Non-Invasive Estimation of Right Atrial Pressure Using a Semi-Automated Echocardiographic Tool for Inferior Vena Cava Edge-Tracking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Data
2.2. Processing of Ultrasound Scans with Edge-Tracking Technique
2.3. Estimation of Right Atrial Pressure
2.4. Statistical Analysis
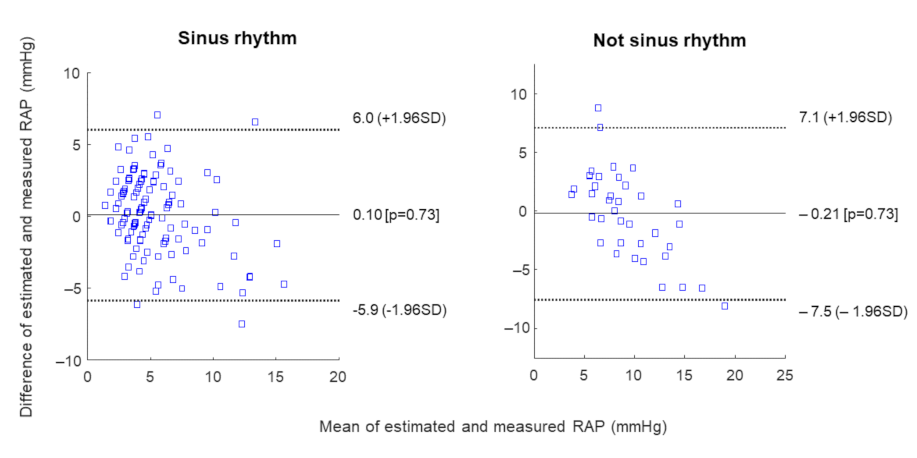
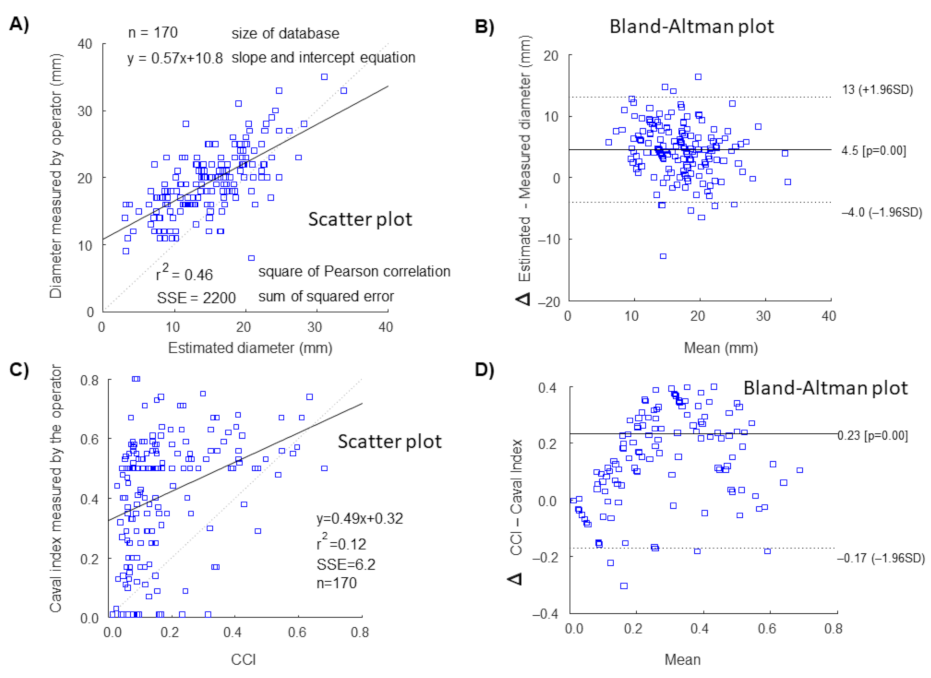
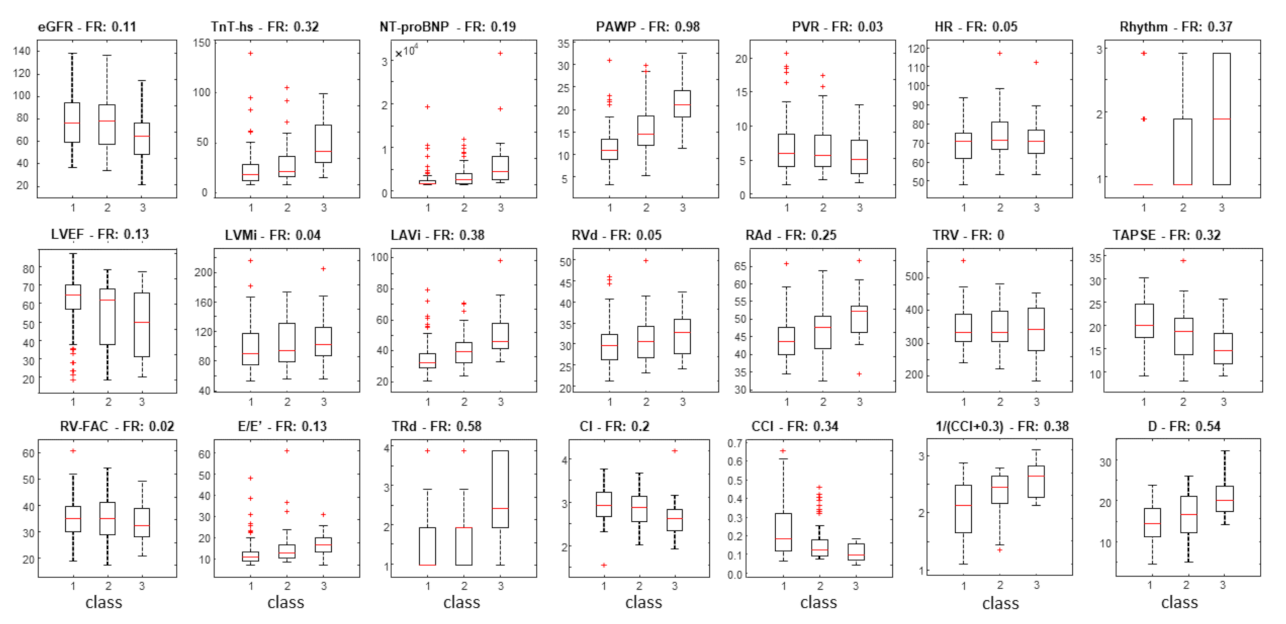
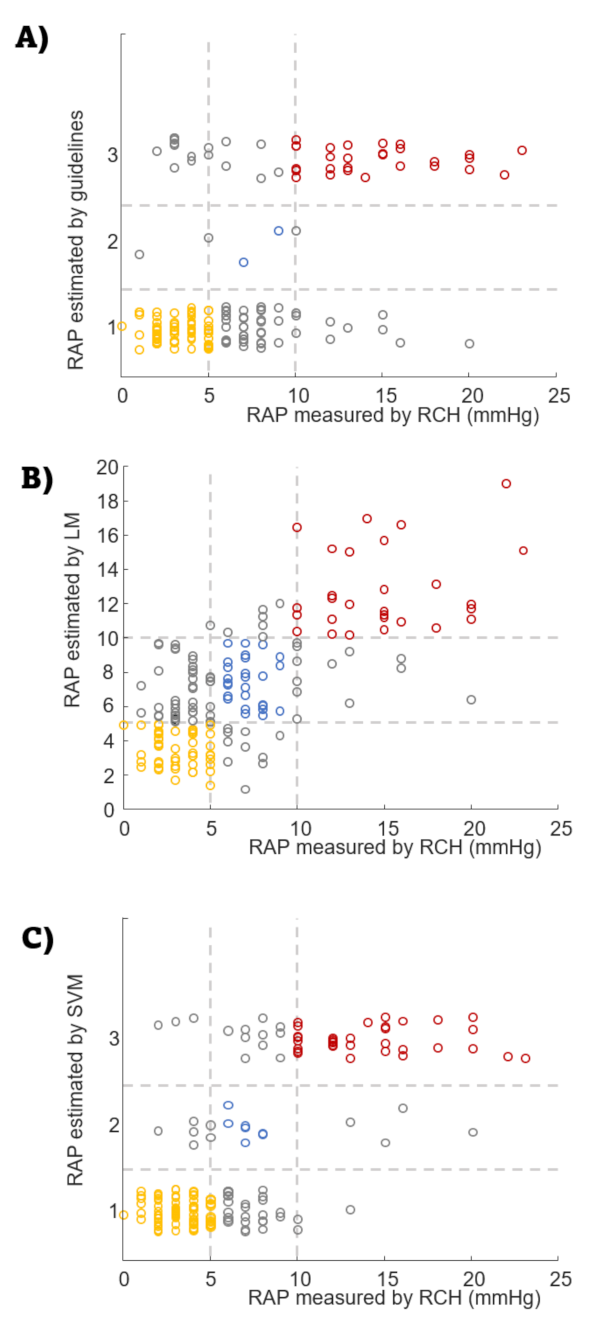
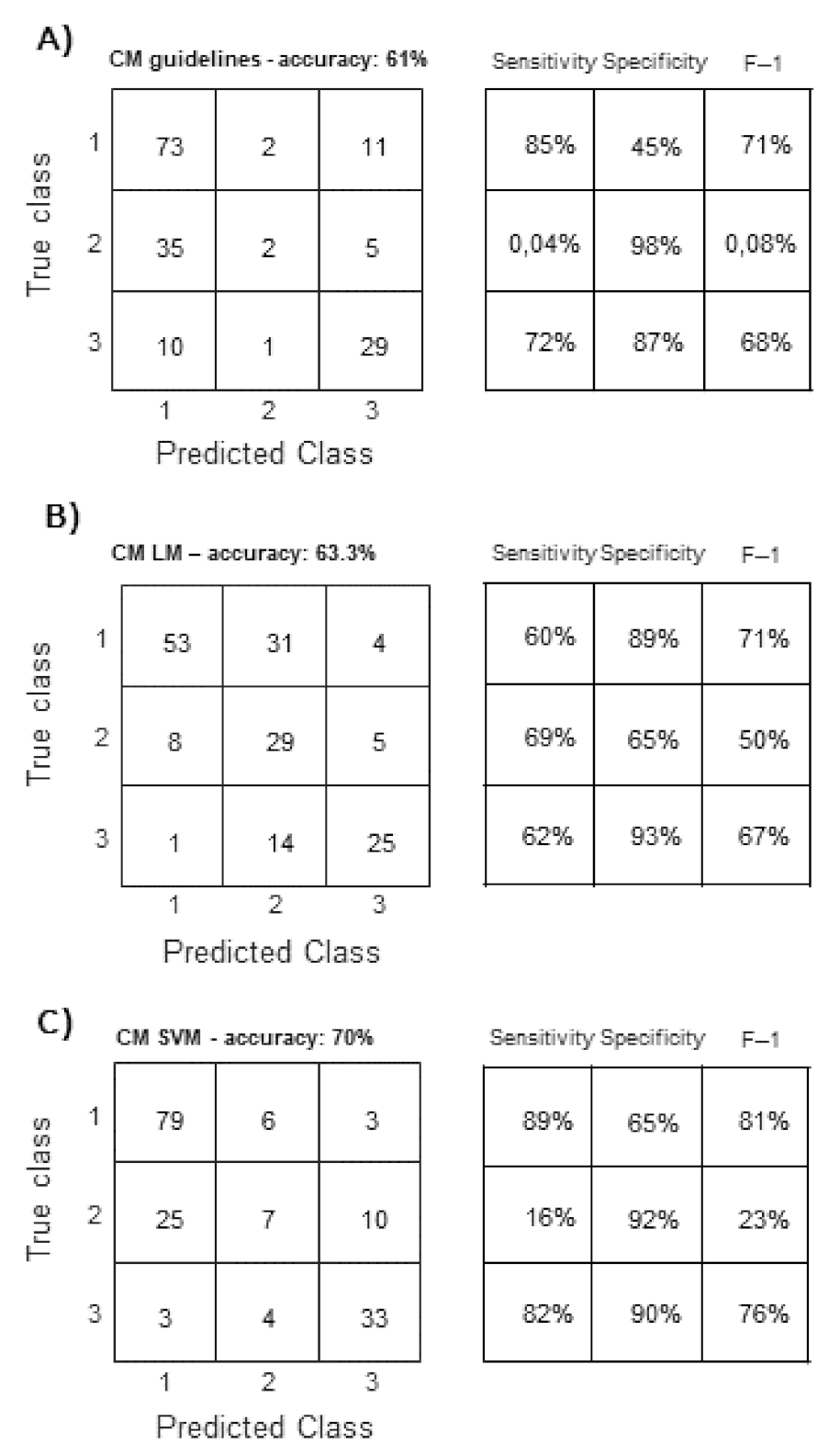
3. Results
4. Discussion
Study Limitations
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Cardiac index |
| CCI | Cardiac caval index |
| eGFR | Estimate glomerular filtration rate |
| hs-TnT | High sensitivity troponin T |
| IVC | Inferior vena cava |
| IVCd | Inferior vena cava diameter |
| LAVI | Left atria volume index |
| LVEF | Left ventricular ejection fraction |
| LVMI | Left ventricular mass index |
| NYHA | New York Heart Association |
| NT-proBNP | Amino terminal pro brain natriuretic peptide |
| PAWP | Precapillary artery wedge pressure |
| PVR | Pulmonary vascular resistances |
| RAd | Right atrial diameter |
| RAP | Right atrial pressure |
| RVd | Right ventricular diameter |
| RV-FAC | Right ventricular fractional area change |
| TAPSE | Tricuspid annular plane excursion |
| TRd | Tricuspid regurgitation degree |
| TVR | Tricuspid regurgitation velocity |
| US | Ultrasound |
Appendix A. Echocardiographic Assessment
Appendix B. Right Heart Catheterization
Appendix C. Further Results

References
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.N.; Miyoshi, T.; Addetia, K.; Henry, M.P.; Citro, R.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; et al. Normal Values of Cardiac Output and Stroke Volume According to Measurement Technique, Age, Sex, and Ethnicity: Results of the World Alliance of Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 1077–1085.e1. [Google Scholar] [CrossRef] [PubMed]
