Effects of Smart Drugs on Cholinergic System and Non-Neuronal Acetylcholine in the Mouse Hippocampus: Histopathological Approach
Abstract
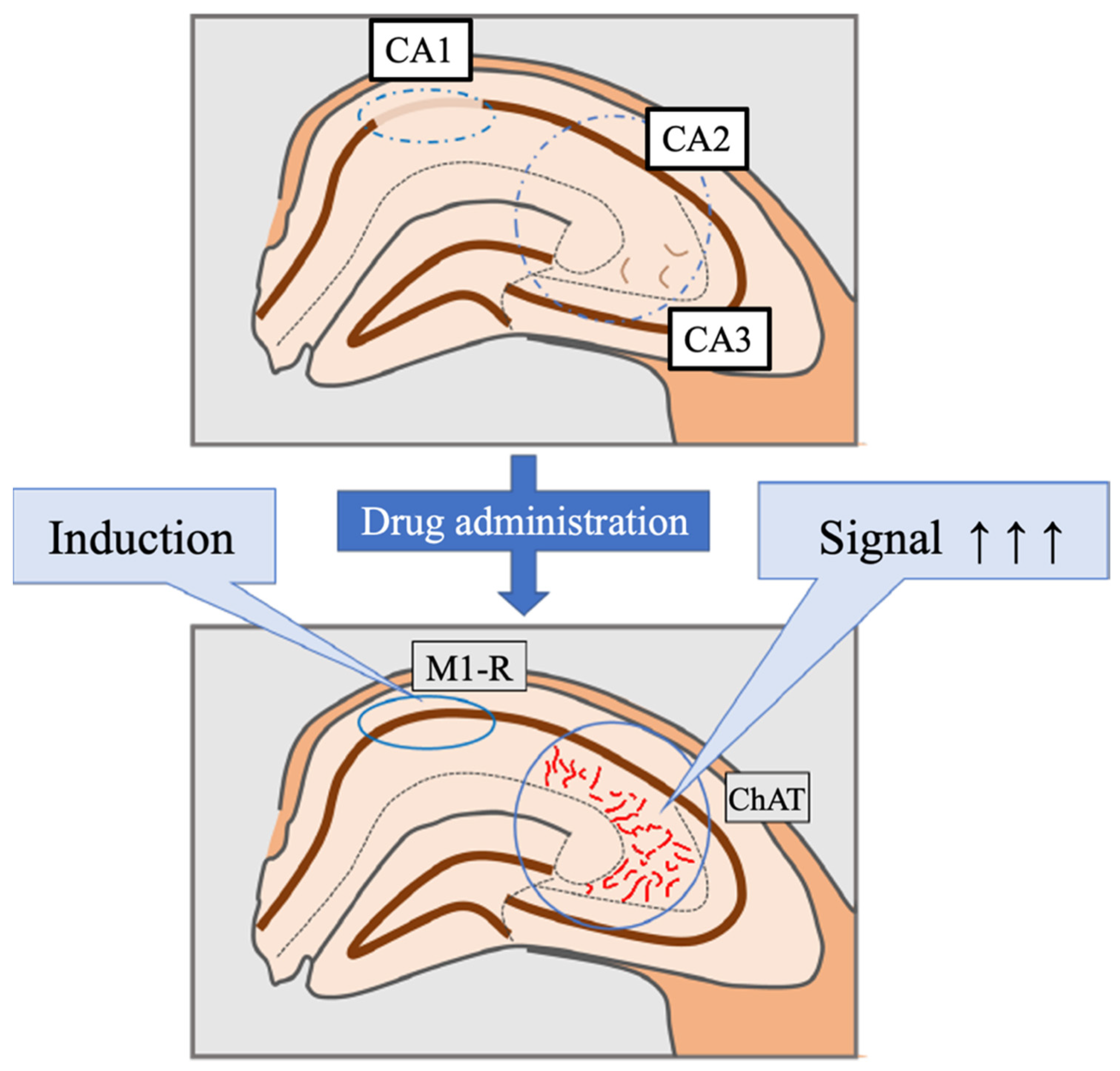
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Administration
2.3. Tissue Preparation
2.4. Histological and Immunohistochemical Analysis
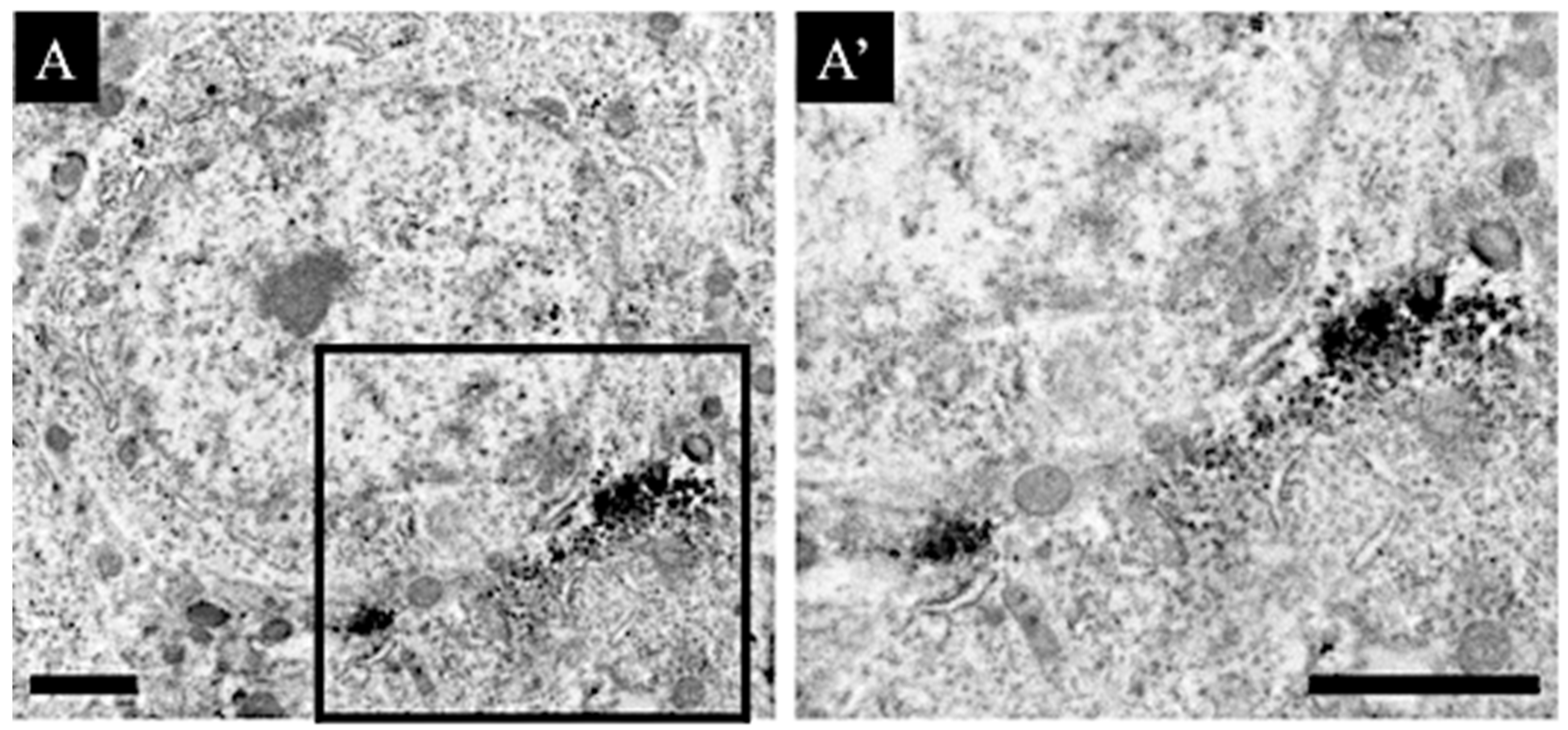
2.5. Immunoelectron Microscopic Analysis
2.6. Western Blot Analysis
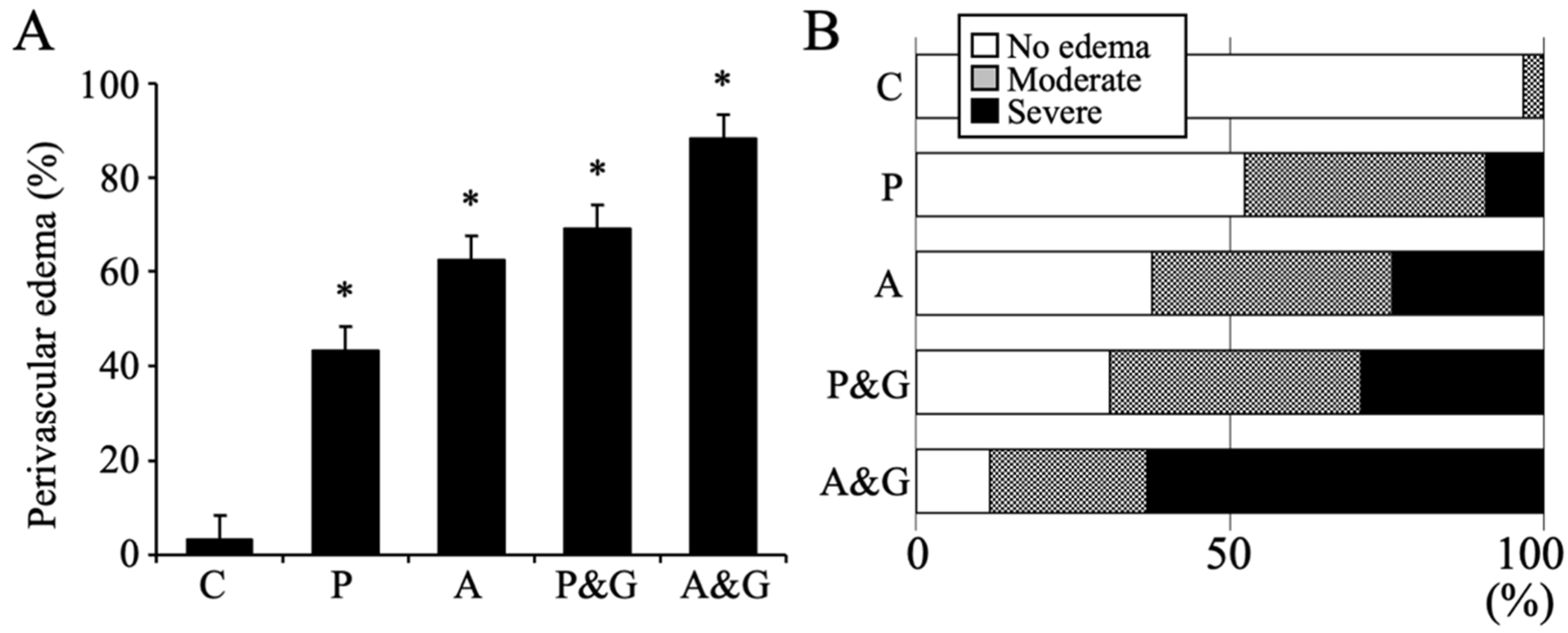
2.7. Data Analysis and Measurement of Perivascular Edema
3. Results
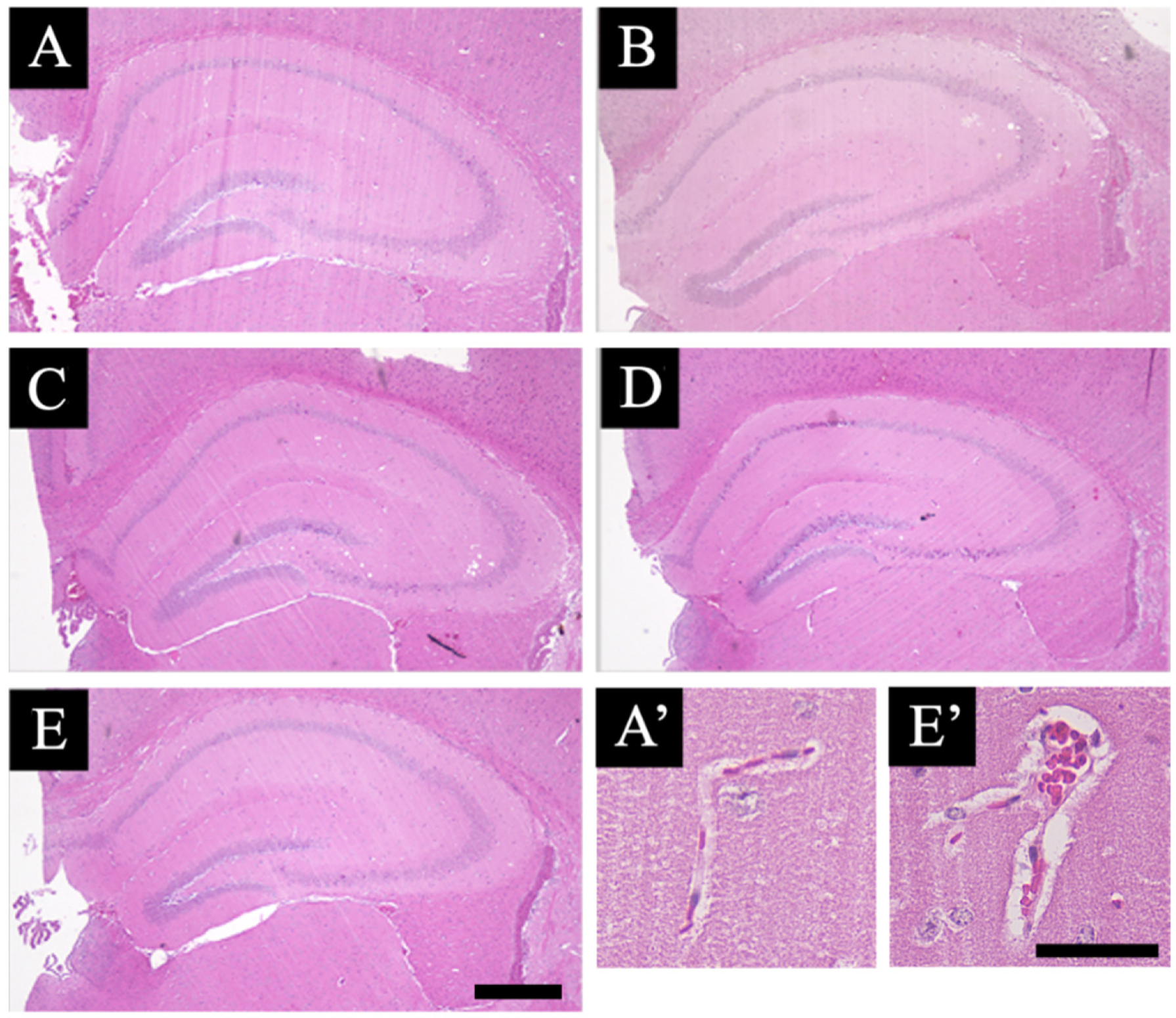
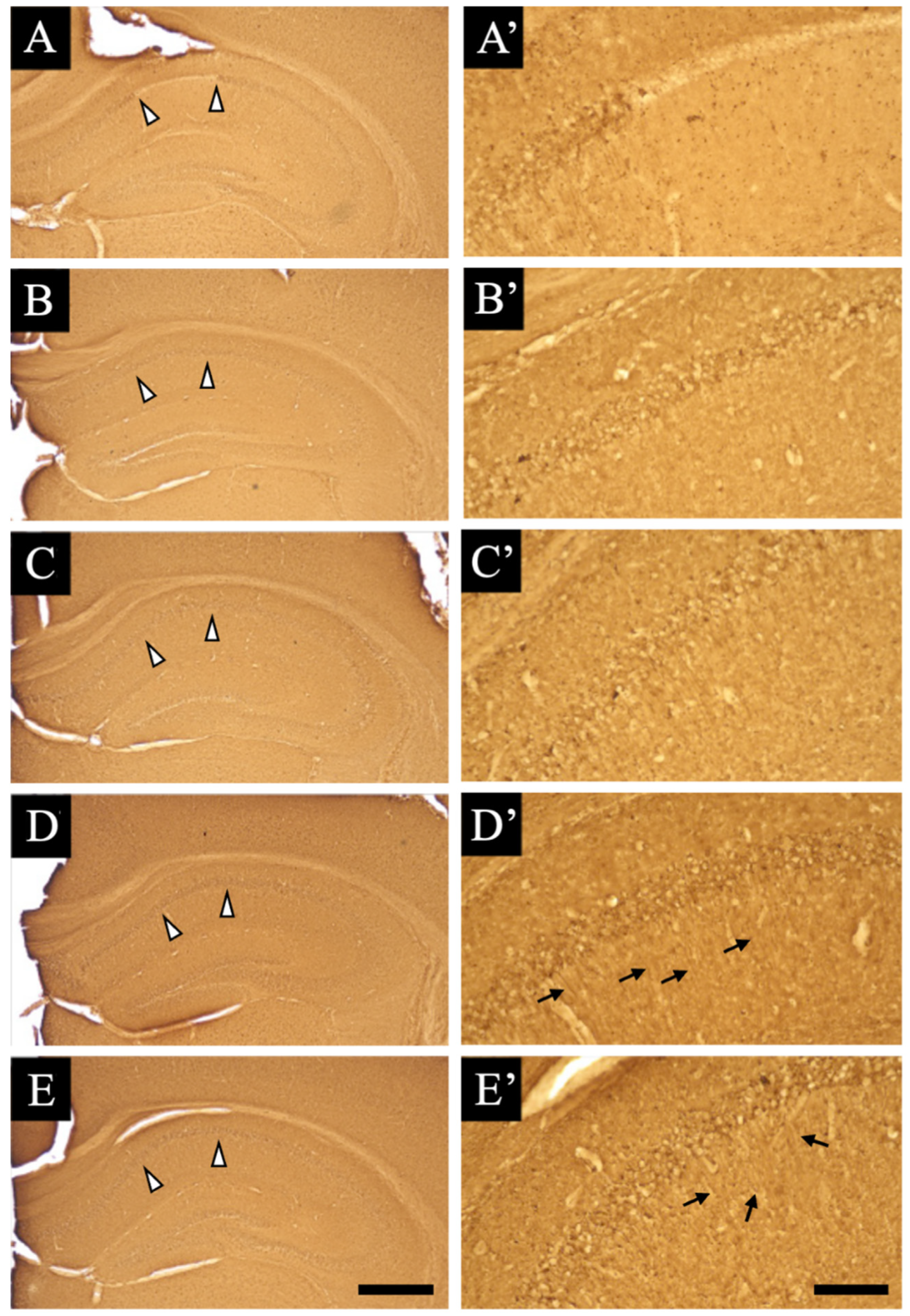
3.1. Pathological Analysis
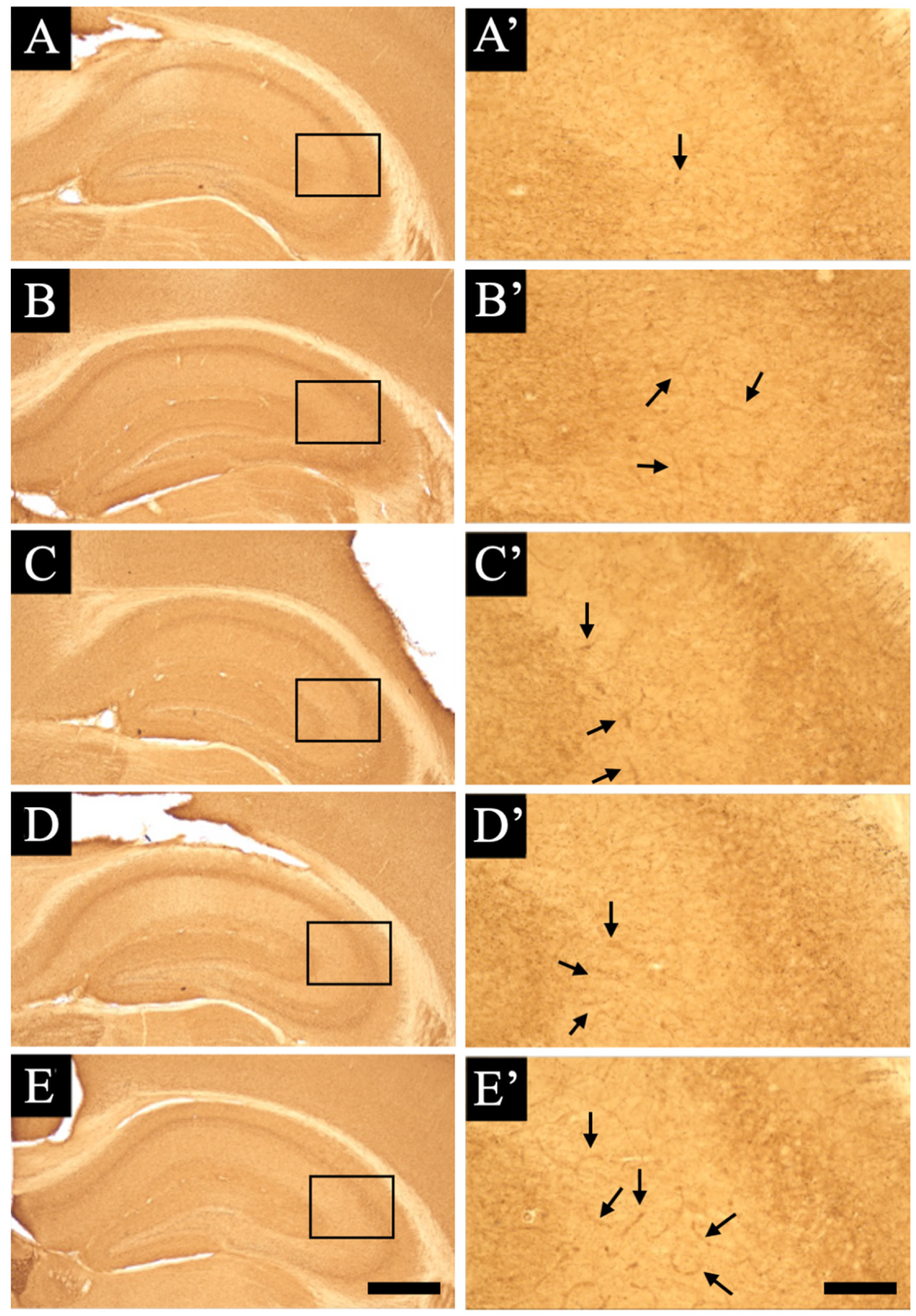
3.2. Distribution Changes in Acetylcholinesterase
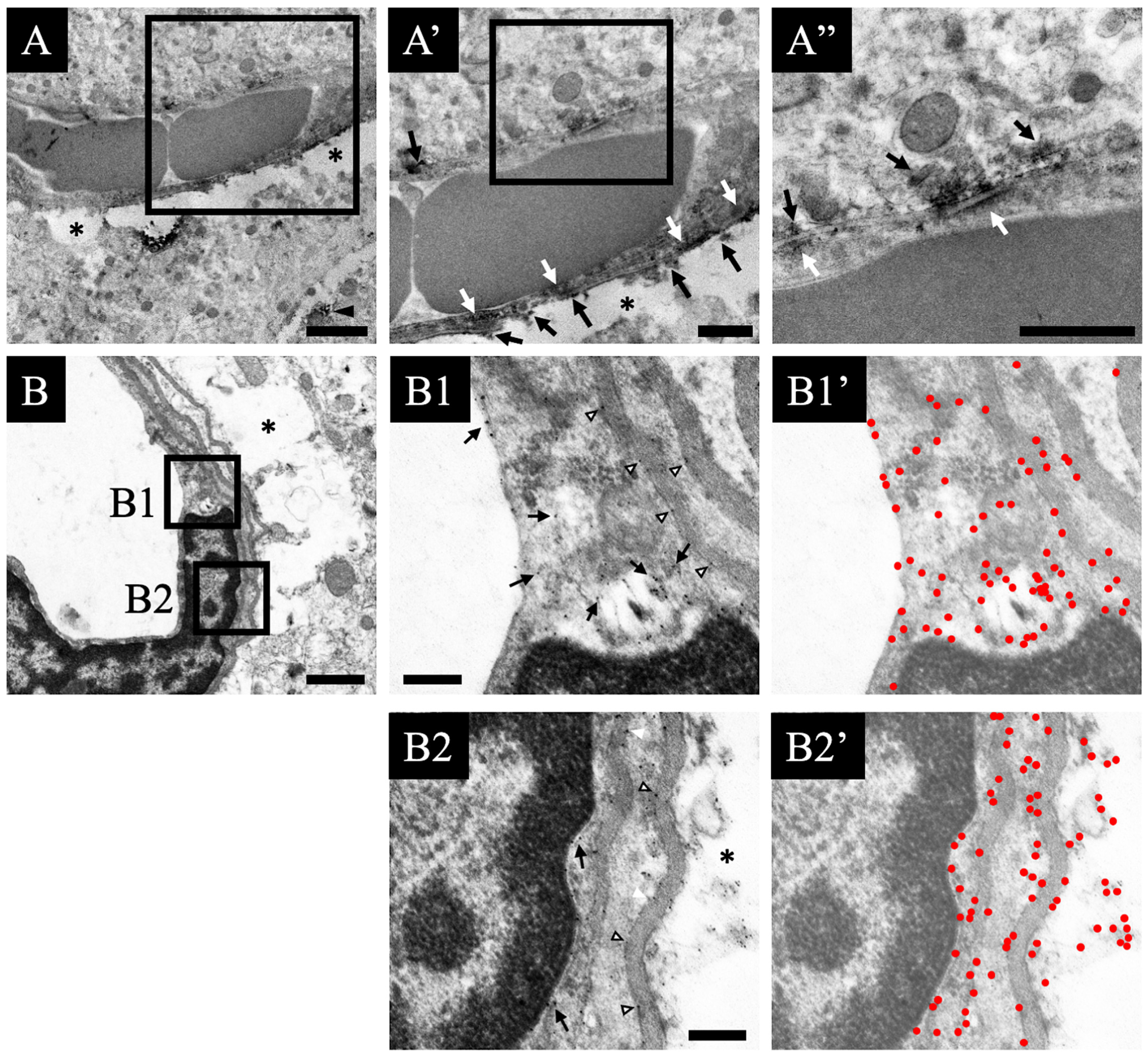
3.3. Distribution Changes in Choline Acetyltransferase (Immunohistochemical and Immuno-Ultrastructural Analysis)
3.4. Distribution Changes in the M1 Muscarinic Acetylcholine Receptors (Immunohistochemical and Immuno-Ultrastructural Analysis)
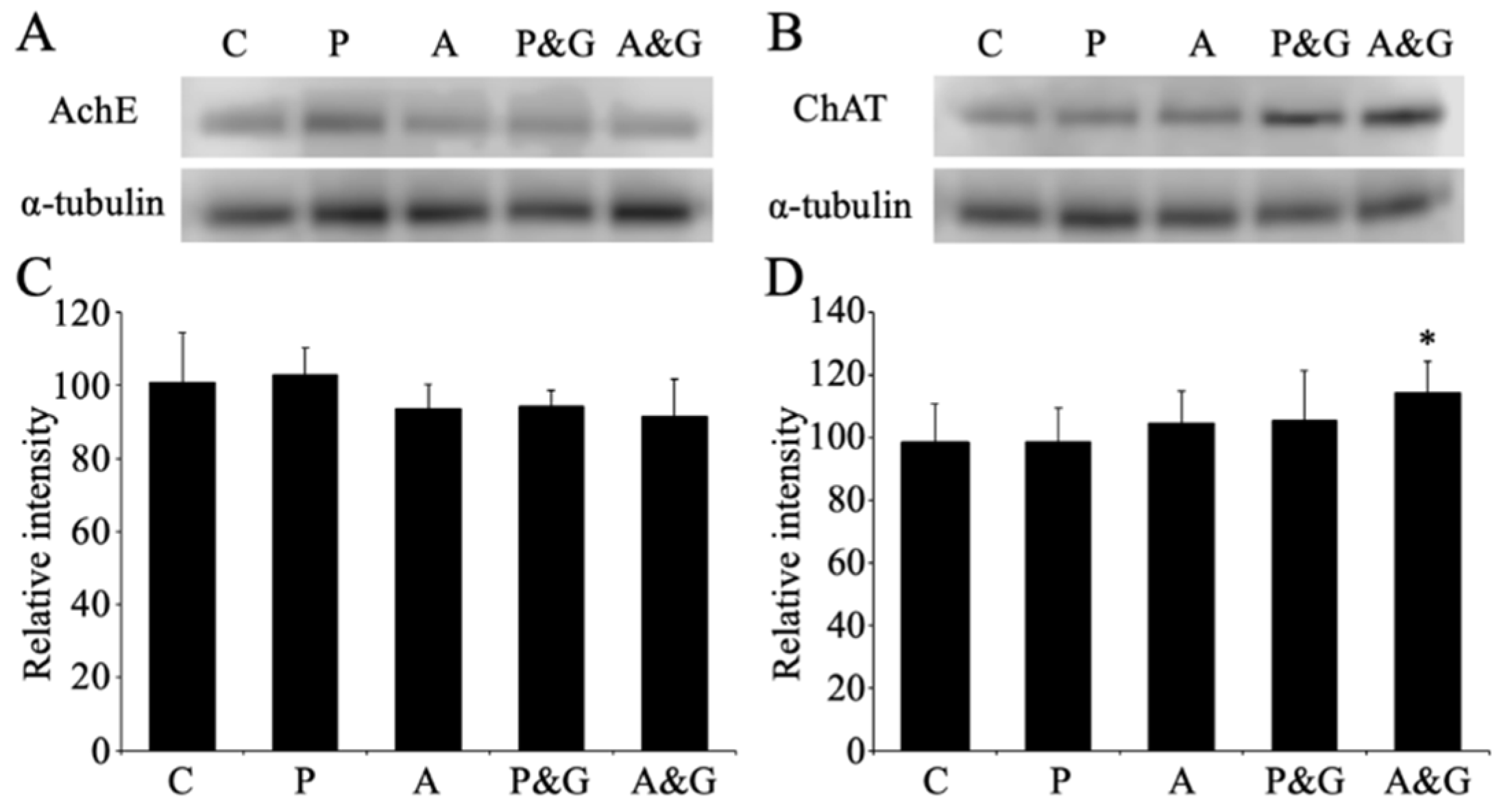
3.5. Changes in the Expression of Acetylcholinesterase and Choline Acetyltransferase after Smart Drug Administration
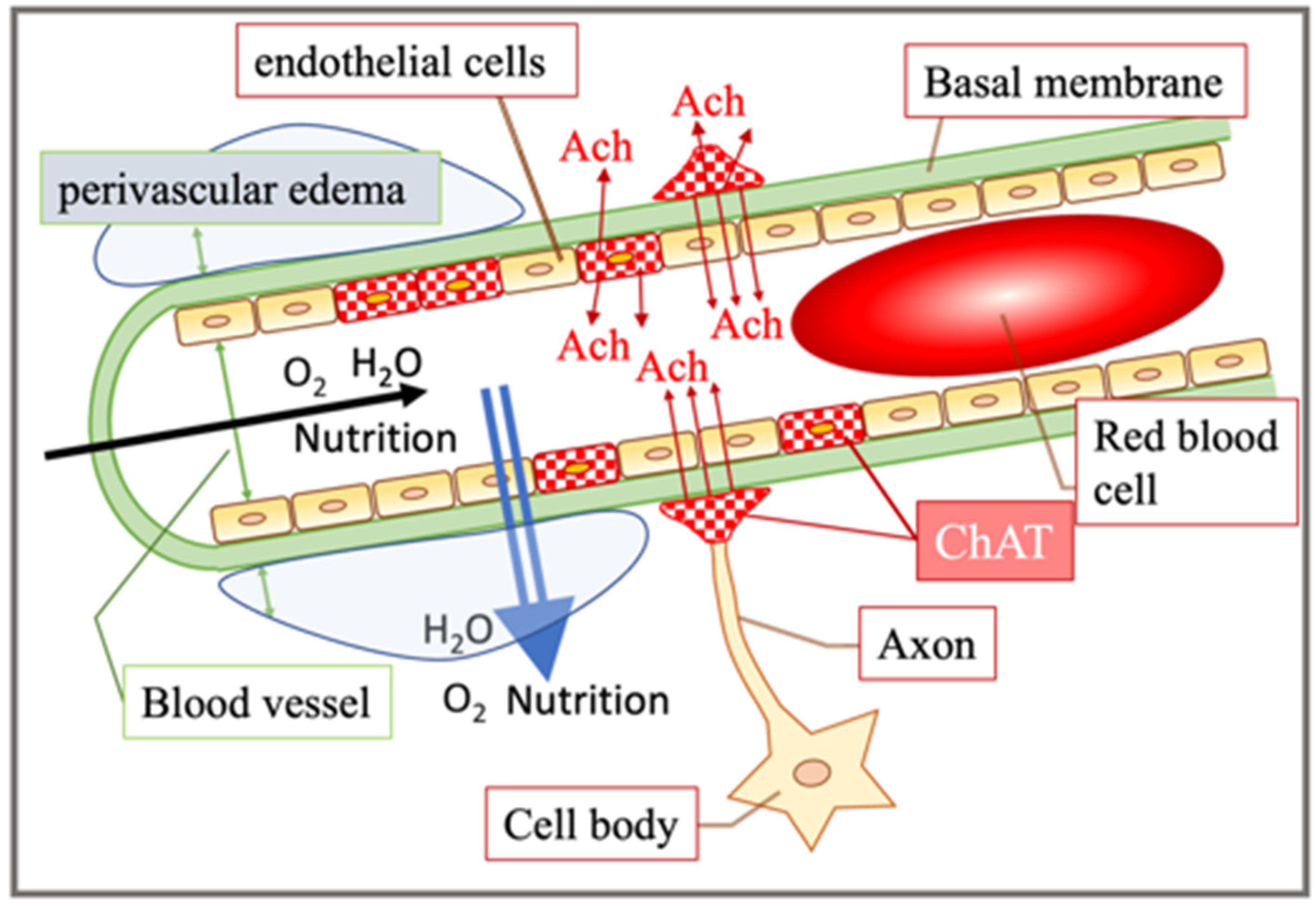
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alster, P.; Dunalska, A.; Migda, B.; Madetko, N.; Krolicki, L. The Rate of Decrease in Brain Perfusion in Progressive Supranuclear Palsy and Corticobasal Syndrome May be Impacted by Glycemic Variability—A Pilot Study. Front. Neurol. 2021, 12, 767480. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, K.; Morihara, R.; Ohta, Y.; Hishikawa, N.; Kawano, S.; Sasaki, R.; Matsumoto, N.; Nomura, E.; Nakano, Y.; Takahashi, Y.; et al. Clinical Benefits of Antioxidative Supplement Twendee X for Mild Cognitive Impairment: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Prospective Interventional Study. J. Alzheimer’s Dis. 2019, 71, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Guirguis, A.; Fergus, S.; Schifano, F. The Use and Impact of Cognitive Enhancers among University Students: A Systematic Review. Brain Sci. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ishii, Y. Awareness survey of smart drugs amoung undergraduates. Jpn. J. Drug Inform. 2018, 10, 41–46. [Google Scholar]
