gasperetti, A.; Mancone, M.; Kaplan, A.V.; Gobbi, C.; Mascioli, G.; Busana, M.; Saguner, A.M.; Mitacchione, G.; Giacomelli, A.; et al. Redefining the Prognostic Value of High-Sensitivity Troponin in COVID-19 Patients: The Importance of Concomitant Coronary Artery Disease. J. Clin. Med. 2020, 9, 3263. [Google Scholar] [CrossRef]
- Modin, D.; Claggett, B.; Sindet-Pedersen, C.; Lassen, M.C.H.; Skaarup, K.G.; Jensen, J.U.S.; Fralick, M.; Schou, M.; Lamberts, M.; Gerds, T.; et al. Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation 2020, 142, 2080–2082. [Google Scholar] [CrossRef]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article-Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Chilazi, M.; Duffy, E.Y.; Thakkar, A.; Michos, E.D. COVID and Cardiovascular Disease: What We Know in 2021. Curratheroscler Rep. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, L.; Pink, I.; Kühne, J.F.; Beushausen, K.; Keil, J.; Christoph, S.; Sauer, A.; Boblitz, L.; Schmidt, J.; David, S.; et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 2021, 6, 418. [Google Scholar] [CrossRef]
- Wu, K.K.; Thiagarajan, P. Role of endothelium in thrombosis and hemostasis. Annu. Rev. Med. 1996, 47, 315–331. [Google Scholar]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Barnes, M.; Heywood, A.E.; Mahimbo, A.; Rahman, B.; Newall, A.T.; Macintyre, C.R. Acute myocardial infarction and influenza: A meta-analysis of case-control studies. Heart 2015, 101, 1738–1747. [Google Scholar] [CrossRef] [Green Version]
- Pillai, B.; Trikkur, S.; Farooque, U.; Ramakrishnan, D.; Kakkra, J.J.; Kashyap, G.; Lalwani, C.; Mani, A.B.; Vishwanath, J. Type II Myocardial Infarction: Predisposing Factors, Precipitating Elements, and Outcomes. Cureus 2020, 12, e9254. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Sandoval, Y. The many faces of type 2 myocardial infarction. J. Am. Coll. Cardiol. 2017, 70, 1569–1572. [Google Scholar] [CrossRef]
- Yang, L.C.; Zhang, R.T.; Guo, L.J.; Xiao, H.; Zu, L.Y.; Zhang, Y.Y.; Cheng, Q.; Zhao, Z.L.; Ge, Q.G.; Gao, W. Hypoxia and inflammation are risk factors for acute myocardial injury in patients with coronavirus disease 2019. J. Peking Univ. Health Sci. 2020, 53, 159–166. [Google Scholar]
- Soliman, E.Z.; Safford, M.M.; Muntner, P.; Khodneva, Y.; Dawood, F.Z.; Zakai, N.A.; Thacker, E.L.; Judd, S.; Howard, V.J.; Howard, G.; et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern. Med. 2014, 174, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Kawakami, S.; Noguchi, T.; Tanaka, T.; Asaumi, Y.; Kanaya, T.; Nagai, T.; Nakao, K.; Fujino, M.; Nagatsuka, K.; et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation 2015, 132, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romiti, S.; Totaro, M.; Laderchi, A.; Peruzzi, M.; Vinciguerra, M.; Greco, E. Case Report: Emergency CABG Following Failure of PTCA in a COVID-19 Patient. Front Cardiovasc. Med. 2021, 7, 620610. [Google Scholar] [CrossRef] [PubMed]
- Courand, P.Y.; Harbaoui, B.; Bonnet, M.; Lantelme, P. Spontaneous Coronary Artery Dissection in a Patient with COVID-19. JACC Cardiovasc. Interv. 2020, 13, e107–e108. [Google Scholar] [CrossRef]
- Albiero, R.; Seresini, G. Atherosclerotic spontaneous coronary artery dissection (A-SCAD) in a patient with COVID-19: Case report and possible mechanisms. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Hinterseer, M.; Zens, M.; Wimmer, R.J.; Delladio, S.; Lederle, S.; Kupatt, C.; Hartmann, B. Acute myocardial infarction due to coronary stent thrombosis in a symptomatic COVID-19 patient. Clin. Res. Cardiol. 2021, 110, 302–306. [Google Scholar] [CrossRef]
