Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objective Olfactory Dysfunction
2.2. Olfactory Training
2.3. Olfactory Quality of Life
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features
3.2. Olfactory Training Results
3.2.1. Compliance
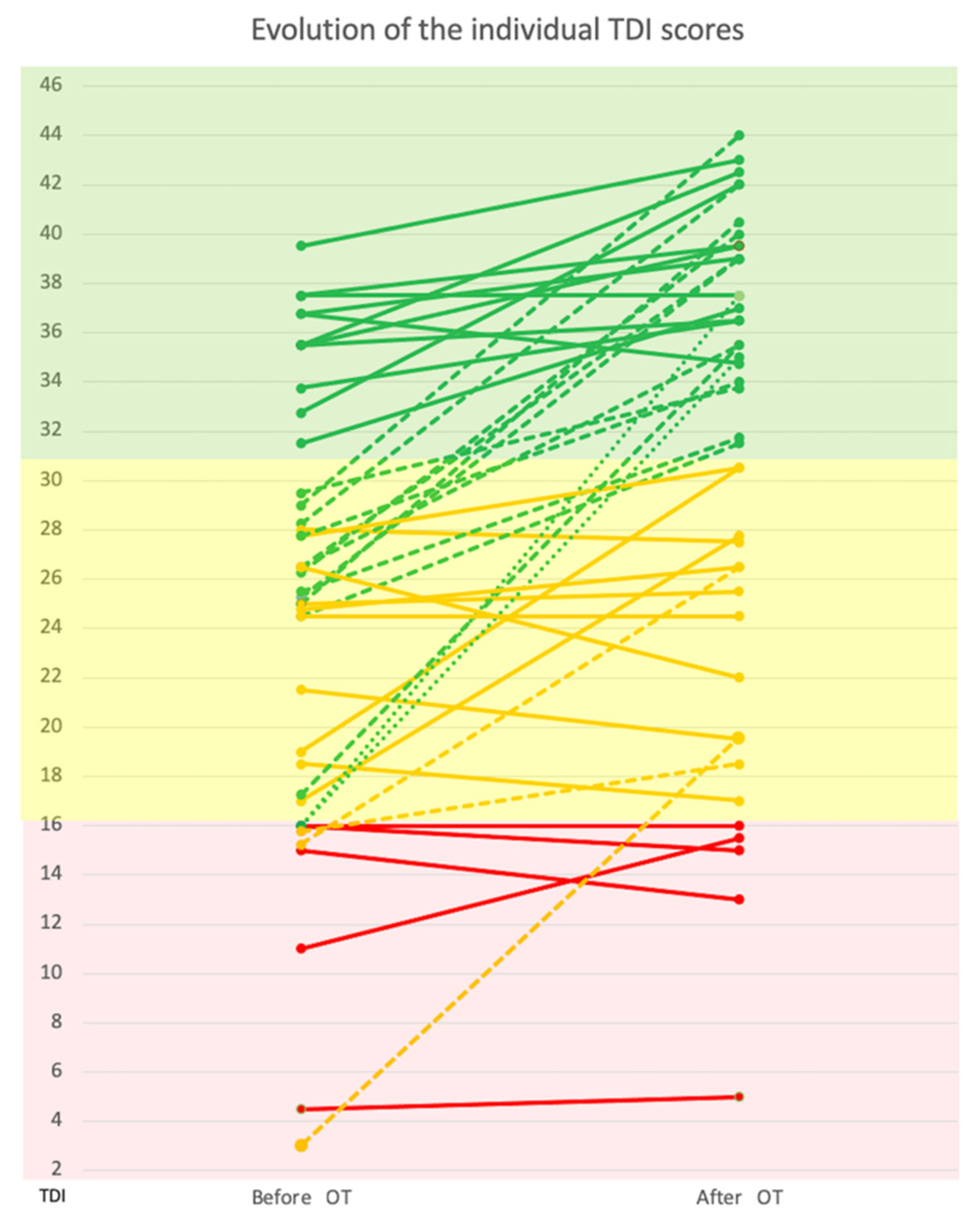
3.2.2. TDI
3.2.3. Qualitative Dysosmia
3.3. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riestra-Ayora, J.; Yanes-Diaz, J.; Esteban-Sanchez, J.; Vaduva, C.; Molina-Quiros, C.; Larran-Jimenez, A.; Martin-Sanz, E. Long-Term Follow-up of Olfactory and Gustatory Dysfunction in COVID-19: 6 Months Case—Control Study of Health Workers. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4831–4837. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, D.; Molinari, G.; Silvestri, M.; De Corso, E.; Guaraldi, G.; Mussini, C.; Presutti, L.; Fernandez, I.J. Patient-Reported Olfactory Recovery after SARS-CoV-2 Infection: A 6-Month Follow-Up Study. Int. Forum Allergy Rhinol. 2021, 11, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Surda, P.; Vaira, L.A.; Lechien, J.R.; Safarian, M.; Saussez, S.; Kumar, N. Six Month Follow-Up of Self-Reported Loss of Smell during the COVID-19 Pandemic. Rhinol. J. 2021, 59, 26–31. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Antonucci, P.; Capriotti, V.; Sacchet, E.; Cragnolini, F.; D’Alessandro, A.; Zanelli, E.; et al. Self-Reported Smell and Taste Recovery in Coronavirus Disease 2019 Patients: A One-Year Prospective Study. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 515–520. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Beckers, E.; Mustin, V.; Ducarme, M.; Journe, F.; Marchant, A.; Jouffe, L.; Barillari, M.R.; Cammaroto, G.; et al. Prevalence and 6-Month Recovery of Olfactory Dysfunction: A Multicentre Study of 1363 COVID-19 Patients. J. Intern. Med. 2021, 290, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Petrocelli, M.; Cutrupi, S.; Salzano, G.; Maglitto, F.; Salzano, F.A.; Lechien, J.R.; Saussez, S.; Boscolo-Rizzo, P.; De Riu, G.; Vaira, L.A. Six-Month Smell and Taste Recovery Rates in Coronavirus Disease 2019 Patients: A Prospective Psychophysical Study. J. Laryngol. Otol. 2021, 135, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, T.; Evelina, T.; Mats, J.O.; Nina, G.-N.; Sebastian, H.; Charlotte, T.; Johan, N.L. High Prevalence of Olfactory Disorders 18 Months after Contracting COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Vandersteen, C.; Payne, M.; Dumas, L.-E.; Metelkina-Fernandez, V.; Plonka, A.; Chirio, D.; Demonchy, E.; Risso, K.; Askenazy-Gittard, F.; Guevara, N.; et al. Persistent Olfactory Complaints after COVID-19: A New Interpretation of the Psychophysical Olfactory Scores. Rhinol. Online 2021, 4, 66–72. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.-S.; Hummel, T. The Influence of Olfactory Loss on Dietary Behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef]
- Valsamidis, K.; Printza, A.; Constantinidis, J.; Triaridis, S. The Impact of Olfactory Dysfunction on the Psychological Status and Quality of Life of Patients with Nasal Obstruction and Septal Deviation. Int. Arch. Otorhinolaryngol. 2020, 24, e237–e246. [Google Scholar] [CrossRef] [Green Version]