- Beigel, R.; Cercek, B.; Arsanjani, R.; Siegel, R.J. Echocardiography in the Use of Noninvasive Hemodynamic Monitoring. J. Crit. Care 2014, 29, 184.e1–184.e8. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Yock, P.G.; Popp, R.L. Noninvasive Estimation of Right Ventricular Systolic Pressure by Doppler Ultrasound in Patients with Tricuspid Regurgitation. Circulation 1984, 70, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Sattin, M.; Burhani, Z.; Jaidka, A.; Millington, S.; Arntfield, R. Stroke Volume Determination by Echocardiography. Chest 2022. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Galderisi, M.; Edvardsen, T.; Donal, E.; Goliasch, G.; Cardim, N.; Magne, J.; Laginha, S.; Hagendorff, A.; Haland, T.F.; et al. Echo-Doppler Estimation of Left Ventricular Filling Pressure: Results of the Multicentre EACVI Euro-Filling Study. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Chubuchny, V.; Pugliese, N.R.; Taddei, C.; Poggianti, E.; Spini, V.; Barison, A.; Formichi, B.; Airò, E.; Bauleo, C.; Prediletto, R.; et al. A Novel Echocardiographic Method for Estimation of Pulmonary Artery Wedge Pressure and Pulmonary Vascular Resistance. ESC Heart Fail. 2021, 8, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.E.; Franey, L.M.; Marwick, T.; Maeder, M.T.; Kaye, D.M.; Vlahos, A.P.; Serra, W.; Al-Azizi, K.; Schiller, N.B.; Lester, S.J. Noninvasive Assessment of Pulmonary Vascular Resistance by Doppler Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 1170–1177. [Google Scholar] [CrossRef]
- Scalia, G.M.; Scalia, I.G.; Kierle, R.; Beaumont, R.; Cross, D.B.; Feenstra, J.; Burstow, D.J.; Fitzgerald, B.T.; Platts, D.G. ePLAR—The Echocardiographic Pulmonary to Left Atrial Ratio—A Novel Non-Invasive Parameter to Differentiate Pre-Capillary and Post-Capillary Pulmonary Hypertension. Int. J. Cardiol. 2016, 212, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alto, M.; Romeo, E.; Argiento, P.; Pavelescu, A.; Mélot, C.; D’Andrea, A.; Correra, A.; Bossone, E.; Calabrò, R.; Russo, M.G.; et al. Echocardiographic Prediction of Pre- versus Postcapillary Pulmonary Hypertension. J. Am. Soc. Echocardiogr. 2015, 28, 108–115. [Google Scholar] [CrossRef]