- Gouliaev, A.H.; Senning, A. Piracetam and other structurally related nootropics. Brain Res. Rev. 1994, 19, 180–222. [Google Scholar] [CrossRef]
- Wilsher, C.; Atkins, G.; Manfield, P. Piracetam as an aid to learning in dyslexia. Psychopharmacology 1979, 65, 107–109. [Google Scholar] [CrossRef]
- Mindus, P.; Cronholm, B.; Levander, S.E.; Schalling, D. Piracetam-induced improvement of mental performance. A controlled study on normally aging individuals. Acta Psychiatr. Scand. 1976, 54, 150–160. [Google Scholar] [CrossRef]
- Dimond, S.J.; Brouwers, E.M. Increase in the power of human memory in normal man through the use of drugs. Psychopharmacology 1976, 49, 307–309. [Google Scholar] [CrossRef]
- Leuner, K.; Kurz, C.; Guidetti, G.; Orgogozo, J.M.; Muller, W.E. Improved mitochondrial function in brain aging and Alzheimer disease—The new mechanism of action of the old metabolic enhancer piracetam. Front. Neurosci. 2010, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Waegemans, T.; Wilsher, C.R.; Danniau, A.; Ferris, S.H.; Kurz, A.; Winblad, B. Clinical efficacy of piracetam in cognitive impairment: A meta-analysis. Dement. Geriatr. Cogn. Disord. 2002, 13, 217–224. [Google Scholar] [CrossRef]
- Reynolds, C.D.; Jefferson, T.S.; Volquardsen, M.; Pandian, A.; Smith, G.D.; Holley, A.J.; Lugo, J.N. Oral aniracetam treatment in C57BL/6J mice without pre-existing cognitive dysfunction reveals no changes in learning, memory, anxiety or stereotypy. F1000Research 2017, 6, 1452. [Google Scholar] [CrossRef] [PubMed]
- Elston, T.W.; Pandian, A.; Smith, G.D.; Holley, A.J.; Gao, N.; Lugo, J.N. Aniracetam does not alter cognitive and affective behavior in adult C57BL/6J mice. PLoS ONE 2014, 9, e104443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, H.; McDowell, A.; Mielby, B.S.; Tucker, I.G.; Colombo, M. Aniracetam does not improve working memory in neurologically healthy pigeons. PLoS ONE 2019, 14, e0215612. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, J.S.; Nicoll, R.A. Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. Proc. Natl. Acad. Sci. USA 1991, 88, 10936–10940. [Google Scholar] [CrossRef] [Green Version]