- Reffo, E.; Stritoni, V.; Di Salvo, G. Inflammatory syndrome in children associated with COVID-19 complicated by acute myocardial infarction. Eur. Heart J. 2021, 42, 2136. [Google Scholar] [CrossRef]
- Persson, J.; Shorofsky, M.; Leahy, R.; Friesen, R.; Khanna, A.; Cole, L.; Kim, J.S. ST-Elevation Myocardial Infarction due to Acute Thrombosis in an Adolescent With COVID-19. Pediatrics 2021, 148, e2020049793. [Google Scholar] [CrossRef] [PubMed]
- Le, C.K.; Nguyen, M.B.; Vo, A.T. ST-elevation in an adolescent with COVID-19: Myopericarditis or myocardial infarction? Am. J. Emerg. Med. 2022, 52, 271. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, P.; Wong, J.; Pushparajah, K.; Mathur, S.K.; Simpson, J.M.; Pascall, E.; Cleary, A.; Stewart, K.; Adhvaryu, K.; Savis, A.; et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020, 17, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Kogan, E.A.; Kukleva, A.D.; Berezovskiy, Y.S.; Blagova, O.V.; Zharkov, N.V.; Ainetdinova, D.K.; Demyashkin, G.A. Kliniko-morfologicheskayakharakteristika SARS-CoV-2-assotsiirovannogo miokardita, podtverzhdennogonalichiem RNK ibelkovvirusa v tkanimiokarda [Clinical and morphological characteristics of SARS-CoV-2-related myocarditis proven by the presence of viral RNA and proteins in myocardial tissue]. Arkh. Patol. 2021, 83, 5–13. [Google Scholar]
- Hu, L.; Chen, S.; Fu, Y.; Gao, Z.; Long, H.; Ren, H.W.; Zuo, Y.; Wang, J.; Li, H.; Xu, Q.B.; et al. Risk Factors Associated with Clinical Outcomes in 323 Coronavirus Disease 2019 (COVID-19) Hospitalized Patients in Wuhan, China. Clin. Infect. Dis. 2020, 71, 2089–2098. [Google Scholar] [CrossRef]
- Chen, D.; Li, X.; Song, Q.; Hu, C.; Su, F.; Dai, J.; Ye, Y.; Huang, J.; Zhang, X. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Netw. Open 2020, 3, e2011122. [Google Scholar] [CrossRef]
- Lakkireddy, D.R.; Chung, M.K.; Gopinathannair, R.; Patton, K.K.; Gluckman, T.J.; Turagam, M.; Cheung, J.W.; Patel, P.; Sotomonte, J.; Lampert, R.; et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020, 17, e233–e241. [Google Scholar]
- Peltzer, B.; Manocha, K.K.; Ying, X.; Kirzner, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Safford, M.M.; et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 3077–3085. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Merchant, F.M.; Lakkireddy, D.R.; Etheridge, S.P.; Feigofsky, S.; Han, J.K.; Kabra, R.; Natale, A.; Poe, S.; Saha, S.A.; et al. COVID-19 and cardiac arrhythmias: A global perspective on arrhythmia characteristics and management strategies. J. Interv. Card. Electrophysiol. 2020, 59, 329–336. [Google Scholar] [CrossRef]
- Yadav, R.; Bansal, R.; Budakoty, S.; Barwad, P. COVID-19 and sudden cardiac death: A new potential risk. Indian Heart J. 2020, 72, 333–336. [Google Scholar] [CrossRef] [PubMed]
- The Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1—epidemiology, pathophysiology, and diagnosis. Eur. Heart J. 2022, 43, 1033–1058. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Brady, W.J.; Bridwell, R.E.; Ramzy, M.; Montrief, T.; Singh, M.; Gottlieb, M. Electrocardiographic manifestations of COVID-19. Am. J. Emerg. Med. 2021, 41, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Akwe, J.; Halford, B.; Kim, E.; Miller, A. A review of Cardiac and Non-Cardiac Causes of Troponin Elevation and Clinical Relevance Part II: Non Cardiac Causes. J. Cardiol. Curr. Res. 2018, 11, 00364. [Google Scholar]
