Durable Mechanical Circulatory Support in Adult Congenital Heart Disease: Reviewing Clinical Considerations and Experience
Abstract
:1. Introduction
General Considerations
2. Experience with Durable Mechanical Circulatory Support in ACHD Patients
3. ACHD-Specific Anatomic Considerations
3.1. Congenital Heart Disease Associated with Systemic Right Ventricle
3.2. Durable Mechanical Circulatory Support Considerations in Systemic Right Ventricles
3.3. Single Ventricle Circulations
3.4. Durable Mechanical Circulatory Support Considerations in Single Ventricle Circulations
4. Temporary Mechanical Circulatory Support
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.S.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014, 130, 749–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomer, A.C.; Vaartjes, I.; van der Velde, E.T.; de Jong, H.M.; Konings, T.C.; Wagenaar, L.J.; Heesen, W.F.; Eerens, F.; Baur, L.H.; Grobbee, D.; et al. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int. J. Cardiol. 2013, 168, 2487–2493. [Google Scholar] [CrossRef] [PubMed]
- Cedars, A.; Benjamin, L.; Vyhmeister, R.; Harris, K.; Bradley, E.A.; Wadia, S.; Awad, A.J.; Novak, E. Contemporary Hospitalization Rate Among Adults with Complex Congenital Heart Disease. World J. Pediatric Congenit. Heart Surg. 2016, 7, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Verheugt, C.L.; Uiterwaal, C.S.; van der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; van Dijk, A.P.; Vliegen, H.W.; Grobbee, D.E.; Grobbee, B.J. Mortality in adult congenital heart disease. Eur. Heart J. 2010, 31, 1220–1229. [Google Scholar] [CrossRef] [Green Version]
- Marelli, A.J.; Mackie, A.S.; Ionescu-Ittu, R.; Rahme, E.; Pilote, L. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation 2007, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.F.; Chung, K.; Anderson, A.S.; Siegler, M.; Huang, E.S.; Churpek, M.M. Practice Changes at U.S. Transplant Centers After the New Adult Heart Allocation Policy. J. Am. Coll. Cardiol. 2020, 75, 2906–2916. [Google Scholar] [CrossRef]
- Alshawabkeh, L.I.; Hu, N.; Carter, K.; Opotowsky, A.R.; Light-McGroary, K.; Cavanaugh, J.E.; Bartlett, H.L. Wait-List Outcomes for Adults With Congenital Heart Disease Listed for Heart Transplantation in the U.S. J. Am. Coll. Cardiol. 2016, 68, 908–917. [Google Scholar] [CrossRef]
- Karamlou, T.; Hirsch, J.; Welke, K.; Ohye, R.G.; Bove, E.L.; Devaney, E.J.; Gajarski, R.J. A United Network for Organ Sharing analysis of heart transplantation in adults with congenital heart disease: Outcomes and factors associated with mortality and retrans-plantation. J. Thorac. Cardiovasc. Surg. 2010, 140, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Mylotte, D.; Pilote, L.; Ionescu-Ittu, R.; Abrahamowicz, M.; Khairy, P.; Therrien, J.; Mackie, A.S.; Marelli, A. Specialized adult congenital heart disease care: The impact of policy on mortality. Circulation 2014, 129, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e81–e192. [Google Scholar] [CrossRef]
- Hosseinpour, A.R.; Cullen, S.; Tsang, V.T. Transplantation for adults with congenital heart disease. Eur. J. Cardio-Thorac. Surg. 2006, 30, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.J.; Law, Y.; Book, W.M.; Broberg, C.S.; Burchill, L.; Cecchin, F.; Chen, J.M.; Delgado, D.; Dimopoulos, K.; Everitt, M.D.; et al. Transplantation and mechanical circulatory support in congenital heart disease: A scientific statement from the American Heart Association. Circulation 2016, 133, 802–820. [Google Scholar] [CrossRef] [PubMed]
- Warnes, C.A. The Adult with Congenital Heart Disease: Born to Be Bad? J. Am. Coll. Cardiol. 2005, 46, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Givertz, M.M.; DeFilippis, E.M.; Landzberg, M.J.; Pinney, S.P.; Woods, R.K.; Valente, A.M. Advanced heart failure therapies for adults with congenital heart disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 2295–22312. [Google Scholar] [CrossRef]
- VanderPluym, C.J.; Cedars, A.; Eghtesady, P.; Maxwell, B.G.; Gelow, J.M.; Burchill, L.J.; Maltais, S.; Koehl, D.A.; Cantor, R.S.; Blume, E.D. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: An analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J. Heart Lung Transplant. 2018, 37, 89–99. [Google Scholar] [CrossRef]