- Kolta, A.; Lynch, G.; Ambros-Ingerson, J. Effects of aniracetam after LTP induction are suggestive of interactions on the kinetics of the AMPA receptor channel. Brain Res. 1998, 788, 269–286. [Google Scholar] [CrossRef]
- Satoh, M.; Ishihara, K.; Iwama, T.; Takagi, H. Aniracetam augments, and midazolam inhibits, the long-term potentiation in guinea-pig hippocampal slices. Neurosci. Lett. 1986, 68, 216–220. [Google Scholar] [CrossRef]
- Kim, S.K.; Ko, Y.H.; Lee, S.Y.; Jang, C.G. Memory-enhancing effects of 7,3′,4′-trihydroxyisoflavone by regulation of cholinergic function and BDNF signaling pathway in mice. Food Chem. Toxicol. 2020, 137, 111160. [Google Scholar] [CrossRef]
- Said, E.S.; Elsayed, A.M.; Rashed, L.A.; Nadwa, E.H.; Alsuhaibani, N.A.; Alfuraih, B.S.; Mahmoud, R.H. Evaluation of nootropic activity of telmisartan and metformin on diazepam-induced cognitive dysfunction in mice through AMPK pathway and amelioration of hippocampal morphological alterations. Eur. J. Pharmacol. 2021, 912, 174511. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Oswald, R.E. Piracetam defines a new binding site for allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. J. Med. Chem. 2010, 53, 2197–2203. [Google Scholar] [CrossRef] [Green Version]
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Andersen, P.; Morris, R.; Amaral, D.; Bliss, T.; O’Keefe, J. The Hippocampus Book; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Lee, J.C.; Park, J.H.; Ahn, J.H.; Park, J.; Kim, I.H.; Cho, J.H.; Shin, B.N.; Lee, T.K.; Kim, H.; Song, M.; et al. Effects of chronic scopolamine treatment on cognitive impairment and neurofilament expression in the mouse hippocampus. Mol. Med. Rep. 2018, 17, 1625–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukatsu, T.; Miyake-Takagi, K.; Nagakura, A.; Omino, K.; Okuyama, N.; Ando, T.; Takagi, N.; Furuya, Y.; Takeo, S. Effects of nefiracetam on spatial memory function and acetylcholine and GABA metabolism in microsphere-embolized rats. Eur. J. Pharmacol. 