- Efros, O.; Barda, N.; Meisel, E.; Leibowitz, A.; Fardman, A.; Rahav, G.; Klempfner, R.; Grossman, E. Myocardial injury in hospitalized patients with COVID-19 infection-Risk factors and outcomes. PLoS ONE 2021, 16, e0247800. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; et al. ST-Elevation Myocardial Infarction in Patients with COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Yassin, A.; Abdelkader, M.A.; Mohammed, R.M.; Osman, A.M. CT pulmonary angiography in COVID-19 pneumonia: Relationship between pulmonary embolism and disease severity. Egypt. J. Radiol. Nucl. Med. 2021, 52, 10. [Google Scholar] [CrossRef]
- Tanacli, R.; Doeblin, P.; Götze, C.; Zieschang, V.; Faragli, A.; Stehning, C.; Korosoglou, G.; Erley, J.; Weiss, J.; Berger, A.; et al. COVID-19 vs. Classical Myocarditis Associated Myocardial Injury Evaluated by Cardiac Magnetic Resonance and Endomyocardial Biopsy. Front Cardiovasc. Med. 2021, 8, 737257. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Fardman, A.; Zahger, D.; Orvin, K.; Oren, D.; Kofman, N.; Mohsen, J.; Tsafrir, O.; Asher, E.; Rubinshtein, R.; Jamal, J.; et al. Acute myocardial infarction in the COVID-19 era: Incidence, clinical characteristics and in-hospital outcomes-A multicenter registry. PLoS ONE 2021, 16, e0253524. [Google Scholar] [CrossRef]
- Choudry, F.A.; Hamshere, S.M.; Rathod, K.S.; Akhtar, M.M.; Archbold, R.A.; Guttmann, O.P.; Woldman, S.; Jain, A.K.; Knight, C.J.; Baumbach, A.; et al. Thrombus Burden in Patients WithCOVID-19 Presenting With ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Skorupski, W.J.; Grygier, M.; Lesiak, M.; Kałużna-Oleksy, M. Coronary Stent Thrombosis in COVID-19 Patients: A Systematic Review of Cases Reported Worldwide. Viruses 2022, 14, 260. [Google Scholar] [CrossRef]
- Iakovou, I.; Schmidt, T.; Bonizzoni, E.; Ge, L.; Sangiorgi, G.M.; Stankovic, G.; Airoldi, F.; Chieffo, A.; Montorfano, M.; Carlino, M.; et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005, 293, 2126–2130. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Yang, Q. Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: Implications from clinical features to pathologic findings. Circulation 2020, 141, 1736–1738. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical characteristics of Coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Khiali, S.; Entezari-Maleki, T. Anticoagulation in COVID-19: DDI Perspective. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620959457. [Google Scholar] [CrossRef] [PubMed]
- Tomaszuk-Kazberuk, A.; Koziński, M.; Domienik-Karłowicz, J.; Jaguszewski, M.; Darocha, S.; Wybraniec, M. Pharmacotherapy of atrial fibrillation in COVID-19 patients. Cardiol. J. 2021, 28, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Smythe, M.A.; Burns, C.; Liu, Q.; Garwood, C.L. Potential Dexamethasone-Direct Oral Anticoagulant Drug Interaction: Is This a Concern in COVID? Ann. Pharmacother. 2022, 56, 319–329. [Google Scholar] [CrossRef]
- Potere, N.; Candeloro, M.; Porreca, E.; Marinari, S.; Federici, C.; Auciello, R.; Di Nisio, M. Direct oral anticoagulant plasma levels in hospitalized COVID-19 patients treated with dexamethasone. J. Thromb. Thrombolysis 2022, 53, 346–351. [Google Scholar] [CrossRef]