- Steiner, J.M.; Krieger, E.V.; Stout, K.K.; Stempien-Otero, A.; Mahr, C.; Mokadam, N.A.; Hermsen, J.L. Durable mechanical circulatory support in teenagers and adults with congenital heart disease: A systematic review. Int. J. Cardiol. 2017, 245, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Jacob, K.A.; Hjortnaes, J.; Kranenburg, G.; de Heer, F.; Kluin, J. Mortality after cardiac surgery in patients with liver cirrhosis clas-sified by the Child-Pugh score. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.R.; Lam, W.W.; Rodriguez, F.H., III; Ermis, P.R.; Simpson, L.; Frazier, O.H.; Franklin, W.J.; Parekh, D.R. Clinical outcomes after ventricular assist device implantation in adults with complex congenital heart disease. J. Heart Lung Transplant. 2013, 32, 615–620. [Google Scholar] [CrossRef]
- Cedars, A.; Vanderpluym, C.; Koehl, D.; Cantor, R.; Kutty, S.; Kirklin, J.K. An Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of hospitalization, functional status, and mortality after mechanical circulatory support in adults with congenital heart disease. J. Heart Lung Transplant. 2018, 37, 619–630. [Google Scholar] [CrossRef]
- Brickner, M.E.; Hillis, L.D.; Lange, R.A. Congenital heart disease in adults. N. Engl. J. Med. 2000, 342, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Mustard, W.T.; Keith, J.D.; Trusler, G.A.; Fowler, R.; Kidd, L. The surgical management of transposition of the great vessels. J. Thorac. Cardiovasc. Surg. 1964, 48, 953–958. [Google Scholar] [CrossRef]
- Vejlstrup, N.; Sørensen, K.; Mattsson, E.; Thilén, U.; Kvidal, P.; Johansson, B.; Iversen, K.; Søndergaard, L.; Dellborg, M.; Eriksson, P. Long-Term Outcome of Mustard/Senning Correction for Transposition of the Great Arteries in Sweden and Denmark. Circulation 2015, 132, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brida, M.; Diller, G.-P.; Gatzoulis, M.A. Response by Brida et al to Letter Regarding Article, “Systemic Right Ventricle in Adults with Congenital Heart Disease: Anatomic and Phenotypic Spectrum and Current Approach to Management”. Circulation 2018, 138, 326–327. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.A.; Del Nido, P.J.; Vasilyev, N.V. Management of systemic right ventricular failure in patients with congenitally cor-rected transposition of the great arteries. Circulation 2016, 134, 1293–1302. [Google Scholar] [CrossRef]
- Joyce, D.L.; Crow, S.S.; John, R.; Louis, J.D.; Braunlin, E.A.; Pyles, L.A.; Kofflin, P.; Joyce, L.D. Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J. Heart Lung Transplant. 2010, 29, 1302–1305. [Google Scholar] [CrossRef]
- Roche, S.L.; Crossland, D.S.; Adachi, I.; Broda, C.; Jansen, K.; Hickey, E. Mechanical Circulatory Support for the Failing Sub-Aortic Right Ventricle in Adults. Semin. Thorac. Cardiovasc. Surg. Pediatric Card. Surg. Annu. 2021, 24, 2–9. [Google Scholar] [CrossRef]
- Serfas, J.D.; Patel, P.A.; Krasuski, R.A. Heart Transplantation and Mechanical Circulatory Support in Adults with Congenital Heart Disease. Curr. Cardiol. Rep. 2018, 20, 81. [Google Scholar] [CrossRef]
- O’Connor, M.J.; Lorts, A.; Davies, R.R.; Fynn-Thompson, F.; Joong, A.; Maeda, K.; Mascio, C.E.; McConnell, P.I.; Mongé, M.C.; Nandi, D.; et al. Early experience with the HeartMate 3 continuous-flow ventricular assist device in pediatric patients and patients with congenital heart disease: A multicenter registry analysis. J. Heart Lung Transplant. 2020, 39, 573–579. [Google Scholar] [CrossRef]
- Dennis, M.; Zannino, D.; Du Plessis, K.; Bullock, A.; Disney, P.J.; Radford, D.J.; Hornung, T.; Grigg, L.; Cordina, R.; D’Udekem, Y.; et al. Clinical Outcomes in Adolescents and Adults After the Fontan Procedure. J. Am. Coll. Cardiol. 2018, 71, 1009–1017. [Google Scholar] [CrossRef]
- Frescura, C.; Thiene, G. The new concept of univentricular heart. Front. Pediatrics 2014, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Redington, A. The physiology of the Fontan circulation. Prog. Pediatric Cardiol. 2006, 22, 179–186. [Google Scholar] [CrossRef]