2002, 453, 59–67. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Ardjmand, A.; Ahmadi, S.; Rezayof, A. Activation of dopamine D1 receptors in the medial septum improves scopolamine-induced amnesia in the dorsal hippocampus. Behav. Brain Res. 2012, 229, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mitsushima, D.; Sano, A.; Takahashi, T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat. Commun. 2013, 4, 2760. [Google Scholar] [CrossRef] [Green Version]
- Nakadate, K.; Imamura, K.; Watanabe, Y. Cellular and subcellular localization of alpha-1 adrenoceptors in the rat visual cortex. Neuroscience 2006, 141, 1783–1792. [Google Scholar] [CrossRef]
- Ehara, A.; Taguchi, D.; Nakadate, K.; Ueda, S. Attractin deficiency causes metabolic and morphological abnormalities in slow-twitch muscle. Cell Tissue Res. 2021, 384, 745–756. [Google Scholar] [CrossRef]
- Taguchi, D.; Ehara, A.; Kadowaki, T.; Sakakibara, S.I.; Nakadate, K.; Hirata, K.; Ueda, S. Minocycline Alleviates Cluster Formation of Activated Microglia and Age-dependent Dopaminergic Cell Death in the Substantia Nigra of Zitter Mutant Rat. Acta Histochem. Cytochem. 2020, 53, 139–146. [Google Scholar] [CrossRef]
- Nakadate, K.; Kamata, S. Severe Acute Hepatic Dysfunction Induced by Ammonium Acetate Treatment Results in Choroid Plexus Swelling and Ventricle Enlargement in the Brain. Int. J. Mol. Sci. 2022, 23, 2010. [Google Scholar] [CrossRef]
- Nakadate, K. Developmental changes in the flotillin-1 expression pattern of the rat visual cortex. Neuroscience 2015, 292, 101–111. [Google Scholar] [CrossRef]
- Nakadate, K.; Imamura, K.; Watanabe, Y. c-Fos activity mapping reveals differential effects of noradrenaline and serotonin depletion on the regulation of ocular dominance plasticity in rats. Neuroscience 2013, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Nakadate, K.; Hanada, K. Minocycline prevents and repairs the skin disorder associated with afatinib, one of the epidermal growth