- Albani, S.; Stolfo, D.; Venkateshvaran, A.; Chubuchny, V.; Biondi, F.; De Luca, A.; Lo Giudice, F.; Pasanisi, E.M.; Petersen, C.; Airò, E.; et al. Echocardiographic Biventricular Coupling Index to Predict Precapillary Pulmonary Hypertension. J. Am. Soc. Echocardiogr. 2022. [Google Scholar] [CrossRef]
- Kircher, B.J.; Himelman, R.B.; Schiller, N.B. Noninvasive Estimation of Right Atrial Pressure from the Inspiratory Collapse of the Inferior Vena Cava. Am. J. Cardiol. 1990, 66, 493–496. [Google Scholar] [CrossRef]
- Brennan, J.M.; Blair, J.E.; Goonewardena, S.; Ronan, A.; Shah, D.; Vasaiwala, S.; Kirkpatrick, J.N.; Spencer, K.T. Reappraisal of the Use of Inferior Vena Cava for Estimating Right Atrial Pressure. J. Am. Soc. Echocardiogr. 2007, 20, 857–861. [Google Scholar] [CrossRef]
- Milan, A.; Magnino, C.; Veglio, F. Echocardiographic Indexes for the Non-Invasive Evaluation of Pulmonary Hemodynamics. J. Am. Soc. Echocardiogr. 2010, 23, 225–239. [Google Scholar] [CrossRef]
- Ruge, M.; Marhefka, G.D. IVC measurement for the noninvasive evaluation of central venous pressure. J. Echocardiogr. 2022. [Google Scholar] [CrossRef]
- Mele, D.; Pestelli, G.; Molin, D.D.; Smarrazzo, V.; Luisi, G.A.; Trevisan, F.; Fiorencis, A.; Flamigni, F.; Ferrari, R. Right Atrial Pressure Is Associated with Outcomes in Patients with Heart Failure and Indeterminate Left Ventricular Filling Pressure. J. Am. Soc. Echocardiogr. 2020, 33, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Velez-Martinez, M.; Ayers, C.R.; Reimold, S.C.; Thibodeau, J.T.; Mishkin, J.D.; Mammen, P.P.A.; Markham, D.W.; Patel, C.B. Relationship of Right- to Left-Sided Ventricular Filling Pressures in Advanced Heart Failure. Circ. Heart Fail. 2013, 6, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Cogswell, R.; Pritzker, M.; De Marco, T. Performance of the REVEAL Pulmonary Arterial Hypertension Prediction Model Using Non-Invasive and Routinely Measured Parameters. J. Heart Lung Transplant. 