- Gewillig, M.; Eyskens, B.; Heying, R.; Ganame, J.; La Gerche, A.; Brown, S.C.; Budts, W.; Gorenflo, M. The Fontan circulation: Who controls cardiac output? Interact. Cardiovasc. Thorac. Surg. 2010, 10, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemler, M.S.; Scott, W.A.; Leonard, S.R.; Stromberg, D.; Ramaciotti, C. Fenestration improves clinical outcome of the Fontan proce-dure: A prospective, randomized study. Circulation 2002, 105, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Book, W.M.; Gerardin, J.; Saraf, A.; Marie Valente, A.; Rodriguez, F., III. Clinical Phenotypes of Fontan Failure: Implications for Management. Congenit. Heart Dis. 2016, 11, 296–308. [Google Scholar] [CrossRef]
- Villa, C.R.; Lorts, A.; Morales, D.L. Ventricular assist device therapy in the Fontan circulation. In Seminars in Thoracic and Cardio-Vascular Surgery: Pediatric Cardiac Surgery Annual; WB Saunders: London, UK, 2021; Volume 24, pp. 19–25. [Google Scholar]
- Moore, R.A.; Madueme, P.C.; Lorts, A.; Morales, D.L.; Taylor, M.D. Virtual implantation evaluation of the total artificial heart and compatibility: Beyond standard fit criteria. J. Heart Lung Transplant. 2014, 33, 1180–1183. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.A.; Lorts, A.; Madueme, P.C.; Taylor, M.D.; Morales, D.L. Virtual implantation of the 50cc SynCardia total artificial heart. J. Heart Lung Transplant. 2016, 35, 824–827. [Google Scholar] [CrossRef]
- Farooqi, K.M.; Saeed, O.; Zaidi, A.; Sanz, J.; Nielsen, J.; Hsu, D.T.; Jorde, U.P. 3D Printing to Guide Ventricular Assist Device Placement in Adults with Congenital Heart Disease and Heart Failure. JACC Heart Fail. 2016, 4, 301–311. [Google Scholar] [CrossRef]
- Ali, L.A.; Cadoni, A.; Rossi, G.; Keilberg, P.; Passino, C.; Festa, P. Effective Cardiac Index and Systemic-Pulmonary Collaterals Evaluated by Cardiac Magnetic Resonance Late After Fontan Palliation. Am. J. Cardiol. 2017, 119, 2069–2072. [Google Scholar] [CrossRef]
- Smith, M.; El-Said, H.; Pretorius, V.; Mendenhall, M.; Thomas, T.; Reeves, R.R.; Enciso, J.S.; Alshawabkeh, L.; Nigro, J.; Adler, E.D.; et al. Significance of Aortopulmonary Collaterals in a Single-Ventricle Patient Supported with a HeartMate 3. Circ. Heart Fail. 2020, 13, e006473. [Google Scholar] [CrossRef]
- Wells, D.A.; Coghill, M.; Szugye, N.; Moore, R.; Lorts, A.; Tweddell, J.S.; Morales, D.L. Transplantation and Arch Repair in Fontan 3 Years after HeartMate 3: Technical Considerations. Ann. Thorac. Surg. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J. Protein-losing enteropathy after Fontan operation. Congenit. Heart Dis. 2007, 2, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Santiago, E.; Téllez, L.; Garrido-Lestache Rodriguez-Monte, E.; Garrido-Gómez, E.; Aguilera-Castro, L.; Álvarez-Fuente, M.; Del Cerro, M.J.; Albillos, A.; Romera, R.; Olavarria, A.; et al. Fontan protein-losing enteropathy is associated with advanced liver disease and a proinflammatory intestinal and systemic state. Liver Int. 2020, 40, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Cedars, A.M.; Schumacher, K.; Kindel, S.; Lorts, A.; Rosenthal, D.; Chen, S.; Morales, D.; Peng, D.; O’Connor, M.; Simpson, K.E.; et al. The Fontan VAD Physiology Project (FVPP). J. Heart Lung Transplant. 2020, 39, S12–S13. [Google Scholar] [CrossRef]
- Cedars, A.; Kutty, S.; Danford, D.; Schumacher, K.; Auerbach, S.R.; Bearl, D.; Chen, S.; Conway, J.; Dykes, J.C.; Jaworski, N.; et al. Systemic ventricular assist device support in Fontan patients: A report by ACTION. J. Heart Lung Transplant. 2021, 40, 368–376. [Google Scholar] [CrossRef]
- Broda, C.R.; Taylor, D.A.; Adachi, I. Progress in experimental and clinical subpulmonary assistance for Fontan circulation. J. Thorac. Cardiovasc. Surg. 2018, 156, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Prêtre, R.; Häussler, A.; Bettex, D.; Genoni, M. Right-Sided Univentricular Cardiac Assistance in a Failing Fontan Circulation. Ann. Thorac. Surg. 2008, 86, 1018–1020. [Google Scholar] [CrossRef]
- Rodefeld, M.D.; Marsden, A.; Figliola, R.; Jonas, T.; Neary, M.; Giridharan, G.A. Cavopulmonary Assist: Long-Term Reversal of the Fontan Paradox. J. Thorac. Cardiovasc. Surg. 2019, 158, 1627–1636. [Google Scholar] [CrossRef]
- Berlin Heart [Internet]. Medical Professionals EXCOR Venous Cannula. Available online: https://www.berlinheart.de/en/medical-professionals/excorr-venous-cannula/ (accessed on 22 March 2022).
