Peritoneal Dialysis as a Renal Replacement Therapy Modality for Patients with Acute Kidney Injury
Abstract
:1. Introduction
2. Mortality Outcomes
3. Effects on Mechanical Ventilation
4. Impact on Renal Recovery
5. Ideal Prescribed Dialysis Dose
6. Infectious and Mechanical Complications
7. Cost and Economic Implications
8. Peritoneal Ultrafiltration for Refractory Heart Failure
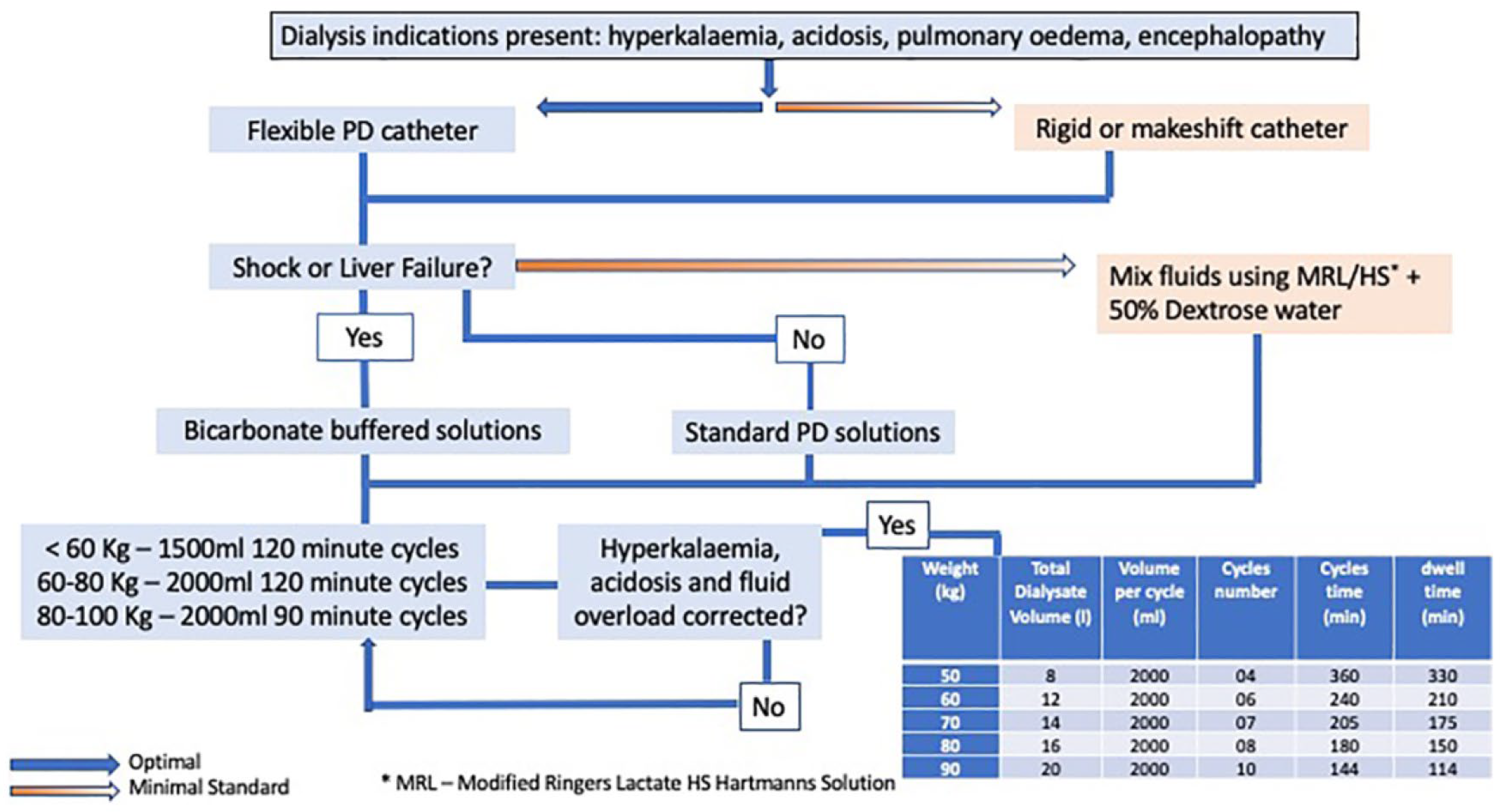
9. Acute PD Prescription
10. Acute Peritoneal Dialysis during the COVID-19 Pandemic; Experiences and Lessons Learnt
11. Future Directions
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Chionh, C.Y.; Soni, S.S.; Finkelstein, F.O.; Ronco, C.; Cruz, D.N. Use of peritoneal dialysis in AKI: A systematic review. Clin. J. Am. Soc. Nephrol. 2013, 8, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Ponce, D.; Balbi, A.; Cullis, B. Acute PD: Evidence, Guidelines, and Controversies. Semin. Nephrol. 2017, 37, 103–112. [Google Scholar] [CrossRef]
- Phu, N.H.; Hien, T.T.; Mai, N.T.; Chau, T.T.; Chuong, L.V.; Loc, P.P.; Winearls, C.; Farrar, J.; White, N.; Day, N. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N. Engl. J. Med. 2002, 347, 895–902. [Google Scholar] [CrossRef]
- George, J.; Varma, S.; Kumar, S.; Thomas, J.; Gopi, S.; Pisharody, R. Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: A pilot study. Perit. Dial. Int. 2011, 31, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Al-Hwiesh, A.; Abdul-Rahman, I.; Finkelstein, F.; Divino-Filho, J.; Qutub, H.; Al-Audah, N.; Abdelrahman, A.; El-akhrany, N.; Nasr El-Din, M.; El-Salamony, T.; et al. Acute kidney injury in critically ill patients: A prospective randomized study of tidal peritoneal dialysis versus continuous renal replacement therapy. Ther. Apher. Dial. 2018, 22, 371–379. [Google Scholar] [CrossRef]
- Gabriel, D.P.; Caramori, J.T.; Martim, L.C.; Barretti, P.; Balbi, A.L. High volume peritoneal dialysis vs daily hemodialysis: A randomized, controlled trial in patients with acute kidney injury. Kidney Int. Suppl. 2008, 73, S87–S93. [Google Scholar] [CrossRef] [Green Version]
- Ponce, D.; Berbel, M.N.; Abrão, J.M.G.; Goes, C.R.; Balbi, A.L. A randomized clinical trial of high volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. Int. Urol. Nephrol. 2013, 45, 869–878. [Google Scholar] [CrossRef]
- Almeida, C.P.; Ponce, D.; de Marchi, A.C.; Balbi, A.L. Effect of peritoneal dialysis on respiratory mechanics in acute kidney injury patients. Perit. Dial. Int. 2014, 34, 544–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, D.; Brito, G.A.; Abrão, J.G.; Balb, A.L. Different prescribed doses of high-volume peritoneal dialysis and outcome of patients with acute kidney injury. Adv. Perit. Dial. Conf. Perit. Dial. 2011, 27, 118–124. [Google Scholar]