2014, 33, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Cleland, J.G.F.; Zhang, J.; Kallvikbacka-Bennett, A.; Urbinati, A.; Shah, P.; Kazmi, S.; Clark, A.L. Cardiac Dysfunction, Congestion and Loop Diuretics: Their Relationship to Prognosis in Heart Failure. Cardiovasc. Drugs Ther. 2016, 30, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Mesin, L.; Albani, S.; Policastro, P.; Pasquero, P.; Porta, M.; Melchiorri, C.; Leonardi, G.; Albera, C.; Scacciatella, P.; Pellicori, P.; et al. Assessment of Phasic Changes of Vascular Size by Automated Edge Tracking-State of the Art and Clinical Perspectives. Front. Cardiovasc. Med. 2022, 8, 775635. [Google Scholar] [CrossRef] [PubMed]
- Albani, S.; Mesin, L.; Roatta, S.; De Luca, A.; Giannoni, A.; Stolfo, D.; Biava, L.; Bonino, C.; Contu, L.; Pelloni, E.; et al. Inferior Vena Cava Edge Tracking Echocardiography: A Promising Tool with Applications in Multiple Clinical Settings. Diagnostics 2022, 12, 427. [Google Scholar] [CrossRef]
- Mesin, L.; Pasquero, P.; Roatta, S. Tracking and Monitoring Pulsatility of a Portion of Inferior Vena Cava from Ultrasound Imaging in Long Axis. Ultrasound Med. Biol. 2019, 45, 1338–1343. [Google Scholar] [CrossRef]
- Mesin, L.; Pasquero, P.; Roatta, S. Multi-directional Assessment of Respiratory and Cardiac Pulsatility of the Inferior Vena Cava from Ultrasound Imaging in Short Axis. Ultrasound Med. Biol. 2020, 46, 3475–3482. [Google Scholar] [CrossRef]
- Mesin, L.; Pasquero, P.; Roatta, S.; Porta, M. Automated Volume Status Assessment Using Inferior Vena Cava Pulsatility. Electronics 2020, 9, 1671. [Google Scholar] [CrossRef]