- Broda, C.; Smith, P.A.; Wang, Y.; Sampaio, L.C.; Adachi, I.; Taylor, D.A. Construction and Evaluation of a Bio-Engineered Pump to Enable Subpulmonary Support of the Fontan Circulation: A Proof-of-Concept Study. J. Heart Lung Transplant. 2020, 39, S176. [Google Scholar] [CrossRef]
- Acheampong, B.; Johnson, J.N.; Stulak, J.M.; Dearani, J.A.; Kushwaha, S.S.; Daly, R.C.; Haile, D.T.; Schears, G.J. Postcardiotomy ECMO Support after High-risk Operations in Adult Congenital Heart Disease. Congenit. Heart Dis. 2016, 11, 751–755. [Google Scholar] [CrossRef]
- Morray, B.H.; Dimas, V.V.; Lim, S.; Balzer, D.T.; Parekh, D.R.; Van Mieghem, N.M.; Ewert, P.; Kim, D.W.; Justino, H.; McElhinney, D.B.; et al. Circulatory support using the impella device in fontan patients with systemic ventricular dysfunction: A multicenter ex-perience. Catheter. Cardiovasc. Interv. 2017, 90, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Fishberger, S.B.; Asnes, J.D.; Rollinson, N.L.; Cleman, M.W. Percutaneous right ventricular support during catheter ablation of intraatrial reentrant tachycardia in an adult with a mustard baffle—A novel use of the Impella device. J. Interv. Card. Electro.