- Hur, K.; Choi, J.S.; Zheng, M.; Shen, J.; Wrobel, B. Association of Alterations in Smell and Taste with Depression in Older Adults. Laryngoscope Investig. Otolaryngol. 2018, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory Disorders and Quality of Life-An Updated Review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, S. Sensory Perception of Food and Ageing. In Food for the Ageing Population; Elsevier: Amsterdam, The Netherlands, 2009; pp. 73–94. ISBN 9781845691936. [Google Scholar]
- Pence, T.S.; Reiter, E.R.; DiNardo, L.J.; Costanzo, R.M. Risk Factors for Hazardous Events in Olfactory-Impaired Patients. JAMA Otolaryngol. Neck Surg. 2014, 140, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position Paper on Olfactory Dysfunction. Rhinol. J. 2017, 54, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Webster, K.E.; O’Byrne, L.; MacKeith, S.; Philpott, C.; Hopkins, C.; Burton, M.J. Interventions for the Prevention of Persistent Post-COVID-19 Olfactory Dysfunction. Cochrane Database Syst. Rev. 2021, 7, CD013877. [Google Scholar] [CrossRef]
- Hummel, T.; Rissom, K.; Reden, J.; Hähner, A.; Weidenbecher, M.; Hüttenbrink, K.-B. Effects of Olfactory Training in Patients with Olfactory Loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef]
- Damm, M.; Pikart, L.K.; Reimann, H.; Burkert, S.; Göktas, Ö.; Haxel, B.; Frey, S.; Charalampakis, I.; Beule, A.; Renner, B.; et al. Olfactory Training Is Helpful in Postinfectious Olfactory Loss: A Randomized, Controlled, Multicenter Study. Laryngoscope 2014, 124, 826–831. [Google Scholar] [CrossRef]
- Kattar, N.; Do, T.M.; Unis, G.D.; Migneron, M.R.; Thomas, A.J.; McCoul, E.D. Olfactory Training for Postviral Olfactory Dysfunction: Systematic Review and Meta-Analysis. Otolaryngol. Neck Surg. 2021, 164, 244–254. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks Normative Data Based on an Extended Sample of 9139 Subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, C.; Chiesa-Estomba, C.M.; Horoi, M.; Le Bon, S.D.; Hans, S.; Distinguin, L.; Chekkoury-Idrissi, Y.; Circiu, M.P.; Khalife, M.; Saussez, S.; et al. Validity and Reliability of the French Short Version of the Questionnaire of Olfactory Disorders-Negative Statements (SQOD-NS). Ear Nose Throat J. 2021, 014556132110320. [Google Scholar] [CrossRef]
- Leplège, A.; Ecosse, E.; Coste, J.; Pouchot, J.; Perneger, T. Le Questionnaire MOS SF-36: Manuel de l’utilisateur et Guide d’interprétation Des Scores; Editions ESTEM: Paris, France, 2001; ISBN 2843711185/9782843711183. [Google Scholar]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kollndorfer, K.; Fischmeister, F.P.S.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Trattnig, S.; Schöpf, V. Olfactory Training Induces Changes in Regional Functional Connectivity in Patients with Long-Term Smell Loss. NeuroImage Clin. 2015, 9, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokowska, A.; Drechsler, E.; Karwowski, M.; Hummel, T. Effects of Olfactory Training: A Meta-Analysis. Rhinol. J. 2017, 55, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleszkiewicz, A.; Hanf, S.; Whitcroft, K.L.; Haehner, A.; Hummel, T. Examination of Olfactory Training Effectiveness in Relation to Its Complexity and the Cause of Olfactory Loss. Laryngoscope 2018, 128, 1518–1522. [Google Scholar] [CrossRef]