- Albani, S.; Pinamonti, B.; Giovinazzo, T.; de Scordilli, M.; Fabris, E.; Stolfo, D.; Perkan, A.; Gregorio, C.; Barbati, G.; Geri, P.; et al. Accuracy of right atrial pressure estimation using a multi-parameter approach derived from inferior vena cava semi-automated edge-tracking echocardiography: A pilot study in patients with cardiovascular disorders. Int. J. Cardiovasc. Imaging 2020, 36, 1213–1225. [Google Scholar] [CrossRef]
- Mesin, L.; Albani, S.; Sinagra, G. Non-invasive Estimation of Right Atrial Pressure using the Pulsatility of Inferior Vena Cava. Ultrasound Med. Biol. 2019, 45, 1331–1337. [Google Scholar] [CrossRef]
- Patel, M.R.; Bailey, S.R.; Bonow, R.O.; Chambers, C.E.; Chan, P.S.; Dehmer, G.J.; Kirtane, A.J.; Wann, L.S.; Ward, R.P. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 Appropriate Use Criteria for Diagnostic Catheterization: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 59, 1995–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, F.L.L.; Hagan, A.D.; Holmen, J.R.; Pryor, T.A.; Strickland, R.D.; Castle, C.H. Evaluation of Size and Dynamics of the Inferior Vena Cava as an Index of Right-Sided Cardiac Function. Am. J. Cardiol. 1984, 53, 579–585. [Google Scholar] [CrossRef]
- Mesin, L.; Giovinazzo, T.; D’Alessandro, S.; Roatta, S.; Raviolo, A.; Chiacchiarini, F.; Porta, M.; Pasquero, P. Improved repeatability of the estimation of pulsatility of inferior vena cava. Ultrasound Med. Biol. 2019, 45, 2830–2843. [Google Scholar] [CrossRef]
- Ermini, L.; Seddone, S.; Policastro, P.; Mesin, L.; Pasquero, P.; Roatta, S. The Cardiac Caval Index: Improving Noninvasive Assessment of Cardiac Preload. J. Ultrasound Med. 2021. [Google Scholar] [CrossRef]
- de Scordilli, M.; Pinamonti, B.; Albani, S.; Gregorio, C.; Barbati, G.; Daneluzzi, C.; Korcova, R.; Perkan, A.; Fabris, E.; Geri, P.; et al. Reliability of Noninvasive Hemodynamic Assessment with Doppler Echocardiography: Comparison with the Invasive Evaluation. J. Cardiovasc. Med. 2019, 20, 1. [Google Scholar] [CrossRef]