factor receptor-tyrosine kinase inhibitors for non-small cell lung cancer. BMC Cancer 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Tanaka-Nakadate, S. Three-Dimensional Electron Microscopy Reconstruction of Degenerative Dopaminergic Neurons Surrounded by Activated Microglia in Substantia Nigra. Ultrastruct. Pathol. 2015, 39, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Sato-Yazawa, H.; Kashiwagi, K.; Nakadate, K.; Iwamoto, M.; Kohno, K.; Miyata-Hiramatsu, C.; Masawa, M.; Onozaki, M.; Noda, S.; et al. Endocrine secretory granule production is caused by a lack of REST and intragranular secretory content and accelerated by PROX1. J. Mol. Histol. 2022, 53, 437–448. [Google Scholar] [CrossRef]
- Cohen, P.A.; Zakharevich, I.; Gerona, R. Presence of Piracetam in Cognitive Enhancement Dietary Supplements. JAMA Intern. Med. 2020, 180, 458–459. [Google Scholar] [CrossRef]
- Stancheva, S.L.; Petkov, V.D.; Hadjiivanova, C.I.; Petkov, V.V. Age-related changes of the effects of a group of nootropic drugs on the content of rat brain biogenic monoamines. Gen. Pharmacol. 1991, 22, 873–877. [Google Scholar] [CrossRef]
- Saletu, B.; Grunberger, J. Memory dysfunction and vigilance: Neurophysiological and psychopharmacological aspects. Ann. N. Y. Acad. Sci. 1985, 444, 406–427. [Google Scholar] [CrossRef]
- Winnicka, K.; Tomasiak, M.; Bielawska, A. Piracetam—An old drug with novel properties? Acta Pol. Pharm. 2005, 62, 405–409. [Google Scholar]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Bliss, T.V.; Gardner-Medwin, A.R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 357–374. [Google Scholar] [CrossRef]
- Sumi, T.; Harada, K. Mechanism underlying hippocampal long-term potentiation and depression based on competition between endocytosis and exocytosis of AMPA receptors. Sci. Rep. 2020, 10, 14711. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Dong, Z.; Bagot, R.C.; Howland, J.G.; Phillips, A.G.; Wong, T.P.; Wang, Y.T. Hippocampal long-term depression is required for the consolidation of spatial memory. Proc. Natl. Acad. Sci. USA 2010, 107, 16697–16702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, A.; Manahan-Vaughan, D. Hippocampal long-term depression: Master or minion in declarative memory processes? Trends Neurosci. 