- Simopoulos, E.; Katotomichelakis, M.; Gouveris, H.; Tripsianis, G.; Livaditis, M.; Danielides, V. Olfaction-Associated Quality of Life in Chronic Rhinosinusitis: Adaptation and Validation of an Olfaction-Specific Questionnaire. Laryngoscope 2012, 122, 1450–1454. [Google Scholar] [CrossRef]
- Mattos, J.L.; Edwards, C.; Schlosser, R.J.; Hyer, M.; Mace, J.C.; Smith, T.L.; Soler, Z.M. A Brief Version of the Questionnaire of Olfactory Disorders in Patients with Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 1144–1150. [Google Scholar] [CrossRef]
- Perneger, T.V.; Leplège, A.; Etter, J.-F.; Rougemont, A. Validation of a French-Language Version of the MOS 36-Item Short Form Health Survey (SF-36) in Young Healthy Adults. J. Clin. Epidemiol. 1995, 48, 1051–1060. [Google Scholar] [CrossRef]
- Bordin, A.; Mucignat-Caretta, C.; Gaudioso, P.; Pendolino, A.L.; Leoni, D.; Scarpa, B.; Andrews, P.J.; Cattelan, A.M.; Antonini, A.; Nicolai, P.; et al. Comparison of Self-reported Symptoms and Psychophysical Tests in Coronavirus Disease 2019 (COVID-19) Subjects Experiencing Long-Term Olfactory Dysfunction: A 6-Month Follow-Up Study. Int. Forum Allergy Rhinol. 2021, 11, 1592–1595. [Google Scholar] [CrossRef]
- Niklassen, A.S.; Draf, J.; Huart, C.; Hintschich, C.; Bocksberger, S.; Trecca, E.M.C.; Klimek, L.; Le Bon, S.D.; Altundag, A.; Hummel, T. COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre Study on Smell and Taste. Laryngoscope 2021, 131, 1095–1100. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, p.; Hummel, T.; Hopkins, C.; Dibattista, M.; Menini, A.; Spinato, G.; Fabbris, C.; Emanuelli, E.; D’Alessandro, A.; Marzolino, R.; et al. High Prevalence of Long-Term Olfactory, Gustatory, and Chemesthesis Dysfunction in Post-COVID-19 Patients: A Matched Case-Control Study with One-Year Follow-Up Using a Comprehensive Psychophysical Evaluation. Rhinol. J. 2021, 59, 517–527. [Google Scholar] [CrossRef]
- Iannuzzi, L.; Salzo, A.E.; Angarano, G.; Palmieri, V.O.; Portincasa, P.; Saracino, A.; Gelardi, M.; Dibattista, M.; Quaranta, N. Gaining Back What Is Lost: Recovering the Sense of Smell in Mild to Moderate Patients after COVID-19. Chem. Senses 2020, 45, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Jafar, A.; Lasso, A.; Shorr, R.; Hutton, B.; Kilty, S. Olfactory Recovery Following Infection with COVID-19: A Systematic Review. PLoS ONE 2021, 16, e0259321. [Google Scholar] [CrossRef] [PubMed]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19–Related Anosmia Is Associated with Viral Persistence and Inflammation in Human Olfactory Epithelium and Brain Infection in Hamsters. Sci. Transl. Med. 2021, 13, eabf8396. [Google Scholar] [CrossRef] [PubMed]