-Physiol. 2010, 29, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.C.; Kodavatiganti, R. Mechanical Support with Impella During Malignant Arrhythmia Ablation: A Case Report on the Growing Trend in the Electrophysiology Laboratory. A&A Case Rep. 2017, 8, 282–285. [Google Scholar]
- White, C.W.; Ganapathi, A.; Schroder, J. A Minimally Invasive Approach to HeartMate 3 Implantation for Systemic Ventricular Failure Following the Mustard Procedure for Transposition of the Great Arteries. ASAIO J. 2020, 66, e62–e63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Vaikunth, S.S.; Haeffele, C.; MacArthur, J.W. Extracorporeal membrane oxygenator as a bridge to heart–liver en bloc transplant in a patient with Fontan circulation. JTCVS Tech. 2022, 12, 171–174. [Google Scholar] [CrossRef] [PubMed]
- García Gómez, M.; Uribarri, A.; San Román Calvar, J.A.; Stepanenko, A. Clinical use of percutaneous mechanical circulatory as-sistance in a patient with end-stage right-sided heart failure and massive tricuspid insufficiency due to congenital heart disease: First-in-the-world case report. Eur. Heart J. Case Rep. 2021, 5, ytab269. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, V.D.; Alshami, A.; Patel, I.; Douedi, S.; Quinlan, A.; Thomas, T.; Prentice, J.; Calderon, D.; Asif, A.; Sen, S.; et al. Outcomes of Renal Function in Cardiogenic Shock Patients with or Without Mechanical Circulatory Support. J. Clin. Med. Res. 2021, 13, 283–292. [Google Scholar] [CrossRef]
- Kingsford, P.; Onwuzurike, J.; Li, J.; Bradley, C.; Vaidya, A.; Wolfson, A.; DePasquale, E. Impact of the UNOS Policy Allocation Change on Waitlist Outcomes in Patients Bridged to Heart Transplantation on Impella. J. Heart Lung Transplant. 2021, 40, S268. [Google Scholar] [CrossRef]
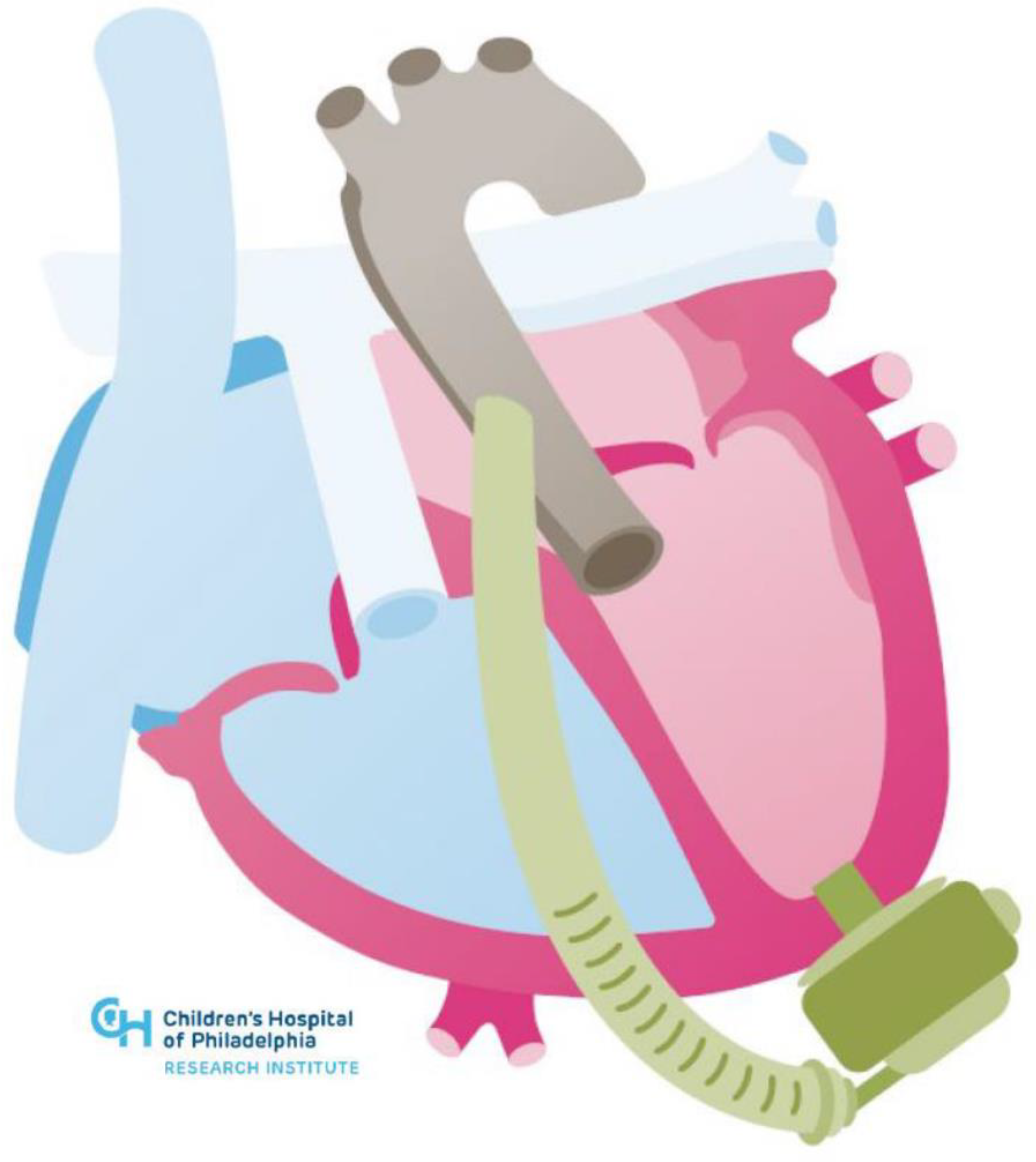
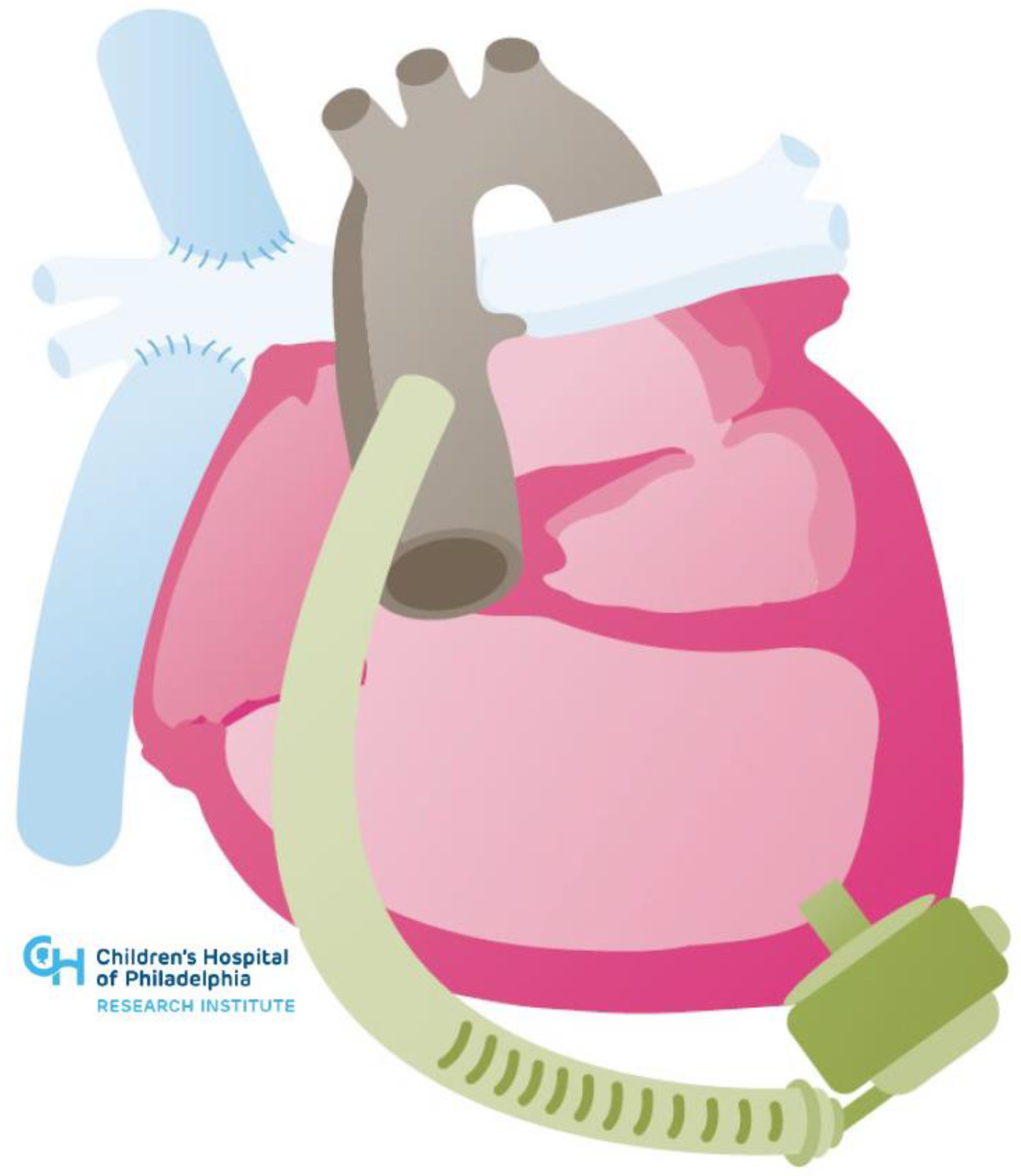

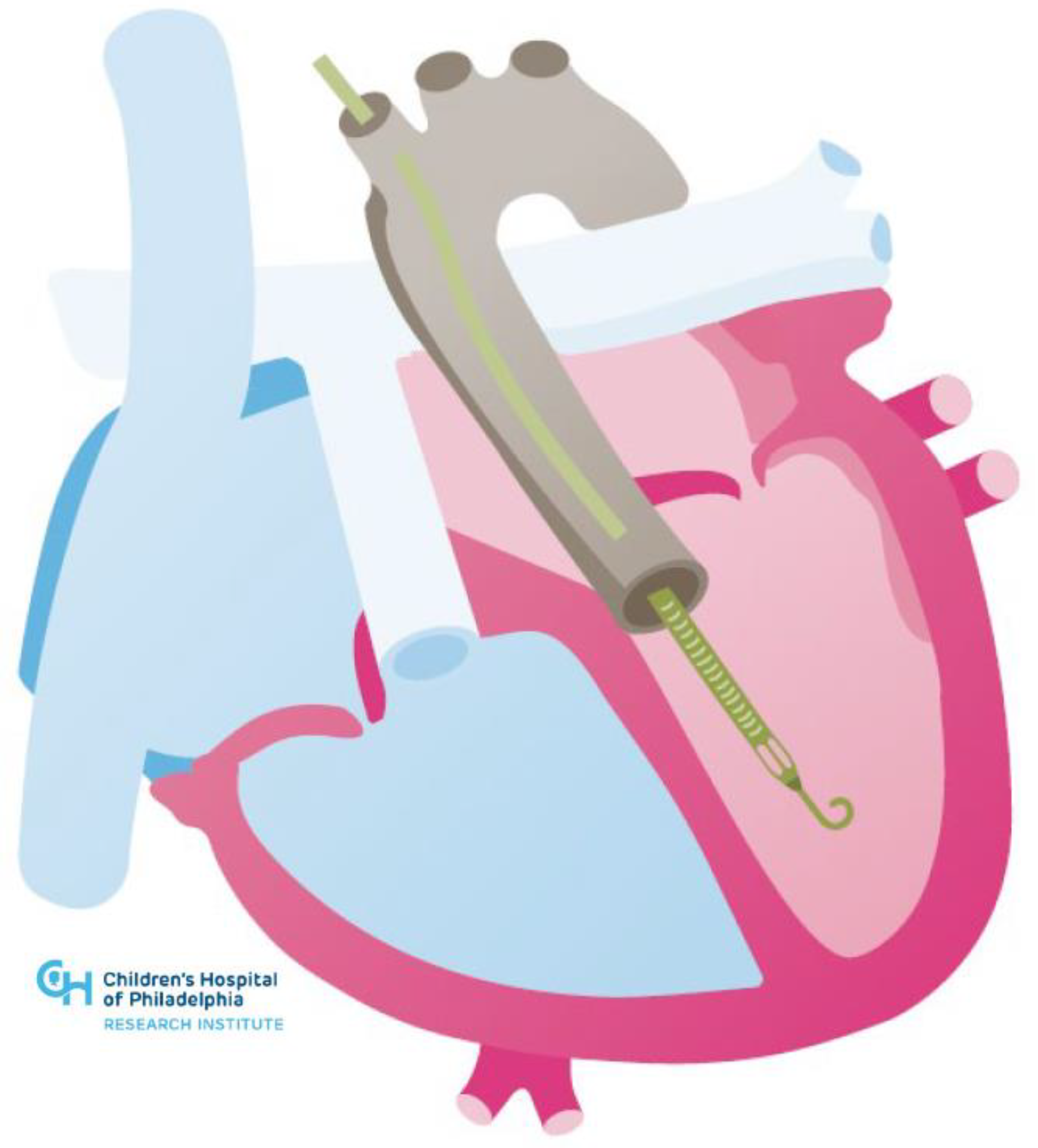
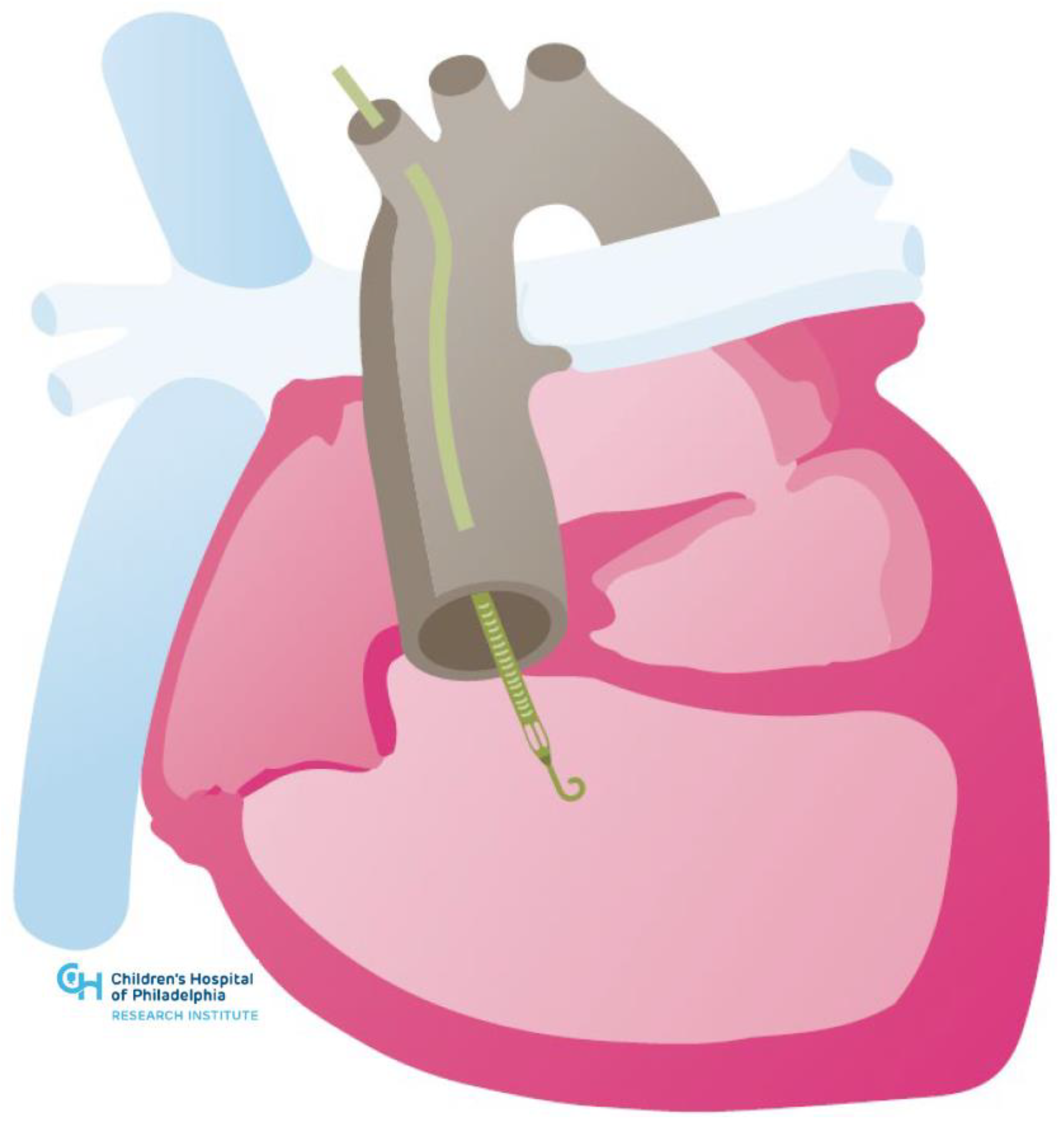
| Systemic Right Ventricle | Single Ventricle | |
|---|---|---|