- Cullis, B.; Al-Hwiesh, A.; Kilonzo, K.; McCulloch, M.; Niang, A.; Nourse, P.; Parapiboon, W.; Ponce, D.; Finkelstein, F.O. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (adults). Perit. Dial. Int. 2021, 41, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Jessup, M.; Brozena, S. Heart failure. N. Engl. J. Med. 2003, 348, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.V.; Musetti, C.; Ciurlino, D.; Basile, C.; Galli, E.; Gambaro, G.; Iadarola, G.; Guastoni, C.; Carlini, A.; Fasciolo, F.; et al. Peritoneal ultrafiltration in refractory heart failure: A cohort study. Perit. Dial. Int. 2014, 34, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, J.E.; Ortega, T.; Rodríguez, C.; Díaz-Molina, B.; Martín, M.; Garcia-Cueto, C.; Vidau, P.; Gago, E.; Ortega, F. Efficacy of peritoneal ultrafiltration in the treatment of refractory congestive heart failure. Nephrol. Dial. Transplant. 2010, 25, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Courivaud, C.; Kazory, A.; Crépin, T.; Azar, R.; Bresson–Vautrin, C.; Chalopin, J.M.; Ducloux, D. Peritoneal dialysis reduces the number of hospitalization days in heart failure patients refractory to diuretics. Perit. Dial. Int. 2014, 34, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Vigiola Cruz, M.; Bellorin, O.; Srivatana, V.; Afaneh, C. Safety and Efficacy of Bedside Peritoneal Dialysis Catheter Placement in the COVID-19 Era: Initial Experience at a New York City Hospital. World J. Surg. 2020, 44, 2464–2470. [Google Scholar] [CrossRef]
- Chen, W.; Caplin, N.; El Shamy, O.; Sharma, S.; Sourial, M.Y.; Ross, M.J.; Sourial, M.H.; Prudhvi, K.; Golestaneh, L.; Srivatana, V.; et al. Use of peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City: A multicenter observational study. Kidney Int. 2021, 100, 2–5. [Google Scholar] [CrossRef]
- Soomro, Q.H.; Mukherjee, V.; Amerling, R.; Caplin, N. Case series of acute peritoneal dialysis in the prone position for acute kidney injury during the Covid-19 pandemic: Prone to complications? Perit. Dial. Int. 2021, 41, 328–332. [Google Scholar] [CrossRef]
- Smoyer, W.E.; Finkelstein, F.O.; McCulloch, M.I.; Carter, M.; Brusselmans, A.; Feehally, J. “Saving Young Lives” with acute kidney injury: The challenge of acute dialysis in low-resource settings. Kidney Int. 2016, 89, 254–256. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Disadvantages |
|---|---|
| Technically simple | Contraindicated in recent abdominal surgery |
| Less infrastructure | Requires intact peritoneal cavity |
| Cost effective | May not be effective in severe acute pulmonary edema/hyperkalemia |
| Avoids vascular access | Peritonitis can occur |
| Biocompatible | Clearance and ultrafiltration unpredictable |
| Continuous renal replacement therapy | Concerns for hyperglycemia |
| Hemodynamic stability | Concerns for impaired respiratory mechanics |
| Gradual solute removal | Concerns for protein loss |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.F. Peritoneal Dialysis as a Renal Replacement Therapy Modality for Patients with Acute Kidney Injury. J. Clin. Med. 2022, 11, 3270. https://doi.org/10.3390/jcm11123270
Khan SF. Peritoneal Dialysis as a Renal Replacement Therapy Modality for Patients with Acute Kidney Injury. Journal of Clinical Medicine. 2022; 11(12):3270. https://doi.org/10.3390/jcm11123270
Chicago/Turabian StyleKhan, Sana Farooq. 2022. "Peritoneal Dialysis as a Renal Replacement Therapy Modality for Patients with Acute Kidney Injury" Journal of Clinical Medicine 11, no. 12: 3270. https://doi.org/10.3390/jcm11123270