2007, 30, 111–118. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markram, H.; Segal, M. Acetylcholine potentiates responses to N-methyl-D-aspartate in the rat hippocampus. Neurosci. Lett. 1990, 113, 62–65. [Google Scholar] [CrossRef]
- Park, P.; Kang, H.; Sanderson, T.M.; Bortolotto, Z.A.; Georgiou, J.; Zhuo, M.; Kaang, B.K.; Collingridge, G.L. On the Role of Calcium-Permeable AMPARs in Long-Term Potentiation and Synaptic Tagging in the Rodent Hippocampus. Front. Synaptic. Neurosci. 2019, 11, 4. [Google Scholar] [CrossRef]
- Park, P.; Kang, H.; Sanderson, T.M.; Bortolotto, Z.A.; Georgiou, J.; Zhuo, M.; Kaang, B.K.; Collingridge, G.L. The Role of Calcium-Permeable AMPARs in Long-Term Potentiation at Principal Neurons in the Rodent Hippocampus. Front. Synaptic. Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Mitsushima, D.; Ishihara, K.; Sano, A.; Kessels, H.W.; Takahashi, T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc. Natl. Acad. Sci. USA 2011, 108, 12503–12508. [Google Scholar] [CrossRef] [Green Version]
- Ito, I.; Tanabe, S.; Kohda, A.; Sugiyama, H. Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam. J. Physiol. 1990, 424, 533–543. [Google Scholar] [CrossRef]
- Shinoe, T.; Matsui, M.; Taketo, M.M.; Manabe, T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J. Neurosci. 2005, 25, 11194–11200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukawa, M.; Ogawa, M.; Nakadate, K.; Maeshima, T.; Ichitani, Y.; Kawai, N.; Okado, N. Serotonin and acetylcholine are crucial to maintain hippocampal synapses and memory acquisition in rats. Neurosci. Lett. 1997, 230, 13–16. [Google Scholar] [CrossRef]
- Koukouli, F.; Maskos, U. The multiple roles of the alpha7 nicotinic acetylcholine receptor in modulating glutamatergic systems in the normal and diseased nervous system. Biochem. Pharmacol. 2015, 97, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Drever, B.D.; Riedel, G.; Platt, B. The cholinergic system and hippocampal plasticity. Behav. Brain Res. 2011, 221, 505–514. [Google Scholar] [CrossRef]