- Ferreli, F.; Gaino, F.; Russo, E.; Di Bari, M.; Rossi, V.; De Virgilio, A.; Di Stadio, A.; Spriano, G.; Mercante, G. Long-Term Olfactory Dysfunction in COVID-19 Patients: 18-Month Follow-Up Study. Int. Forum Allergy Rhinol. 2022, 5, 1–3. [Google Scholar] [CrossRef]
- Hopkins, C.; Surda, P.; Whitehead, E.; Kumar, B.N. Early Recovery Following New Onset Anosmia during the COVID-19 Pandemic—An Observational Cohort Study. J. Otolaryngol.-Head Neck Surg. 2020, 49, 26. [Google Scholar] [CrossRef]
- Vaira, L.A.; Hopkins, C.; Petrocelli, M.; Lechien, J.R.; Chiesa-Estomba, C.M.; Salzano, G.; Cucurullo, M.; Salzano, F.A.; Saussez, S.; Boscolo-Rizzo, P.; et al. Smell and Taste Recovery in Coronavirus Disease 2019 Patients: A 60-Day Objective and Prospective Study. J. Laryngol. Otol. 2020, 134, 703–709. [Google Scholar] [CrossRef]
- Vaira, L.A.; Salzano, G.; Le Bon, S.D.; Maglio, A.; Petrocelli, M.; Steffens, Y.; Ligas, E.; Maglitto, F.; Lechien, J.R.; Saussez, S.; et al. Prevalence of Persistent Olfactory Disorders in Patients with COVID-19: A Psychophysical Case-Control Study with 1-Year Follow-Up. Otolaryngol. Neck Surg. 2021, 23, 019459982110615. [Google Scholar] [CrossRef]
- Le Bon, S.-D.; Konopnicki, D.; Pisarski, N.; Prunier, L.; Lechien, J.R.; Horoi, M. Efficacy and Safety of Oral Corticosteroids and Olfactory Training in the Management of COVID-19-Related Loss of Smell. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3113–3117. [Google Scholar] [CrossRef]
- Saussez, S.; Vaira, L.A.; Chiesa-Estomba, C.M.; Le Bon, S.D.; Horoi, M.; Deiana, G.; Petrocelli, M.; Boelpaep, P.; Salzano, G.; Khalife, M.; et al. Short-Term Efficacy and Safety of Oral and Nasal Corticosteroids in COVID-19 Patients with Olfactory Dysfunction: A European Multicenter Study. Pathogens 2021, 10, 698. [Google Scholar] [CrossRef]
- Abdelalim, A.A.; Mohamady, A.A.; Elsayed, R.A.; Elawady, M.A.; Ghallab, A.F. Corticosteroid Nasal Spray for Recovery of Smell Sensation in COVID-19 Patients: A Randomized Controlled Trial. Am. J. Otolaryngol. 2021, 42, 102884. [Google Scholar] [CrossRef]
- Ojha, P.; Dixit, A. Olfactory Training for Olfactory Dysfunction in COVID-19: A Promising Mitigation amidst Looming Neurocognitive Sequelae of the Pandemic. Clin. Exp. Pharmacol. Physiol. 2022, 49, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Stupka, G.; Haehner, A.; Poletti, S.C. Olfactory Training Changes Electrophysiological Responses at the Level of the Olfactory Epithelium. Rhinology 2018, 56, 330–335. [Google Scholar] [CrossRef]
- Kollndorfer, K.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Pollak, M.; Trattnig, S.; Schöpf, V. Recovery of Olfactory Function Induces Neuroplasticity Effects in Patients with Smell Loss. Neural Plast. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Negoias, S.; Pietsch, K.; Hummel, T. Changes in Olfactory Bulb Volume Following Lateralized Olfactory Training. Brain Imaging Behav. 2017, 11, 998–1005. [Google Scholar] [CrossRef]