| Cardiac Conditions | Dextro-Transposition of the Great Arteries or Congenitally corrected Transposition of the Great Arteries | Complex Congenital Heart Disease not amenable to biventricular repair (e.g., Hypoplastic Left Heart Syndrome, Tricuspid Atresia, Double Inlet Left Ventricle, Unbalanced Atrioventricular Septal Defect) |
| Technical Challenges | Inflow Cannula Placement (e.g., trabeculations, papillary muscles) Concomitant Need for Atrioventricular Valve Surgery or Baffle Revision Abnormal Ventricular Looping Creating Geometric Challenges Scarring and Fibrosis from Prior Sternotomies Achieving Adequate Separation from Ventricular Septum Avoiding Compression of Intrathoracic or Epigastric Structures with Sternotomy Closure | Inflow Cannula Placement (e.g., trabeculations, papillary muscles) Concomitant Need for Atrioventricular Valve Surgery or Fenestration Bleeding Risk (e.g., aortopulmonary or veno-venous collaterals) Scarring and Fibrosis from Multiple Prior Sternotomies Neo-Aortic Pathology Device Fit |
| Management Challenges | Potential for Inflow Obstruction due to Right Ventricular Anatomic and Geometric Features | Preload Dependency due to Cavopulmonary Flow Comorbidities (e.g., Fontan-associated liver disease) |