- Anavekar, N.S.; Oh, J.K. Doppler Echocardiography: A Contemporary Review. J. Cardiol. 2009, 54, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Magnino, C.; Omedè, P.; Avenatti, E.; Presutti, D.; Iannaccone, A.; Chiarlo, M.; Moretti, C.; Gaita, F.; Veglio, F.; Milan, A.; et al. Inaccuracy of Right Atrial Pressure Estimates Through Inferior Vena Cava Indices. Am. J. Cardiol. 2017, 120, 1667–1673. [Google Scholar] [CrossRef]
- Sonoo, T.; Nakamura, K.; Ando, T.; Sen, K.; Maeda, A.; Kobayashi, E.; Sakuma, I.; Doi, K.; Nakajima, S.; Yahagi, N. Prospective analysis of cardiac collapsibility of inferior vena cava using ultrasonography. J. Crit. Care 2015, 30, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Pasquero, P.; Albani, S.; Porta, M.; Roatta, S. Semi-automated tracking and continuous monitoring of inferior vena cava diameter in simulated and experimental ultrasound imaging. Ultrasound Med. Biol. 2015, 41, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Blehar, D.; Resop, D.; Chin, B.; Dayno, M.; Gaspari, R. Inferior vena cava displacement during respirophasic ultrasound imaging. Crit. Ultrasound J. 2012, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Folino, A.; Benzo, M.; Pasquero, P.; Laguzzi, A.; Mesin, L.; Messere, A.; Porta, M.; Roatta, S. Vena Cava Responsiveness to Controlled Isovolumetric Respiratory Efforts. J. Ultrasound Med. 2017, 36, 2113–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Tomida, M.; Ando, T.; Sen, K.; Inokuchi, R.; Kobayashi, E.; Nakajima, S.; Sakuma, I.; Yahagi, N. Cardiac variation of inferior vena cava: New concept in the evaluation of intravascular blood volume. J. Med. Ultrason. 2013, 40, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Goldhammer, E.; Mesnick, N.; Abinader, E.G.; Sagiv, M. Dilated inferior vena cava: A common echocardiographic finding in highly trained elite athletes. J. Am. Soc. Echocardiogr. 1999, 12, 988–993. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Squara, P. Non-Invasive Monitoring of Cardiac Output in Critical Care Medicine. Front. Med. 2017, 4, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellicori, P.; Platz, E.; Dauw, J.; Ter Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.F.; McMurray, J.J.V.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712. [Google Scholar] [CrossRef]