- Yu, W.L.; Toh, H.S.; Liao, C.T.; Chang, W.T. A Double-Edged Sword-Cardiovascular Concerns of Potential Anti-COVID-19 Drugs. Cardiovasc. Drugs Ther. 2021, 35, 205–214. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19: July 2021 update on postdischarge thromboprophylaxis. Blood Adv. 2022, 25, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Green, C.; Greenstein, Y.Y.; Gross, P.L.; Hamburg, N.M.; Haniffa, R.; Goligher, E.C.; Bradbury, C.A.; McVerry, B.J.; Lawler, P.R.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar]
- RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar] [CrossRef]
- Demelo-Rodriguez, P.; Farfán-Sedano, A.I.; Pedrajas, J.M.; Llamas, P.; Sigüenza, P.; Jaras, M.J.; Quintana-Diaz, M.; Fernández-Capitán, C.; Bikdeli, B.; Jiménez, D.; et al. RIETE-BLEEDING Investigators. Bleeding risk in hospitalized patients with COVID-19 receiving intermediate- or therapeutic doses of thromboprophylaxis. J. Thromb. Haemost. 2021, 19, 1981–1989. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar]
- Nadkarni, G.N.; Lala, A.; Bagiella, E.; Chang, H.L.; Moreno, P.R.; Pujadas, E.; Arvind, V.; Bose, S.; Charney, A.W.; Chen, M.D.; et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Rubini-Costa, R.; Bermúdez-Jiménez, F.; Rivera-López, R.; Sola-García, E.; Nagib-Raya, H.; Moreno-Escobar, E.; López-Zúñiga, M.Á.; Briones-Través, A.; Sanz-Herrera, F.; Sequí-Sabater, J.M.; et al. Prevalence of bleeding secondary to anticoagulation and mortality in patients with atrial fibrillation admitted with SARS-CoV-2 infection. Med. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, A.; Núñez-Gil, I.J.; Aparisi, Á.; Arroyo-Espliguero, R.; Maroun Eid, C.; Romero, R.; Becerra-Muñoz, V.M.; Feltes, G.; Molina, M.; García-Aguado, M.; et al. HOPE COVID-19 investigators. Atrial fibrillation in patients with COVID-19. Usefulness of the CHA2DS2-VASc score: An analysis of the international HOPE COVID-19 registry. Rev. Esp. Cardiol. 2021, 74, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Banach, M.; Reiner, Ž.; Pirro, M.; Bianconi, V.; Al-Rasadi, K.; Sahebkar, A. Interaction Between Coronavirus S-Protein and Human ACE2: Hints for Exploring Efficient Therapeutic Targets to Treat COVID-19. Angiology 2021, 72, 122–130. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.; Zhao, Y.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Young, D.; Coupland, C.; Channon, K.M.; Tan, P.S.; Harrison, D.A.; Rowan, K.; Aveyard, P.; Pavord, I.D.; Watkinson, P.J. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart 2020, 106, 1503–1511. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Li, Y.; Zhang, L.; Wang, Y.; Yang, S.; Xiao, X.; Qin, Q. The use of renin-angiotensin-aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1370–1377. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ho, J.H.; Adam, S.; France, M.; Syed, A.; Neely, D.; Rees, A.; Khatib, R.; Cegla, J.; Byrne, C.; et al. Heart UK’s Medical Scientific and Research Committee (2020). Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: An expert panel position statement from HEART UK. Atherosclerosis 2020, 313, 126–136. [Google Scholar] [CrossRef]
- Phadke, M.; Saunik, S. COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev. Res. 2020, 81, 541–543. [Google Scholar] [CrossRef] [Green Version]
- Reiner, Ž.; Hatamipour, M.; Banach, M.; Pirro, M.; Al-Rasadi, K.; Jamialahmadi, T.; Radenkovic, D.; Montecucco, F.; Sahebkar, A. Statins and the COVID-19 main protease: In silico evidence on direct interaction. Arch. Med. Sci. 2020, 16, 490–496. [Google Scholar] [CrossRef]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Hilgenfeld, R.; Drag, M. Substrate specificity profiling of SARS-CoV-2 Mpro protease provides basis for anti-COVID-19 drug design. BioRxiv 2020, 981928. [Google Scholar] [CrossRef]
- Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Zapata-Builes, W.; Guerra-Sandoval, A.L.; Guerra-Almonacid, C.M.; Hincapié-García, J.; Rugeles, M.T.; Hernandez, J.C. Atorvastatin Effectively Inhibits Ancestral and Two Emerging Variants of SARS-CoV-2 in vitro. Front Microbiol. 2022, 13, 721103. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Mohammadi, S.M.; Banach, M.; Beni, F.H.; Guest, P.C.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. Improved COVID-19 Outcomes following Statin Therapy: An Updated Systematic Review and Meta-analysis. Biomed. Res. Int. 2021, 2021, 1901772. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, A.; Niedzielski, M.; Gorzelak-Pabiś, P.; Broncel, M.; Woźniak, E. COVID-19: Direct and Indirect Mechanisms of Statins. Int. J. Mol. Sci. 2021, 22, 4177. [Google Scholar] [CrossRef]
- Durazzo, A.; Sorkin, B.C.; Lucarini, M.; Gusev, P.A.; Kuszak, A.J.; Crawford, C.; Boyd, C.; Deuster, P.A.; Saldanha, L.G.; Gurley, B.J.; et al. Analytical Challenges and Metrological Approaches to Ensuring Dietary Supplement Quality: International Perspectives. Front. Pharmacol. 2022, 12, 714434. [Google Scholar] [CrossRef]
- Islam, M.T.; Quispe, C.; Martorell, M.; Docea, A.O.; Salehi, B.; Calina, D.; Reiner, Ž.; Sharifi-Rad, J. Dietary supplements, vitamins and minerals as potential interventions against viruses: Perspectives for COVID-19. Int. J. Vitam. Nutr. Res. 2022, 92, 49–66. [Google Scholar] [CrossRef]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- Zuin, M.; Rigatelli, G.; Zuliani, G.; Bilato, C.; Zonzin, P.; Roncon, L. Incidence and mortality risk in coronavirus disease 2019 patients complicated by acute cardiac injury: Systematic review and meta-analysis. J. Cardiovasc. Med. 2020, 21, 759–764. [Google Scholar] [CrossRef]
- Santoso, A.; Pranata, R.; Wibowo, A.; Al-Farabi, M.J.; Huang, I.; Antariksa, B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am. J. Emerg. Med. 2021, 44, 352–357. [Google Scholar] [CrossRef]
- Rashid, M.; Wu, J.; Timmis, A.; Curzen, N.; Clarke, S.; Zaman, A.; Nolan, J.; Shoaib, A.; Mohamed, M.O.; de Belder, M.A.; et al. Outcomes of COVID-19-positive acute coronary syndrome patients: A multisource electronic healthcare records study from England. J. Intern. Med. 2021, 290, 88–100. [Google Scholar] [CrossRef]
- Rodriguez-Leor, O.; Cid Alvarez, A.B.; Pérez de Prado, A.; Rossello, X.; Ojeda, S.; Serrador, A.; López-Palop, R.; Martin-Moreiras, J.; Rumoroso, J.R.; Cequier, A.; et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention 2021, 16, 1426–1433. [Google Scholar] [CrossRef]
- Gluckman, T.J.; Wilson, M.A.; Chiu, S.T.; Penny, B.W.; Chepuri, V.B.; Waggoner, J.W.; Spinelli, K.J. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2020, 5, 1419–1424. [Google Scholar] [CrossRef]
- Mafham, M.M.; Spata, E.; Goldacre, R.; Gair, D.; Curnow, P.; Bray, M.; Hollings, S.; Roebuck, C.; Gale, C.P.; Mamas, M.A.; et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020, 396, 381–389. [Google Scholar] [CrossRef]