- McAlpine, L.S.; Fesharaki-Zadeh, A.; Spudich, S. Coronavirus Disease 2019 and Neurodegenerative Disease: What Will the Future Bring? Curr. Opin. Psychiatry 2021, 34, 177–185. [Google Scholar] [CrossRef]
- Erausquin, G.A.; Snyder, H.; Carrillo, M.; Hosseini, A.A.; Brugha, T.S.; Seshadri, S. The Chronic Neuropsychiatric Sequelae of COVID-19: The Need for a Prospective Study of Viral Impact on Brain Functioning. Alzheimer’s Dement. 2021, 17, 1056–1065. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and Long-Term Consequences of COVID-19 Infections for the Development of Neurological Disease. Alzheimers. Res. Ther. 2020, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Beshel, J.; Kay, L.M. An Olfacto-Hippocampal Network Is Dynamically Involved in Odor-Discrimination Learning. J. Neurophysiol. 2007, 98, 2196–2205. [Google Scholar] [CrossRef] [Green Version]
- Hedner, M.; Larsson, M.; Arnold, N.; Zucco, G.M.; Hummel, T. Cognitive Factors in Odor Detection, Odor Discrimination, and Odor Identification Tasks. J. Clin. Exp. Neuropsychol. 2010, 32, 1062–1067. [Google Scholar] [CrossRef]
- Karimi-Galougahi, M.; Yousefi-Koma, A.; Bakhshayeshkaram, M.; Raad, N.; Haseli, S. 18FDG PET/CT Scan Reveals Hypoactive Orbitofrontal Cortex in Anosmia of COVID-19. Acad. Radiol. 2020, 27, 1042–1043. [Google Scholar] [CrossRef]
- Donegani, M.I.; Miceli, A.; Pardini, M.; Bauckneht, M.; Chiola, S.; Pennone, M.; Marini, C.; Massa, F.; Raffa, S.; Ferrarazzo, G.; et al. Brain Metabolic Correlates of Persistent Olfactory Dysfunction after SARS-CoV2 Infection. Biomedicines 2021, 9, 287. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.-Y.; Huang, C.-C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-Based 3-Month Follow-Up Study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Parker, J.K.; Kelly, C.E.; Gane, S.B. Molecular Mechanism of Parosmia. medRxiv 2021. [Google Scholar] [CrossRef]
- Bitter, T.; Siegert, F.; Gudziol, H.; Burmeister, H.P.; Mentzel, H.-J.; Hummel, T.; Gaser, C.; Guntinas-Lichius, O. Gray Matter Alterations in Parosmia. Neuroscience 2011, 177, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Iannilli, E.; Leopold, D.A.; Hornung, D.E.; Hummel, T. Advances in Understanding Parosmia: An FMRI Study. ORL 2019, 81, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Meunier, N.; Briand, L.; Jacquin-Piques, A.; Brondel, L.; Pénicaud, L. COVID 19-Induced Smell and Taste Impairments: Putative Impact on Physiology. Front. Physiol. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vandersteen, C.; Payne, M.; Dumas, L.É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Robert, P.; Fernandez, X.; et al. What about Using Sniffin’ Sticks 12 Items Test to Screen Post-COVID-19 Olfactory Disorders? Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3477–3484. [Google Scholar] [CrossRef]
- Smeets, M.A.M.; Veldhuizen, M.G.; Galle, S.; Gouweloos, J.; de Haan, A.-M.M.J.A.; Vernooij, J.; Visscher, F.; Kroeze, J.H.A. Sense of Smell Disorder and Health-Related Quality of Life. Rehabil. Psychol. 2009, 54, 404–412. [Google Scholar] [CrossRef]