- Fujii, S.; Ji, Z.; Sumikawa, K. Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci. Lett. 2000, 286, 134–138. [Google Scholar] [CrossRef]
- Kawashima, K.; Watanabe, N.; Oohata, H.; Fujimoto, K.; Suzuki, T.; Ishizaki, Y.; Morita, I.; Murota, S. Synthesis and release of acetylcholine by cultured bovine arterial endothelial cells. Neurosci. Lett. 1990, 119, 156–158. [Google Scholar] [CrossRef]
- Parnavelas, J.G.; Kelly, W.; Burnstock, G. Ultrastructural localization of choline acetyltransferase in vascular endothelial cells in rat brain. Nature 1985, 316, 724–725. [Google Scholar] [CrossRef]
- Ikeda, C.; Morita, I.; Mori, A.; Fujimoto, K.; Suzuki, T.; Kawashima, K.; Murota, S. Phorbol ester stimulates acetylcholine synthesis in cultured endothelial cells isolated from porcine cerebral microvessels. Brain Res. 1994, 655, 147–152. [Google Scholar] [CrossRef]
- Kirkpatrick, C.J.; Bittinger, F.; Nozadze, K.; Wessler, I. Expression and function of the non-neuronal cholinergic system in endothelial cells. Life Sci. 2003, 72, 2111–2116. [Google Scholar] [CrossRef]
- Kirkpatrick, C.J.; Bittinger, F.; Unger, R.E.; Kriegsmann, J.; Kilbinger, H.; Wessler, I. The non-neuronal cholinergic system in the endothelium: Evidence and possible pathobiological significance. Jpn. J. Pharmacol. 2001, 85, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Wessler, I.; Kilbinger, H.; Bittinger, F.; Kirkpatrick, C.J. The biological role of non-neuronal acetylcholine in plants and humans. Jpn. J. Pharmacol. 2001, 85, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J.; Racke, K. The cholinergic ‘pitfall’: Acetylcholine, a universal cell molecule in biological systems, including humans. Clin. Exp. Pharmacol. Physiol. 1999, 26, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Kirkpatrick, C.J.; Racke, K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: Expression and function in humans. Pharmacol. Ther. 1998, 77, 59–79. [Google Scholar] [CrossRef]