| Feature | Abbreviation | Number of Missing Values |
|---|---|---|
| Rhythm | ||
| Heart rate | HR | 1 |
| Rhythm (3 classes: sinus rhythm/atrial fibrillation/pacing) | Rhythm | 0 |
| Left chambers | ||
| Left ventricular ejection fraction | LVEF | 0 |
| Cardiac index | CI | 6 |
| Left ventricular mass index | LVMi | 2 |
| E/E | E/E | 0 |
| Pulmonary artery wedge pressure | PAWP | 0 |
| Left atrial volume index | LAVi | 1 |
| Pulmonary circulation | ||
| Pulmonary vascular resistance | PVR | 0 |
| Tricuspid regurgitation velocity | TRV | 0 |
| Right chambers | ||
| Right atrial diameter | RAd | 0 |
| Right ventricular diameter | RVd | 1 |
| Tricuspid annular plane excursion | TAPSE | 3 |
| RV fractional area change | RV-FAC | 0 |
| Tricuspid regurgitation degree | TRd | 0 |
| Cardiac caval index | CCI | 0 |
| Mean IVC diametr | IVCd | 0 |
| Biomarkers | ||
| Amino-terminal pro-B-type natriuretic peptide | NT-proBNP | 2 |
| Troponin-T (high sensitivity) TnT-hs | TnT-hs | 4 |
| Estimated Glomerular Filtration Rate | eGFR | 2 |
| Age (years) | 64 ± 14 |
|---|---|
| Gender | |
| Female (%) | 55.3 |
| Male (%) | 44.7 |
| Clinical referral for RHC | |
| Pulmonary arterial hypertension (%) | 42 |
| Worsening/acute heart failure (%) | 25 |
| Evaluation of dyspnea of new onset (%) | 22 |
| Valvular heart disease (%) | 4 |
| Other (%) | 7 |
| Heart failure with reduced EF (%) | 29 |
| Risk factors and comorbidities | |
| Systemic arterial hypertension | 40 |
| Diabetes | 22 |
| Dyslipidemia | 22 |
| Chronic obstructive pulmonary disease | 17 |
| Chronic kidney disease | 15 |
| NYHA class | |
| I (%) | 20 |
| II (%) | 45 |
| III (%) | 31 |
| IV (%) | 4 |
| Rhythm | |
| Heart rate | 72 ± 13 |
| Sinus rhythm (%) | 65 |
| Atrial fibrillation (%) | 28 |
