Paravalvular Leak Echo Imaging before and during the Percutaneous Procedure
Abstract
:Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, C.E.; Hahn, R.T.; Berrebi, A.; Borer, J.S.; Cutlip, D.E.; Fontana, G.; Gerosa, G.; Ibrahim, R.; Jelnin, V.; Jilaihawi, H.; et al. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis: An Expert Statement. J. Am. Coll. Cardiol. 2017, 69, 2067–2087. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 431–475. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Hahn, R.T.; Weissman, N.J.; Monaghan, M.J. Assessment of paravalvular regurgitation following TAVR: A proposal of unifying grading scheme. JACC Cardiovasc. Imaging 2015, 8, 340–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pysz, P.; Kozłowski, M.; Malczewska, M.; Adamczyk-Filipek, E.; Wojakowski, W.; Smolka, G. Prospective registry validating the reproducibility of mitral paravalvular leak measurements in a standardized real-time three-dimensional transesophageal echocardiography algorithm for optimal choice of the closure device. Postep. Kardiol. Interwencyjnej 2019, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Smolka, G.; Pysz, P.; Kozłowski, M.; Jasiński, M.; Gocoł, R.; Roleder, T.; Kargul, A.; Ochała, A.; Wojakowski, W. Transcatheter closure of paravalvular leaks using a paravalvular leak device—A prospective Polish registry. Postep. Kardiol. Interwencyjnej 2016, 12, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolka, G.; Pysz, P.; Jasiński, M.; Roleder, T.; Peszek-Przybyła, E.; Ochała, A.; Wojakowski, W. Multiplug paravalvular leak closure using Amplatzer Vascular Plugs III: A prospective registry. Catheter. Cardiovasc. Interv. 2016, 87, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Perez, M.; Cruz-González, I.; Gil-Ortega, M.V.; Rasco, A.G.; Sánchez, P.L. Photo-Realistic Echocardiography Imaging During Percutaneous Paravalvular Leak Closure. JACC Cardiovasc. Interv. 2020, 13, e185–e187. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, N.C.; Beigel, R.; Ho, S.Y.; Nietlispach, F.; Cheng, R.; Agricola, E.; Siegel, R.J. Imaging for Mitral Interventions: Methods and Efficacy. JACC Cardiovasc. Imaging 2018, 11, 872–901. [Google Scholar] [CrossRef] [PubMed]
- Hascoet, S.; Smolka, G.; Bagate, F.; Guihaire, J.; Potier, A.; Hadeed, K.; Lavie-Badie, Y.; Bouvaist, H.; Dauphin, C.; Bauer, F.; et al. Multimodality imaging guidance for percutaneous paravalvular leak closure: Insights from the multi-centre FFPP register. Arch. Cardiovasc. Dis. 2018, 111, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Cao, J.; Newton, J.D.; Wilson, N.; Daniels, M.; Ormerod, O.J. Paravalvular leak closure under intracardiac echocardiographic guidance. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 91, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Basman, C.; Parmar, Y.J.; Kronzon, I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr. Cardiol. Rep. 2017, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Zorinas, A.; Zakarkaitė, D.; Janušauskas, V.; Austys, D.; Puodžiukaitė, L.; Zuozienė, G.; Samalavičius, R.S.; Jovaišienė, I.; Davidavičius, G.; Ručinskas, K.; et al. Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures. J. Clin. Med. 2022, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.W.; Rihal, C.S.; Reeder, G.S.; Nishimura, R.A.; Eleid, M.F. Acute invasive hemodynamic effects of percutaneous mitral paravalvular leak closure. Catheter. Cardiovasc. Interv. 2017, 90, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Smolka, G.; Pysz, P.; Ochała, A.; Kozłowski, M.; Zasada, W.; Parma, Z.; Tendera, M.; Wojakowski, W. Transcatheter paravalvular leak closure and hemolysis—A prospective registry. Arch. Med. Sci. 2017, 13, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Millan, X.; Skaf, S.; Joseph, L.; Ruiz, C.; García, E.; Smolka, G.; Noble, S.; Cruz-Gonzalez, I.; Arzamendi, D.; Serra, A.; et al. Transcatheter reduction of paravalvular leaks: A systematic review and meta-analysis. Can. J. Cardiol. 2015, 31, 260–269. [Google Scholar] [CrossRef] [PubMed]



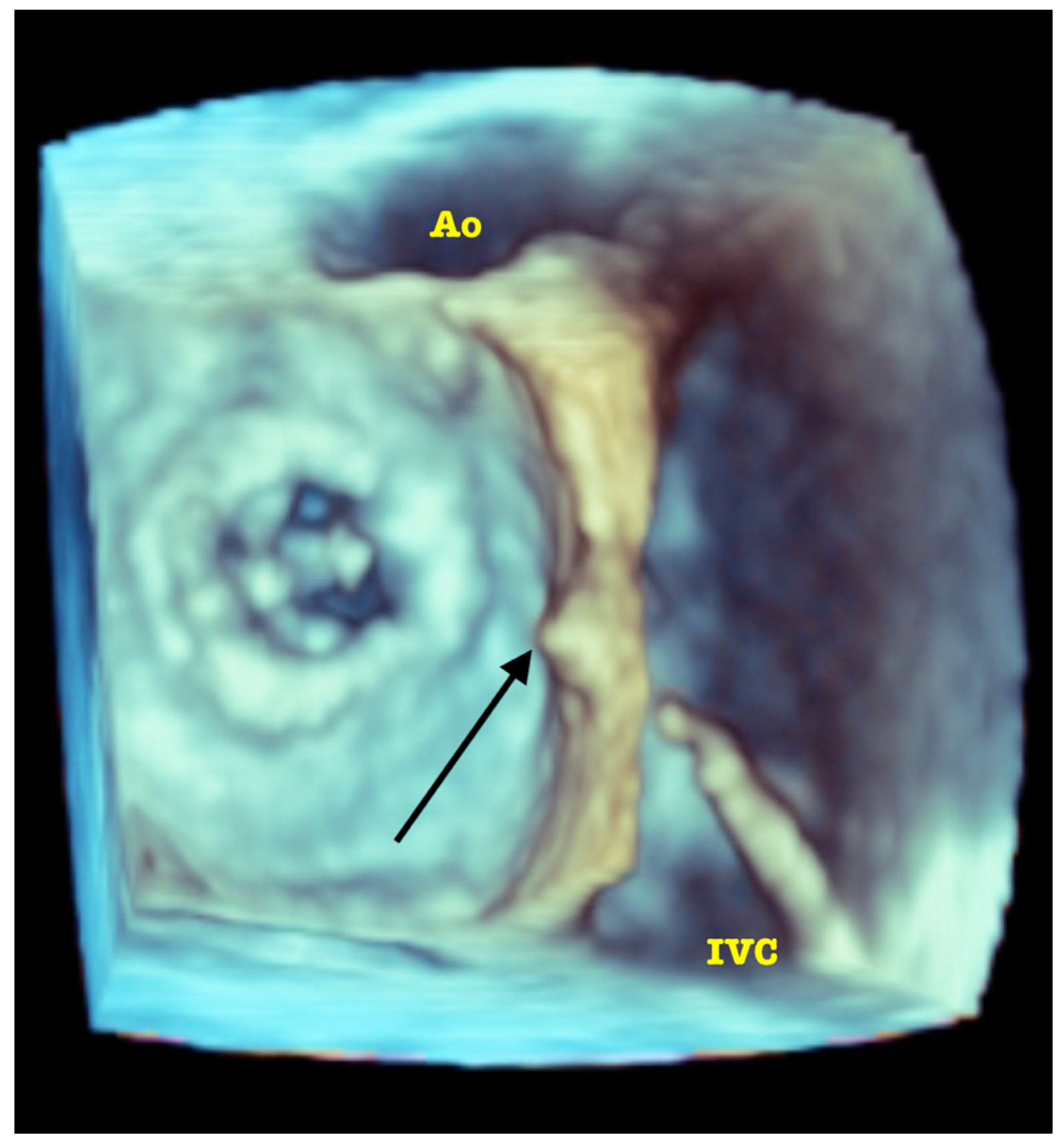
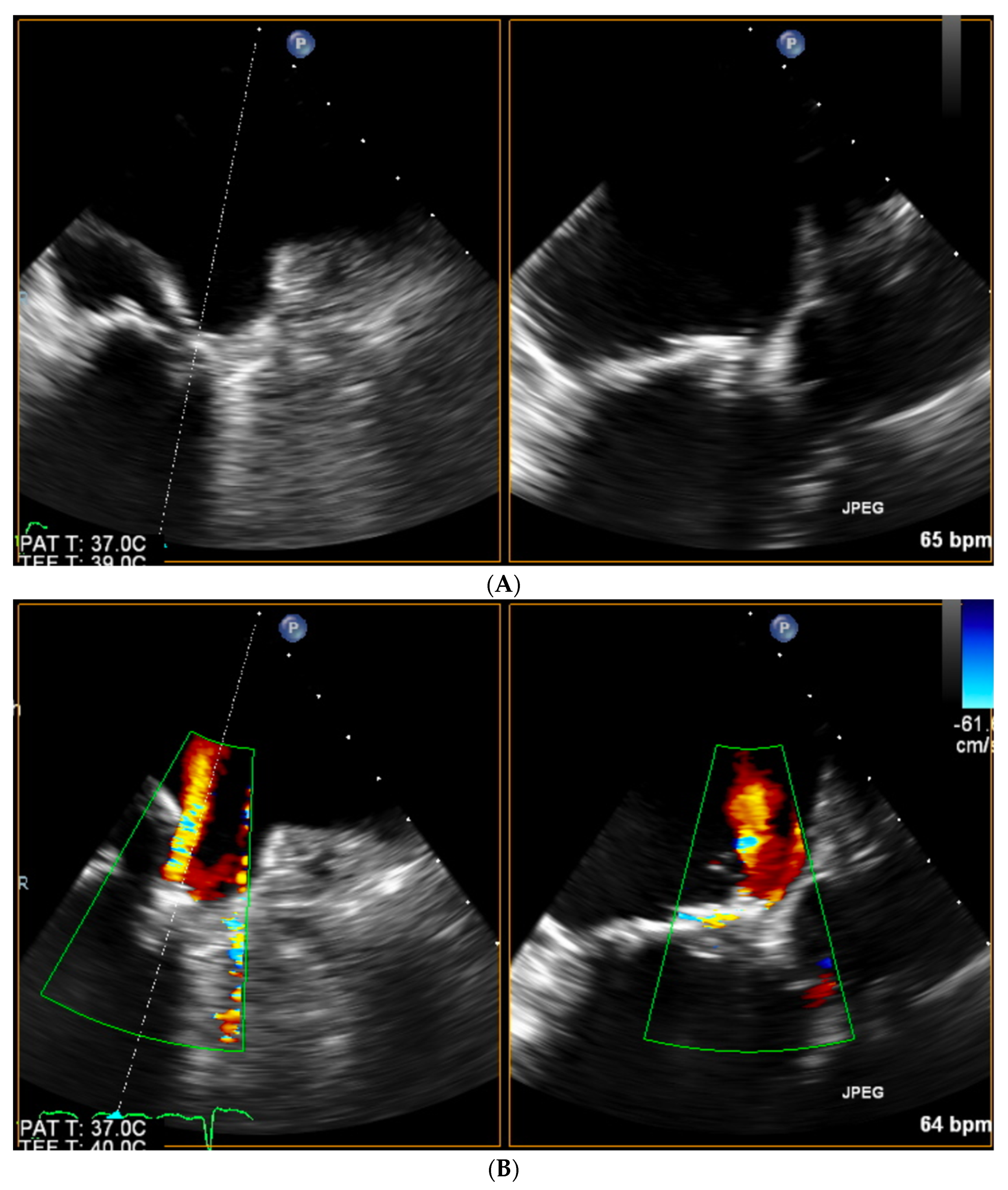
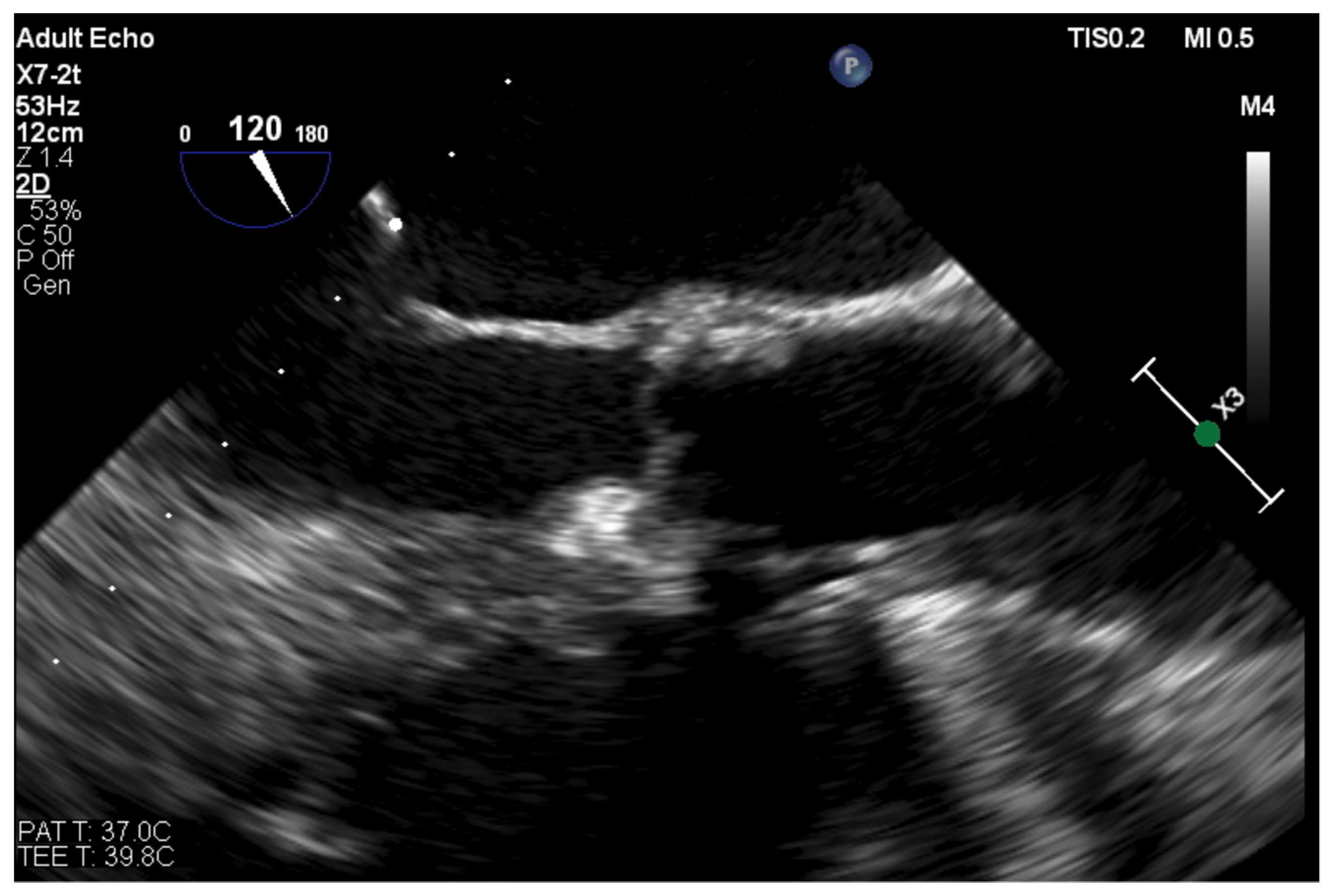
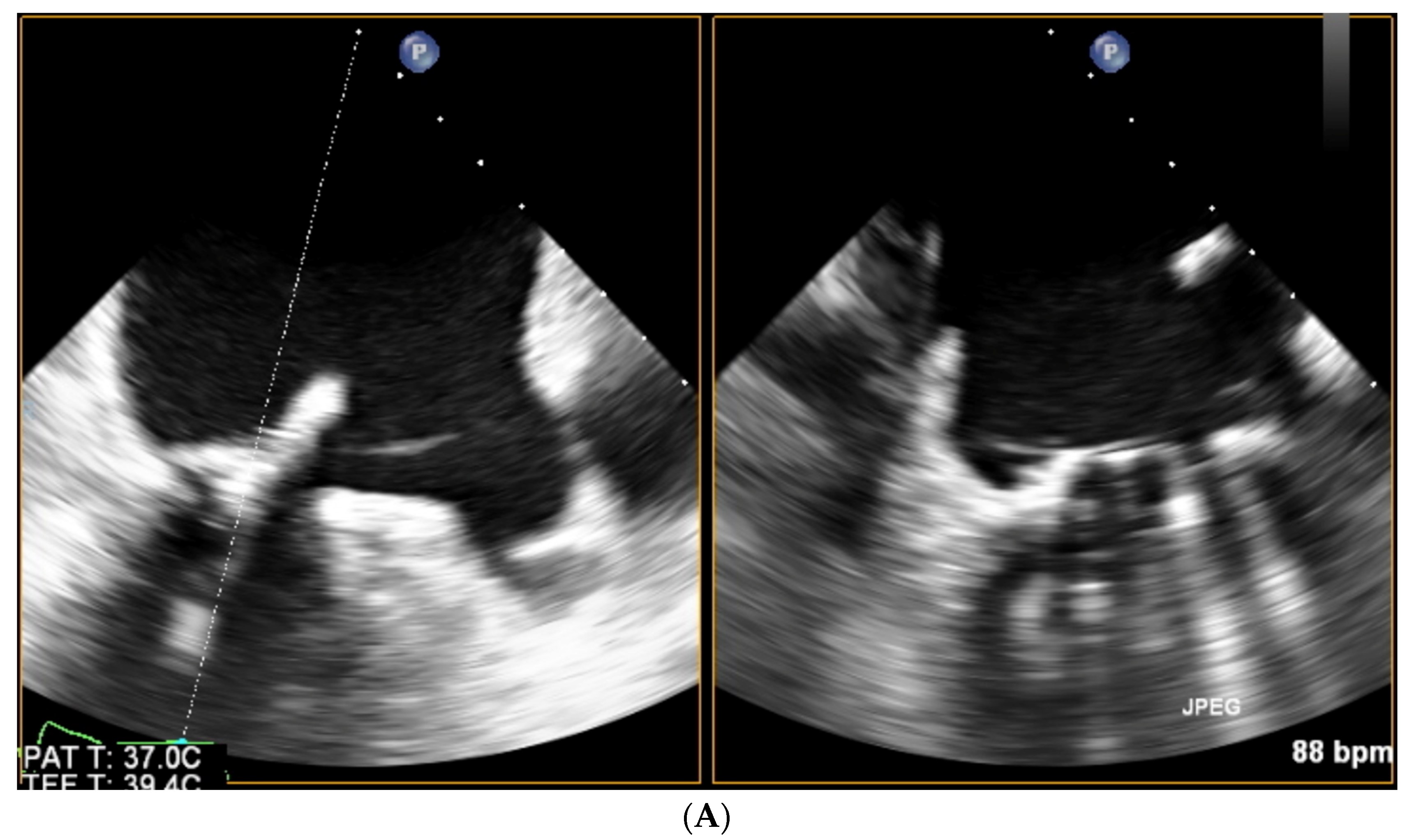

| Trace | Mild | Mild/Moderate | Moderate | Moderate/Severe | Severe | |
|---|---|---|---|---|---|---|
| Flow convergence | Absent | Absent | Absent | Possible | Usually present | Usually present |
| VC, mm | Untraceable | <2 | 2–4 | 4–5 | 5–6 | ≥6 |
| PHT, ms | >500 | >500 | 200–500 | 200–500 | 200–500 | <200 |
| Diastolic flow reversal in descending aorta | Absent | Absent/early diastolic | Intermediary | Intermediary | Holodiastolic (EDV 20–30 cm/s) | Holodiastolic (EDV ≥ 30 cm/s) |
| Circumferential extent of PVL, % | Untraceable | <5 | 5–10 | 10–20 | 20–30 | ≥30 |
| RVol, mL | <10 | <15 | 15–30 | 30–45 | 45–60 | ≥60 |
| RF, % | <15 | <15 | 15–30 | 30–40 | 40–50 | ≥50 |
| EROA, mm2 | <5 | <5 | 5–10 | 10–20 | 20–30 | ≥30 |
| Trace | Mild | Mild/Moderate | Moderate | Moderate/Severe | Severe | |
|---|---|---|---|---|---|---|