- Grando, S.A. Biological functions of keratinocyte cholinergic receptors. J. Investig. Dermatol. Symp. Proc. 1997, 2, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Sastry, B.V.; Sadavongvivad, C. Cholinergic systems in non-nervous tissues. Pharmacol. Rev. 1978, 30, 65–132. [Google Scholar] [PubMed]
- Grando, S.A.; Kawashima, K.; Wessler, I. Introduction: The non-neuronal cholinergic system in humans. Life Sci. 2003, 72, 2009–2012. [Google Scholar] [CrossRef]
- Klapproth, H.; Reinheimer, T.; Metzen, J.; Munch, M.; Bittinger, F.; Kirkpatrick, C.J.; Hohle, K.D.; Schemann, M.; Racke, K.; Wessler, I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn. Schmiedeberg’s Arch. Pharmacol. 1997, 355, 515–523. [Google Scholar] [CrossRef]
- Hirota, C.L.; McKay, D.M. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br. J. Pharmacol. 2006, 149, 463–479. [Google Scholar] [CrossRef]
- Yajima, T.; Inoue, R.; Matsumoto, M.; Yajima, M. Non-neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J. Physiol. 2011, 589, 953–962. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, R.; Kawakami, K.; Nakadate, K. Effects of Smart Drugs on Cholinergic System and Non-Neuronal Acetylcholine in the Mouse Hippocampus: Histopathological Approach. J. Clin. Med. 2022, 11, 3310. https://doi.org/10.3390/jcm11123310
Satoh R, Kawakami K, Nakadate K. Effects of Smart Drugs on Cholinergic System and Non-Neuronal Acetylcholine in the Mouse Hippocampus: Histopathological Approach. Journal of Clinical Medicine. 2022; 11(12):3310. https://doi.org/10.3390/jcm11123310
Chicago/Turabian StyleSatoh, Ryusei, Kiyoharu Kawakami, and Kazuhiko Nakadate. 2022. "Effects of Smart Drugs on Cholinergic System and Non-Neuronal Acetylcholine in the Mouse Hippocampus: Histopathological Approach" Journal of Clinical Medicine 11, no. 12: 3310. https://doi.org/10.3390/jcm11123310
APA StyleSatoh, R., Kawakami, K., & Nakadate, K. (2022). Effects of Smart Drugs on Cholinergic System and Non-Neuronal Acetylcholine in the Mouse Hippocampus: Histopathological Approach. Journal of Clinical Medicine, 11(12), 3310. https://doi.org/10.3390/jcm11123310