- Xiang, D.; Xiang, X.; Zhang, W.; Yi, S.; Zhang, J.; Gu, X.; Xu, Y.; Huang, K.; Su, X.; Yu, B.; et al. Management and Outcomes of PatientsWith STEMI During the COVID-19Pandemic in China. J. Am. Coll. Cardiol. 2020, 76, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Reboldi, G.; Stracci, F.; Pedretti, R.F.E.; Valente, S.; Gensini, G.; Gibson, C.M.; Ambrosio, G. Worldwide differences of hospitalization for ST-segment elevation myocardial infarction during COVID-19: A systematic review and meta-analysis. Int. J. Cardiol. 2022, 347, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Karcher, A.K.; Zahn, R.; Zeymer, U. Collateral damage of COVID-19-lockdown in Germany:Decline of NSTE-ACS admissions. Clin. Res. Cardiol. 2020, 109, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Kennedy, K.F.; Imran, H.; Louis, D.W.; Shippey, E.; Poppas, A.; Wood, K.E.; Abbott, J.D.; Aronow, H.D. Association Between COVID-19 Diagnosis and In-Hospital Mortality in Patients Hospitalized with ST-Segment Elevation Myocardial Infarction. JAMA 2021, 326, 1940–1952. [Google Scholar] [CrossRef]
- He, L.; Lu, F.; Du, X.; Long, D.; Sang, C.; Tang, R.; Dong, J.; Guo, M.; Ma, C. Impact of COVID-19 Pandemic on Hospital Admissions of Acute Coronary Syndrome: A Beijing Inpatient Database Study. Lancet Reg. Health West. Pac. 2022, 19, 100335. [Google Scholar] [CrossRef]
- Aldujeli, A.; Hamadeh, A.; Briedis, K.; Tecson, K.M.; Rutland, J.; Krivickas, Z.; Stiklioraitis, S.; Briede, K.; Aldujeili, M.; Unikas, R.; et al. Delays in Presentation in Patients With Acute Myocardial Infarction During the COVID-19 Pandemic. Cardiol. Res. 2020, 11, 386–391. [Google Scholar] [CrossRef]
- Garcia, S.; Stanberry, L.; Schmidt, C.; Sharkey, S.; Megaly, M.; Albaghdadi, M.S.; Meraj, P.M.; Garberich, R.; Jaffer, F.A.; Stefanescu Schmidt, A.C.; et al. Impact of COVID-19 pandemic on STEMI care: An expanded analysis from the United States. Catheter. Cardiovasc. Interv. 2021, 98, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Gale, C.P.; Kinnaird, T.; Curzen, N.; Ludman, P.; Kontopantelis, E.; Wu, J.; Denwood, T.; Fazal, N.; Deanfield, J.; et al. Impact of COVID-19 on percutaneous coronary intervention for ST-elevation myocardial infarction. Heart 2020, 106, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasica, R.; Djukanovic, L.; Mrdovic, I.; Savic, L.; Ristic, A.; Zdravkovic, M.; Simic, D.; Krljanac, G.; Popovic, D.; Simeunovic, D.; et al. Acute Coronary Syndrome in the COVID-19 Era—Differences and Dilemmas Compared to the Pre-COVID-19 Era. J. Clin. Med. 2022, 11, 3024. https://doi.org/10.3390/jcm11113024
Lasica R, Djukanovic L, Mrdovic I, Savic L, Ristic A, Zdravkovic M, Simic D, Krljanac G, Popovic D, Simeunovic D, et al. Acute Coronary Syndrome in the COVID-19 Era—Differences and Dilemmas Compared to the Pre-COVID-19 Era. Journal of Clinical Medicine. 2022; 11(11):3024. https://doi.org/10.3390/jcm11113024
Chicago/Turabian StyleLasica, Ratko, Lazar Djukanovic, Igor Mrdovic, Lidija Savic, Arsen Ristic, Marija Zdravkovic, Dragan Simic, Gordana Krljanac, Dejana Popovic, Dejan Simeunovic, and et al. 2022. "Acute Coronary Syndrome in the COVID-19 Era—Differences and Dilemmas Compared to the Pre-COVID-19 Era" Journal of Clinical Medicine 11, no. 11: 3024. https://doi.org/10.3390/jcm11113024
APA StyleLasica, R., Djukanovic, L., Mrdovic, I., Savic, L., Ristic, A., Zdravkovic, M., Simic, D., Krljanac, G., Popovic, D., Simeunovic, D., Rajic, D., & Asanin, M. (2022). Acute Coronary Syndrome in the COVID-19 Era—Differences and Dilemmas Compared to the Pre-COVID-19 Era. Journal of Clinical Medicine, 11(11), 3024. https://doi.org/10.3390/jcm11113024