| Device Potential | Ventricular Assist Devices | Ventricular Assist Devices and Cavopulmonary Assist Devices |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saef, J.; Montgomery, R.; Cedars, A.; Tang, W.H.W.; Rossano, J.W.; Maeda, K.; Kim, Y.Y.; Vaikunth, S.S. Durable Mechanical Circulatory Support in Adult Congenital Heart Disease: Reviewing Clinical Considerations and Experience. J. Clin. Med. 2022, 11, 3200. https://doi.org/10.3390/jcm11113200
Saef J, Montgomery R, Cedars A, Tang WHW, Rossano JW, Maeda K, Kim YY, Vaikunth SS. Durable Mechanical Circulatory Support in Adult Congenital Heart Disease: Reviewing Clinical Considerations and Experience. Journal of Clinical Medicine. 2022; 11(11):3200. https://doi.org/10.3390/jcm11113200
Chicago/Turabian StyleSaef, Joshua, Robert Montgomery, Ari Cedars, Wai H. Wilson Tang, Joseph W. Rossano, Katsuhide Maeda, Yuli Y. Kim, and Sumeet S. Vaikunth. 2022. "Durable Mechanical Circulatory Support in Adult Congenital Heart Disease: Reviewing Clinical Considerations and Experience" Journal of Clinical Medicine 11, no. 11: 3200. https://doi.org/10.3390/jcm11113200