- Yom-Tov, E.; Lekkas, D.; Jacobson, N.C. Association of COVID19-Induced Anosmia and Ageusia with Depression and Suicidal Ideation. J. Affect. Disord. Rep. 2021, 5, 100156. [Google Scholar] [CrossRef]

| Mean | SD | |
|---|---|---|
| Age (years) | 41 | 13 |
| Months post-COVID-19 | 5.8 | 3.2 |
| n | % | |
| Total | 43 | 100 |
| Sex | ||
| Female | 26 | 61 |
| Male | 17 | 39 |
| Medical history | ||
| Smokers | 8 | 18.6 |
| Type II diabetes | 2 | 4.6 |
| HTA * | 1 | 2.3 |
| GERD ** | 3 | 7 |
| Neurological diseases | 3 | 7 |
| Self-immune diseases | 3 | 7 |
| Chronic rhinosinusitis | ||
| Allergic | 16 | 37.2 |
| CRSnNP † | 5 | 11.6 |
| CRSwNP ‡ | 0 | 0 |
| Neurologic diseases | 3 | 7 |
| COVID-19 Severity | ||
| Mild to moderate illness | 40 | 93 |
| Severe illness | 3 | 7 |
| Chemo sensorial complain | 37 | 86 |
| Flavors impairment | 35 | 81.4 |
| Taste impairment | 10 | 23.3 |
| BEFORE OT | AFTER OT | ||||
|---|---|---|---|---|---|
| Quality of Life Score | Mean | SD | Mean | SD | p |
| Short-QOD-NS | |||||
| Total score | 10.44 | 5.97 | 13.65 | 6.49 | <0.001 *** |
| Social subdomain | 4.58 | 2.7 | 5.88 | 2.86 | 0.001 ** |
| Food subdomain | 2.98 | 2.22 | 3.72 | 2.42 | 0.036 * |
| Anxiety subdomain | 1.91 | 1.02 | 2.44 | 1.12 | 0.020 * |
| Annoyance subdomain | 0.98 | 1.03 | 1.60 | 1.20 | 0.020 * |
| SF36 | |||||
| Physical functioning | 79.53 | 25.42 | 85.81 | 23.20 | 0.009 ** |
| Social functioning | 66.28 | 28.68 | 74.13 | 26.78 | 0.013 * |
| Physical role | 66.28 | 40.42 | 71.51 | 38.80 | 0.407 |
| Emotional role | 55.81 | 41.61 | 64.34 | 42.04 | 0.049 * |
| General mental health | 62.05 | 21.60 | 61.11 | 21.33 | 0.844 |
| Vitality | 44.54 | 24.07 | 51.86 | 21.63 | 0.023 * |
| Bodily pain | 66.61 | 31.64 | 69.63 | 33.51 | 0.337 |
| General health perception | 63.63 | 26.09 | 69.56 | 23.22 | 0.045 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandersteen, C.; Payne, M.; Dumas, L.-É.; Cancian, É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; et al. Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. J. Clin. Med. 2022, 11, 3275. https://doi.org/10.3390/jcm11123275
Vandersteen C, Payne M, Dumas L-É, Cancian É, Plonka A, D’Andréa G, Chirio D, Demonchy É, Risso K, Askenazy-Gittard F, et al. Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. Journal of Clinical Medicine. 2022; 11(12):3275. https://doi.org/10.3390/jcm11123275
Chicago/Turabian StyleVandersteen, Clair, Magali Payne, Louise-Émilie Dumas, Élisa Cancian, Alexandra Plonka, Grégoire D’Andréa, David Chirio, Élisa Demonchy, Karine Risso, Florence Askenazy-Gittard, and et al. 2022. "Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification" Journal of Clinical Medicine 11, no. 12: 3275. https://doi.org/10.3390/jcm11123275
APA StyleVandersteen, C., Payne, M., Dumas, L.-É., Cancian, É., Plonka, A., D’Andréa, G., Chirio, D., Demonchy, É., Risso, K., Askenazy-Gittard, F., Savoldelli, C., Guevara, N., Robert, P., Castillo, L., Manera, V., & Gros, A. (2022). Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. Journal of Clinical Medicine, 11(12), 3275. https://doi.org/10.3390/jcm11123275