| Flow convergence | Absent | Absent/minimal | Absent/minimal | Intermediary | Intermediary | Large |
| VC, mm | Untraceable | <2 | 2–3 | 3–5 | 5–7 | ≥7 |
| Circumferential extent of PVL, % | Untraceable | <5 | 5–10 | 10–20 | 20–30 | ≥30 |
| RVol, mL | <10 | <15 | 15–30 | 30–45 | 45–60 | ≥60 |
| RF% | <15 | <15 | 15–30 | 30–40 | 40–50 | ≥50 |
| EROA mm2 | <5 | <5 | 5–20 | 20–30 | 30–40 | ≥40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pysz, P.; Wojakowski, W.; Smolka, G. Paravalvular Leak Echo Imaging before and during the Percutaneous Procedure. J. Clin. Med. 2022, 11, 3155. https://doi.org/10.3390/jcm11113155
Pysz P, Wojakowski W, Smolka G. Paravalvular Leak Echo Imaging before and during the Percutaneous Procedure. Journal of Clinical Medicine. 2022; 11(11):3155. https://doi.org/10.3390/jcm11113155
Chicago/Turabian StylePysz, Piotr, Wojtek Wojakowski, and Grzegorz Smolka. 2022. "Paravalvular Leak Echo Imaging before and during the Percutaneous Procedure" Journal of Clinical Medicine 11, no. 11: 3155. https://doi.org/10.3390/jcm11113155
APA StylePysz, P., Wojakowski, W., & Smolka, G. (2022). Paravalvular Leak Echo Imaging before and during the Percutaneous Procedure. Journal of Clinical Medicine, 11(11), 3155. https://doi.org/10.3390/jcm11113155