| Pacing (%) | 7 |
| Left chambers | |
| LVEF (%) | 52 ± 18 |
| CI (L/min/mq) | 2.7 ± 0.7 |
| LVMI (g/mq) | 103 ± 36 |
| E/E | 12 ± 8 |
| PAWP (mmHg) | 15 ± 7 |
| LAVi (mL/mq) | 39 ± 14 |
| Pulmonary circulation | |
| PVR (w.u.) | 3.3 ± 2.2 |
| TRV (cm/s) | 336 ± 65 |
| TRd | 1.876 ± 0.864 |
| Right chambers | |
| RAd (mm) | 46 ± 8 |
| RVd (mm) | 29 ± 5 |
| TAPSE (mm) | 19 ± 6 |
| RV-FAC (%) | 35 ± 9 |
| CCI (%) | 42 ± 20 |
| IVCd (mm) | 19 ± 5 |
| Biomarkers | |
| NT-proBNP (ng/L) | 785 (231–2429) |
| hs-TnT (ng/L) | 16.34 (8.57–30.52) |
| eGFR (mL/min/1.73 mq) MDRD | 74.4 ± 26.9 |
| Serum creatinine (mg/dL) | 0.98 ± 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesin, L.; Policastro, P.; Albani, S.; Petersen, C.; Sciarrone, P.; Taddei, C.; Giannoni, A. Non-Invasive Estimation of Right Atrial Pressure Using a Semi-Automated Echocardiographic Tool for Inferior Vena Cava Edge-Tracking. J. Clin. Med. 2022, 11, 3257. https://doi.org/10.3390/jcm11123257
Mesin L, Policastro P, Albani S, Petersen C, Sciarrone P, Taddei C, Giannoni A. Non-Invasive Estimation of Right Atrial Pressure Using a Semi-Automated Echocardiographic Tool for Inferior Vena Cava Edge-Tracking. Journal of Clinical Medicine. 2022; 11(12):3257. https://doi.org/10.3390/jcm11123257
Chicago/Turabian StyleMesin, Luca, Piero Policastro, Stefano Albani, Christina Petersen, Paolo Sciarrone, Claudia Taddei, and Alberto Giannoni. 2022. "Non-Invasive Estimation of Right Atrial Pressure Using a Semi-Automated Echocardiographic Tool for Inferior Vena Cava Edge-Tracking" Journal of Clinical Medicine 11, no. 12: 3257. https://doi.org/10.3390/jcm11123257
APA StyleMesin, L., Policastro, P., Albani, S., Petersen, C., Sciarrone, P., Taddei, C., & Giannoni, A. (2022). Non-Invasive Estimation of Right Atrial Pressure Using a Semi-Automated Echocardiographic Tool for Inferior Vena Cava Edge-Tracking. Journal of Clinical Medicine, 11(12), 3257. https://doi.org/10.3390/jcm11123257







